Abstract
Background: Accumulated studies indicated a crucial role of astrocytes in neuropathic pain (NPP) development, spread and potentiation by a communication with the nervous system. Increased GFAP expression in dorsal horn of the spinal cord indicated the participation of astrocyte in NPP. However, the underlying mechanism is still in need of further investigations. Methods: In our study, the spared nerve injury (SNI) model was established with partial sciatic nerve ligation. The expression status of FGFR3 was studied in spinal dorsal horn of SNI models. The molecular mechanism of spinal astrocytic FGFR3 activation in mechanical hypersensitivity was investigated. Results: SNI rats showed with hind paw mechanical hypersensitivity and increased GFAP expression in their spinal cords. Increased FGFR3 expression was observed in spinal dorsal horn of SNI models, which was consistent with increased GFAP expression. Elevated FGFR3 upregulates GFAP and TNF-α expression in astrocytes in vivo and in vitro. FGFR3 inhibition by PD173074 lead to downregulation of GFAP and TNF-α and increased withdrawal threshold of SNI models. Mechanically, FGFR3-TBX3 axis activation enhanced TNF-α expression in cultured primary spinal astrocytes. Spinal TNF-α synthesis induced mechanical hypersensitivity in SNI rat models. Conclusion: FGFR3 is involved in NPP maintenance via FGFR3-TBX3 axis activation induced TNF-α synthesis. FGFR3 and correlated signaling pathways of astrocytes are potential molecular targets for NPP administration.
Keywords: Neuropathic pain, astrocyte, fibroblast growth factor receptor-3, TBX3, TNF-α
Introduction
Neuronal damage or dysfunction of nervous system induces a variety of chronic pain, which is referred to as neuropathic pain (NPP) [1]. The typical manifestation of NPP is being allodynic, hyperalgesic, or spontaneous [1]. The incidence of NPP accounts for more than 30% of all chronic pain [2]. The underlying pathophysiologic mechanism of NPP is nerve injury or localized inflammation [3]. Immune cell aggregation and activation are induced by localized inflammation in injured tissues. The release of immunoreactive substances leads to local immunoreactions and promotes a more extensive inflammatory response [4]. The neuroinflammatory environment activates glial cells in the brain and spinal cord and plays an important role in sensory nerve injury.
Previous studies predominantly focused on neuron and accumulated studies indicated a crucial role of glial cells in NPP development, spread, and potentiation by a communication with nervous system [5]. Glial cells account for about 90% of all cells in central nervous system, of which astrocytes are the predominant subgroup of glial cells [6]. Astrocytes play an important role in maintaining the stability of central nervous system and the differentiation and repair of nerve cells. As a specific biomarker of astrocytes, GFAP showed significant elevation during astrocyte activation. Increased GFAP suggests the development of the development of NPP [7]. Accumulated studies supported that astrocyte activation was involved in NPP development and maintenance [8]. Activated astrocytes release cytokines and neuroactive substances, such as nerve growth factor, neurotrophic factor, IL-1, IL-6, TNF-α, ROS, and NO, which sensitizes nerves in the dorsal horn of the spinal cord, and even directly produces chronic pain [9].
Fibroblast growth factor receptors (FGFRs) are members of transmembrane tyrosine kinase receptors, which mediate FGF signal transduction. A total of four members have been identified, FGFR1-4, which are coded by independent genes. Activated FGFRs by FGFs are involved in embryogenesis, tumorigenesis, and damaged tissue renovation, as well as bone regeneration [10]. During nerve cell development, FGF/FGFRs signaling is involved in cell development, differentiation, dendritic branches induction, and neuronal survival of the nervous system [11]. Among these receptors, FGFR3 expression is observed in a variety of neuron cells of different regions [12,13]. Previous studies have shown that activated FGFR3 promotes the specialization, proliferation, and development of astrocytes [14,15]. However, further studies are still needed for the functional role and underlying mechanism of FGFR3 in NPP. Here, in this study, we identified the functional role and molecular mechanism of FGFR3 in the development and maintenance of NPP by influencing the activity of astrocyte.
Material and methods
Animals and SNI model establishment
Sprague Dawley rats (180-220 g) were obtained from the Animal Center of Xinqiao Hospital, which were maintained at a constant ambient environment: temperature (22 ± 0.5°C), humidity (60-70%), light/dark cycle (lights on 7 AM), and standard laboratory diet and water. SNI surgery was performed as previous report [16]. Random grouped rats were fixed to operating table after anesthesia and incised at the right mid-thigh level of the femur. Three peripheral branches of the sciatic nerve (the sural, common peroneal, and tibial nerves) were separated in the trifurcation without injury. Ligation with silk suture was performed in the tibial and common peroneal nerves, while the sural nerve maintains intact. Suturing of the incision was carried out at the last stage. In the sham group, the sciatic nerve was exposed without ligation. All animal experiments were conducted in accordance with the “Guidelines for the Care and Use of Laboratory Animals” which was established by the Army Medical University. Experimental procedures were approved by the Ethics Committee of the Army Medical University.
Mechanical withdrawal threshold measurement
The mechanical withdrawal threshold was measured with the right hind paw using von Frey filaments. In ascending order of force, each filament was applied to each hind paw of the mid-plantar area for five times and each application was held for 5 s. Animals that exhibited no response to stimulation of the filament were assigned as cutoff value. All assessments were performed blinded.
Immunofluorescence staining
The spinal cord tissues were collected after euthanizing the SNI and Sham rats. Then, they were fixed in 4% paraformaldehyde for 3 days and incubated in 30% sucrose in PBS overnight at 4°C. Serial tissue sections were prepared with a frozen section machine in a thickness of 18 μm. Tissue sections were fixed with acetone and blocked with goat serum. Then, they were incubated with primary antibodies against FGFR3 and GFAP overnight at 4°C. The second antibody used in this study was FITC-conjugated goat anti-rabbit IgG and Cy5-conjugated goat anti-mouse IgG. The cell nuclei were stained with 4’-6-diamidino-2-phenylindole (DAPI). Immunofluorescence analysis was performed with a fluorescence microscope (Carl-Zeiss Jena, Germany).
Primary astrocytes culture
Cultured spinal astrocytes referred previous report [17]. Spinal cords isolated from Sprague Dawley rats were minced and incubated with trypsin and DNase I. Dissociated cells were suspended cultured in tissue culture flasks with Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% fetal calf serum. After 10 days culture, microglial cells were washed away. Remaining cells were seeded to new flasks with the treatment of 1 μM of cytosine b-Darabinofuranoside (Sigma Chemical Co., St. Louis, MO) to maintain astrocytes.
Transfection of primary cultured astrocytes with lentivirus
Lentivirus of FGFR3 was established with FGFR3 plasmid (#75730, Addgene, USA) in SunBio (Shanghai, China). The siRNA (Seq: GGAGGAGCTGATGGAAGTT) was designed according to NM_053429. FGFR3 knockdown lentivirus (shFgfr3) and negative control (scrambled shRNA) were also established from SunBio (Shanghai, China). For cell infection, the primary cells were cultured for 24 h and infected with FGFR3 lentivirus or control lentivirus for another 24 h as previous report [21]. For analysis of signaling inhibition, infected cells were treated with 10 μM PD173074 (Tocris Bioscience, Ellisville, MO) for 48 h before cell harvest.
RT-PCR assays
RT-PCR assays were performed as previous report [18]. Harvested cells were prepared for cDNA synthesis, and RT-PCR assays were performed with specific primers and PrimeScript RT Master Perfect Real Time Kit (TaKaRa, Japan). The sequences of the primers are described in Table 1.
Table 1.
Primers used in RT-PCR assays
| Genes | Primers | |
|---|---|---|
| Gfap | Forward | 5’-GTGAAGGTCTATTCCTGGTTGC-3’ |
| Reverse | 5’-TCTAGGCGATACTCCGTACATG-3’ | |
| Tnf-α | Forward | 5’-GTAGCAAACCACCAAGCGG-3’ |
| Reverse | 5’-GGTATGAAATGGCAAATCGG-3’ | |
| Fgfr3 | Forward | 5’-AGGCTTCAAGTGCTAAACGC-3’ |
| Reverse | 5’-TGAGGACGGAGCATCTGTTAC-3’ | |
| β-actin | Forward | 5’-CGTAAAGACCTCTATGCCAACA-3’ |
| Reverse | 5’-GGACTCATCGTACTCCTGCTTG-3’ | |
| Ifn-β1 | Forward | 5’-TCTTCTTTGGGTATTGTTTGG-3’ |
| Reverse | 5’-TGCCTTCTTGGGACTGATGT-3’ | |
| Il-6 | Forward | 5’-TGCCTTCTTGGGACTGATGT-3’ |
| Reverse | 5’-AATGACTCTGGCTTTGTCTTTCT-3’ | |
| Ccl2 | Forward | 5’-GCATCCACGTGTTGGCTCA-3’ |
| Reverse | 5’-CTCCAGCCTACTCATTGGGATCA-3’ | |
| Ccl7 | Forward | 5’-CCACATGCTGCTATGTCAAGA-3’ |
| Reverse | 5’-ACACCGACTACTGGTGATCCT-3’ | |
| Il-1b | Forward | 5’-TGGCCTTCTACAGTAACAGCA-3’ |
| Reverse | 5’-GCATGAATACCGCCTTAAAGGAC-3’ | |
| Il-17 | Forward | 5’-GAAGTTGGACCACCACATGA-3’ |
| Reverse | 5’-TCCCTCTTCAGGACCAGGAT-3’ | |
| Tbx3 | Forward | 5’-GGTCAGGAGATGGCTAA-3’ |
| Reverse | 5’-AGGACGGTTCTATGGTG-3’ |
Western blot
Western blot assays were performed as previous report [18]. Cultured spinal astrocytes and spinal tissues were solubilized in immunoprecipitation assay buffer. Then proteins were prepared for immunoblotting analysis. Primary antibodies used in this study are described as below: FGFR3 (Santa Cruz Biotechnology, CA), GFAP (Cell Signaling Technology, Danvers, MA), TNF-α (Cell Signaling Technology, Danvers, MA), TBX3 (GeneTex, San Antonio, TX), and β-actin (Cell Signaling Technology, Danvers, MA).
Rat intrathecal injection
Intrathecal injections were performed on unanesthetized rats as previously described [17]. Rats were restrained on the operating table to identify L5 and L6 vertebrate. Intrathecal drug injection was performed between the L5 and the L6 vertebrae with a 27-guage needle attached to a Hamilton micro-syringe. The entry of the needle was confirmed with the presence of a tail flick. A total of 5 μl drugs or lentivirus was injected each time. PD173074 (10 μM) and TNF-α (100 ng/mL) were suspended in 1:1 (vol/vol) in combined treatment.
Statistical analysis
Data are described as mean ± SEM. Comparisons between paired groups were performed with a one-way analysis of variance (ANOVA) with a pairwise comparison by the Tukey-Kramer method. Comparisons between the two groups were performed using Student’s t test. Potential effects of PD173074 treatment on mechanical hypersensitivity was analyzed by two-way repeated-measures ANOVA. P<0.05 was considered as significant difference.
Results
Increased FGFR3 expression in spinal dorsal horn following partial sciatic nerve ligation
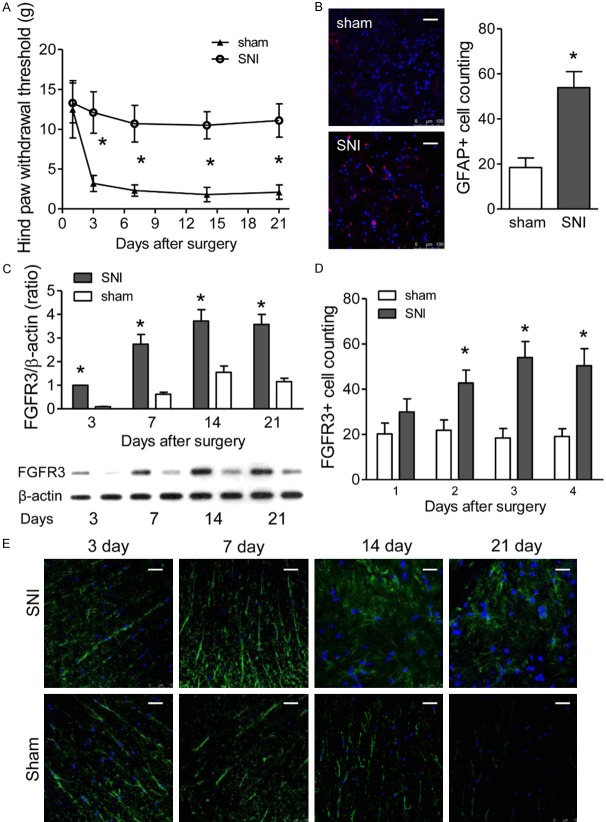
To investigate the potential role of FGFR3 in NPP, SNI models were established. Decreased withdrawal thresholds were observed in ipsilateral hind paw beginning at 3 days after partial sciatic nerve ligation (PSNL) surgery than sham group, which persisted up to 21 days following surgery (P<0.01 vs. sham; Figure 1A). By contrast, the rats with sham operation did not show significant mechanical hypersensitivity (P>0.05 vs. day 1; Figure 1A). Immunofluorescence analysis was performed to identify the expression of GFAP, which showed elevated GFAP expression in the ipsilateral spinal dorsal horn of SNI group (Figure 1B), whereas there was no significant change in the sham group (Figure 1B).
Figure 1.
Partial sciatic nerve ligation leads to hind paw mechanical hypersensitivity and elevated astrocytic FGFR3 expression in rat spinal dorsal horn. (A) Ipsilateral hind paw withdrawal thresholds (g) over time measured with von Frey filaments. Rats were tested 0 and 3, 7, 14, and 21 days after either sham or PSNL surgery (SNI). Data represent the mean ± SEM. *P<0.01 vs. sham mice at the corresponding time point. n = 6/group. (B) Immune Fluorescent Staining of GFAP in ipsilateral dorsal spinal cord at 14 days after surgery. Quantification analysis of GFAP positive cells as shown in the right. (C) The expression of FGFR3 in the ipsilateral spinal dorsal horn of SNI and sham rat quantified by western blotting over time (days). The upper quantitative graphs are from representative immunoblot photographs as shown below. Data represent the mean ± SEM. *P<0.01. N = 6/group. (D, E) immunofluorescence for FGFR3 (green) in the ipsilateral spinal dorsal horn of SNI and sham rat (E). Quantification analysis of GFAP positive cells as shown in (D).
Furthermore, western blot assays were conducted to examine FGFR3 expression in the spinal dorsal horn at the onset of mechanical hypersensitivity (3 days post-PSNL) and during the maintenance of mechanical hypersensitivity (7, 14, and 21 days post-PSNL). Levels of FGFR3 expression in ipsilateral dorsal horn were significantly increased 7, 14, and 21 days after PSNL, compared to sham-operated mice. No significant difference was observed between groups in 3 days after PSNL (P<0.01 vs. sham; Figure 1C). In addition, FGFR3 immunofluorescence intensity was also increased in SNI models, compared to sham rats (Figure 1D, 1E). Thus, our results indicated that elevated FGFR3 expression was due to spared nerve injury in SNI rats, rather than the artifact of tissue processing.
FGFR3 increases GFAP and TNF-α expression in astrocytes in vivo and in vitro
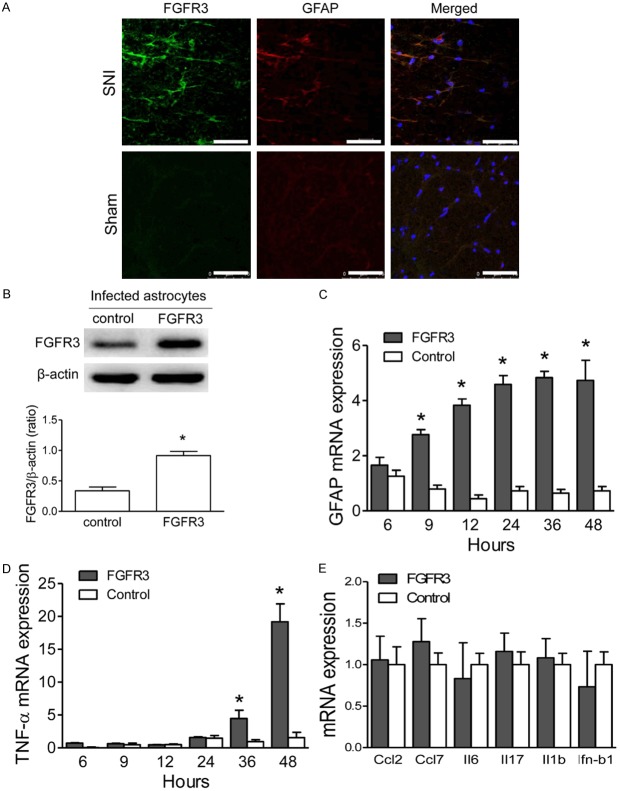
Further analysis was performed to investigate the correlation of FGFR3 expression and astrocytes activation. Immunofluorescence images indicated the colocalization of FGFR3 and GFAP in the ipsilateral spinal dorsal horn of SNI rats (Figure 2B). Furthermore, FGFR3 lentivirus infection significantly increased expression of FGFR3 protein in cultured spinal astrocytes, which was comparable with those from the ipsilateral dorsal horn of PSNL model (Figure 2B). Notably, exogenous FGFR3 expression increased the expression of GFAP and TNF-α mRNA with the treatment of fibroblast growth factor-2 (FGF-2), which was maintained for at least 48 hr after FGFR3 transfection (Figure 2C). By contrast, cells transfected with empty vector showed no change in GFAP expression (Figure 2C). Moreover, increased TNF-α mRNA expression was also observed in FGFR3 transfected astrocytes (Figure 2D), which was significantly increased beginning 24 hr after FGFR3 transfection (Figure 2D). In addition, exogenous FGFR3 expression had no effect on Ccl2, Ccl7, interleukin-6 (Il-6), Il-17, IL-1b, and interferon-β1 (Ifn-β1) mRNA expression (Figure 2E). Our results indicated that elevated FGFR3 expression played a potential role in astrocytes activation during the maintaining of NPP.
Figure 2.
Elevated FGFR3 expression induces gene expression changes in spinal astrocytes. A. Immunofluorescence for FGFR3 (green) and GFAP (red) in the ipsilateral spinal dorsal horn of SNI and sham rat. B. Expression levels of FGFR3 in cultured spinal astrocytes which were infected with FGFR3 lentivirus or control lentivirus. Β-ACTIN was used as loading control. C, D. GFAP, and TNF-α mRNA levels were tested with q-RT-PCR in FGF2 (2 μM) treated for the periods indicated. Cultured spinal astrocytes were infected with FGFR3 lentivirus or controls. E. Effects of FGFR3 transfection on mRNA expression in cultured spinal astrocytes, including CCL-2/3, MCP-3, CXCL1, GDNF, and IL-1b. The astrocytes were treated with FGF2 (2 μM) for 48 hours. Results of three independent determinations are expressed as the mean ± SEM. *P<0.05.
FGFR3 inhibition leads to downregulation of GFAP and TNF-α and increases withdrawal threshold
Further investigation was performed for the function of FGFR3 during NPP. FGFR3 inhibition with PD173074 was administrated in cultured spinal astrocytes for 24 hours, which showed decreased FGFR3 expression protein levels (Figure 3A). However, no significant change was observed in mRNA levels (Figure 3B). Notably, the expression of GFAP mRNA and TNF-α mRNA expression were significantly decreased with PD173074 treatment (Figure 3B). Further analysis was also performed with intrathecal treatment with PD173074 in SNI models. The withdrawal thresholds were significantly decreased in SNI rats of intrathecal treatment with PD173074, compared with vehicle controls (Figure 3C). Furthermore, decreased GFAP and TNF-α levels were also observed in SNI rats following intrathecal treatment with PD173074 than vehicle (Figure 3D). Our results indicated that FGFR3 activation participated in astrocytes activation in NPP models.
Figure 3.
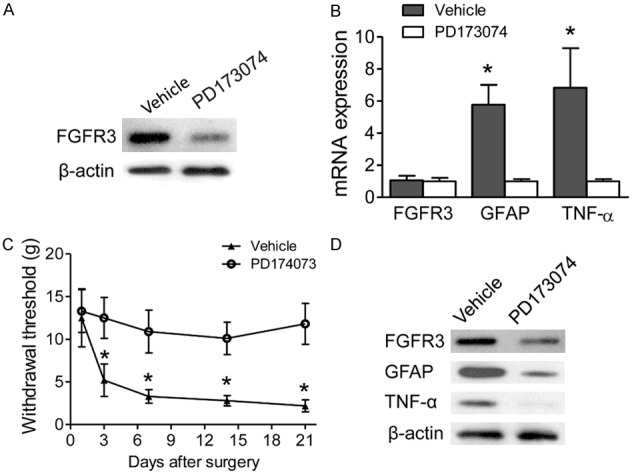
FGFR3 inhibition decreases TNF-α expression in spinal astrocytes. A. After cultured spinal astrocytes were treated with PD173074 for 24 hr, FGFR3 protein levels were measured. Β-ACTIN was used as control. B. Effects of PD173074 on FGFR3, GFAP, and TNF-α mRNA expression in cultured spinal astrocytes, which was infected with FGFR3 lentivirus and treated with FGF2 for 24 hrs. Results of three independent determinations are expressed as the mean ± SEM. *P<0.05. C. Intrathecal injection of SNI models with either PD173074 or vehicle control. Withdrawal thresholds were measured and compared between groups in the indicated days after surgery. Data represent the mean ± SEM. *P<0.01 vs. sham mice at the corresponding time point. n = 6/group. D. Expression levels of FGFR3, GFAP, and TNF-α in the ipsilateral spinal dorsal horn were measured by western blotting 14 days after PD173074 injection.
FGFR3-TBX3 axis activation participates in TNF-α expression in cultured spinal astrocytes
We further determined the correlation between Tbx3 and FGF signaling in cultured spinal astrocytes. We examined Tbx3 expression in cultured spinal astrocytes which were treated with FGF2 combined with or without PD173074. Indeed, the protein expression of Tbx3 was modestly decreased in cultured spinal astrocytes treated with PD173074 (Figure 4A), although the mRNA expression showed no significant change (Figure 4B). To rule out a nonspecific effect of the PD173074, FGFR3 knockdown was performed with lentivirus in cultured astrocytes. Consistent with the findings in PD173074 treated cells, Tbx3 protein expression was decreased in response to FGFR3 knockdown in astrocytes (Figure 4C). Furthermore, FGFR3 knockdown reversed the effect of FGF2 treatment-that is, decreased GFAP and TNF-α mRNA expression (Figure 4D). Thus, our results confirmed the FGFR3-TBX3 axis participated in astrocyte activation and TNF-α synthesis in spinal dorsal horn.
Figure 4.
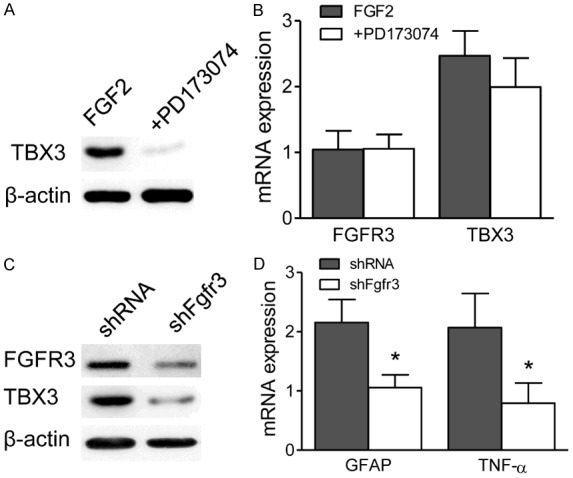
FGFR3-TBX3 axis activation participates in TNF-α expression in cultured spinal astrocytes. A. After cultured spinal astrocytes were treated with PD173074 for 24 hr, TBX3 protein levels were measured with western blot assays. B. FGFR3 and TBX3 mRNA levels were tested with q-RT-PCR in PD173074 and FGF2 (2 μM) combined treated cultured spinal astrocytes for 24 hours. C. Cultured spinal astrocytes were treated with shFgfr3 or control lentivirus. Western blot assays were performed to measure the expression of FGFR3 and TBX3 after 24 hours treatment. D. Expression levels of GFAP and TNF-α was examined in shFgfr3 or control lentivirus treated spinal astrocytes by RT-PCR assays. Β-ACTIN was used as control.
Spinal TNF-α synthesis participates in FGFR3 induced mechanical hypersensitivity
The possible role of TNF-α in mechanical hypersensitivity was further investigated with SNI rats. Intrathecally injected with FGFR3-targeting shFgfr3 was administrated in SNI rats for seven days. FGFR3 levels were detected with immunofluorescence, which showed significant lower FGFR3 expression in the spinal dorsal horn of siFGFR3 treated rats (Figure 5A). The expression of both GFAP and TNF-α protein was significantly decreased in spinal dorsal horn than controls in western blot assays (Figure 5B). Furthermore, intrathecal treatment with PD173074 led to attenuate mechanical hypersensitivity (Figure 5C). Intrathecal injection TNF-α significantly reduced withdrawal thresholds in SNI rats combined treated with PD173074 (Figure 5C). A significant nociceptive effect was observed beginning 3 days following injection and sustained for at least 21 days after treatment (Figure 5C). Furthermore, elevated GFAP expression was observed in spinal dorsal horn of TNF-α treated rat than other two groups by immunofluorescence (Figure 5D). Taken together, our results indicated that elevated TNF-α produced by FGFR3 signaling activation in spinal dorsal horn astrocytes induced mechanical hypersensitivity.
Figure 5.
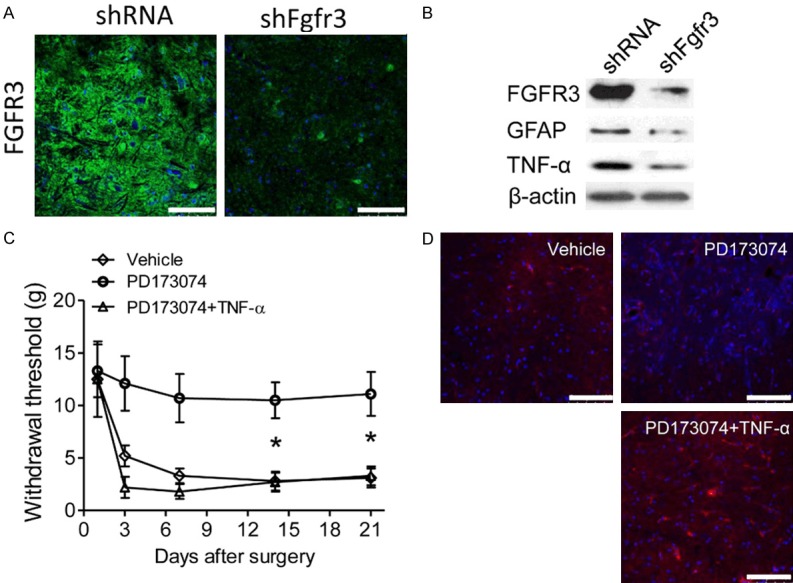
Spinal TNF-α synthesis participates in FGFR3 induced mechanical hypersensitivity. (A) Intrathecal injection of SNI models with shFgfr3 or control lentivirus. Immunofluorescence images showed the expression of FGFR3 in the ipsilateral dorsal spinal cord at 14 days after surgery. (B) Expression levels of FGFR3, GFAP and TNF-α in the ipsilateral spinal dorsal horn were measured by western blot assays 14 days after shFgfr3 or control lentivirus intrathecal treatment. Β-ACTIN was used as control. (C) Intrathecal injection of SNI models with vehicle or PD173074 or PD173074 combined with TNF-α. Withdrawal thresholds were measured and compared between groups in the indicated days after surgery. Data represent the mean ± SEM. *P<0.05 vs. sham mice at the corresponding time point. n = 6/group. (D) Expression levels of GFAP in the ipsilateral spinal dorsal horn were measured by immunofluorescence in the rat treated as (C).
Discussion
NPP is a kind of chronic pain with complicated pathophysiological mechanism and etiology. Accumulated studies have indicated that astrocytes activation participated in NPP by regulating synaptic neurotransmission [6]. Our study found the correlation of GFAP and FGFR3 expression in astrocytes in SNI models. Activated FGFR3-TBX3 signaling increased the expression of GFAP and TNF-α in astrocytes, which played an important role in NPP maintaining. Furthermore, intrathecal injection of PD173074, a FGFR3 inhibitor [19], efficiently relieves NPP in SNI models, which was induced by GFAP and TNF-α reduction. Our study supported FGFR3 to be a potential molecular target for NPP administration.
Previous studies have indicated that increased GFAP expressions are observed in astrocytes during the development and maintenance of NPP. The SNI model established by selective sciatic nerve ligation is internationally recognized, which simulates NPP pathological process well [20]. In our study, SNI model rats showed claudication, slight adduction malformation, and hind limb protective posture and obviously decreased pain threshold in behavior detection of the intraoperative ipsilateral hind paw. More importantly, increased GFAP expression was observed in the astrocytes of spinal cord, which was increased with the passage of time. The activation of astrocyte during NPP was consistent with pain threshold reduction.
FGFRs are a class of transmembrane tyrosine kinase receptor with autophosphorylation activity. Four independent genes encoding FGFRs, FGFR1, FGFR2, FGFR3, and FGFR4, are widely expressed in the nervous system [20]. Previous studies showed that elevated FGFR1 expression in dorsal root ganglion after ligation of sciatic nerve, which suggests FGFR1 phosphorylation is involved in the generation of NPP [21]. FGFR3 expression is observed in a variety of dispersed cells of the central nervous system, such as ventricles [22]. FGFR3 targeted deletion significantly upregulates GFAP expression in grey matter astrocytes [22], whereas some studies indicated that FGFR3 was involved in the development of the cortex [23]. In this study, we also found elevated FGFR3 expression in spinal cord of SNI rat models. FGFR3 signaling activation enhanced GFAP expression in astrocytes of spinal cord, which indicated the functional role of FGFR3 in astrocytes activation.
Increased nutrients are secreted after nerve injury, such as nerve growth factor and glia-derived neurotrophic factors [24]. These factors activate a series of tyrosine kinase receptors, triggering the cascade phosphorylation and protein synthesis to increase excitability and survival of primary sensory neurons [25]. Previous studies indicated that FGFs showed high affinity to tyrosine kinase receptor FGFRs in peripheral nervous system, to modulate nerve system development [26]. FGFR3 specifically bind to FGFs to regulate the specialization and proliferation of the nervous system [14]. Tbx3 transcription factor is downstream of FGF signaling, which is required for propagation of FGF and Wnt signals in histological development [27]. Previous studies indicated that FGFR3 was activated in the dorsal root ganglion during nerve injury [21], as well as Schwann cells and macrophages after sciatic nerve injury and spinal cord injury. In this study, we found FGFR3/TBX3 signal axis activation enhanced GFAP and TNF-α expression in astrocytes, which was the underlying molecular mechanism of FGFR3 in NPP development.
Activated astrocytes released a large amount of proinflammatory cytokines, such as IL-1, IL-6, IL-10, TNF-α, excitatory amino acids, nitrogen monoxidum, and prostaglandins [9]. These inflammatory cytokines diffuse into peripheral neurons and glia cells to induce a pain hypersensitive status [28]. FGFR3/TBX3 axis and its downstream signaling activation transfer phosphorylation signals to other protein kinases and transcriptional factors and release many inflammatory factors to induce sensitization of central nervous system [29]. In this study, we found that FGFR3 and its downstream signaling pathway induced TNF-α synthesis. Furthermore, as a selective tyrosine kinase inhibitor for FGFR3, PD173074 competes with ATP to bind FGFR3 to inhibit autophosphorylation of FGFR3 [19]. Different from other FGFR3 inhibitors, such as SU5402 and GHIR-258, PD173074 also decreased FGFR3 expression [30]. In this study, intrathecal injection of PD173074 or shFgfr3 inhibited the expression of GFAP and TNF-α in SNI models. The reduction of inflammatory factors achieves an analgesic effect of mechanical pain threshold. Therefore, FGFR3 inhibition relieves pain of SNI models, which shed light to the study of NPP administration and drug development. However, further studies are still needed for the clinical treatment of NPP by inhibiting FGFR3 and related signaling pathways.
In conclusion, our study elucidated the biological functions of FGFR3 in the development and maintenance of NPP. FGFR3 and related signaling are potential molecular targets for the development of NPP drugs in the future.
Acknowledgements
This research was supported by Grants from the Scientific Research of Health and Family Planning Commission, Sichuan Province 2018 (18PJ164).
Disclosure of conflict of interest
None.
References
- 1.Woolf CJ, Mannion RJ. Neuropathic pain: aetiology, symptoms, mechanisms, and management. Lancet. 1999;353:1959–1964. doi: 10.1016/S0140-6736(99)01307-0. [DOI] [PubMed] [Google Scholar]
- 2.Treede RD, Jensen TS, Campbell JN, Cruccu G, Dostrovsky JO, Griffin JW, Hansson P, Hughes R, Nurmikko T, Serra J. Neuropathic pain - Redefinition and a grading system for clinical and research purposes. Neurology. 2008;70:1630–1635. doi: 10.1212/01.wnl.0000282763.29778.59. [DOI] [PubMed] [Google Scholar]
- 3.Moalem G, Tracey DJ. Immune and inflammatory mechanisms in neuropathic pain. Brain Res Rev. 2006;51:240–64. doi: 10.1016/j.brainresrev.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Clark AK, Old EA, Malcangio M. Neuropathic pain and cytokines: current perspectives. J Pain Res. 2013;6:803–814. doi: 10.2147/JPR.S53660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hossain MZ, Unno S, Ando H, Masuda Y, Kitagawa J. Neuron-glia crosstalk and neuropathic pain: involvement in the modulation of motor activity in the orofacial region. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Almad A, Maragakis NJ. A stocked toolbox for understanding the role of astrocytes in disease. Nat Rev Neurol. 2018;14:351–362. doi: 10.1038/s41582-018-0010-2. [DOI] [PubMed] [Google Scholar]
- 7.Hergenroeder GW, Redell JB, Choi HA, Schmitt L, Donovan W, Francisco GE, Schmitt K, Moore AN, Dash PK. Increased levels of circulating glial fibrillary acidic protein and collapsin response mediator protein-2 autoantibodies in the acute stage of spinal cord injury predict the subsequent development of neuropathic pain. J Neurotrauma. 2018;35:2530–2539. doi: 10.1089/neu.2018.5675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cao H, Zhang YQ. Spinal glial activation contributes to pathological pain states. Neurosci Biobehav Rev. 2008;32:972–983. doi: 10.1016/j.neubiorev.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 9.Eto K, Kim SK, Takeda I, Nabekura J. The roles of cortical astrocytes in chronic pain and other brain pathologies. Neurosci Res. 2018;126:3–8. doi: 10.1016/j.neures.2017.08.009. [DOI] [PubMed] [Google Scholar]
- 10.Anteby EY, Natanson-Yaron S, Hamani Y, Sciaki Y, Goldman-Wohl D, Greenfield C, Ariel I, Yagel S. Fibroblast growth factor-10 and fibroblast growth factor receptors 1-4: expression and peptide localization in human decidua and placenta. Eur J Obstet Gynecol Reprod Biol. 2005;119:27–35. doi: 10.1016/j.ejogrb.2004.05.014. [DOI] [PubMed] [Google Scholar]
- 11.Grothe C, Nikkhah G. The role of basic fibroblast growth factor in peripheral nerve regeneration. Anat Embryol (Berl) 2001;204:171–177. doi: 10.1007/s004290100205. [DOI] [PubMed] [Google Scholar]
- 12.Mott NN, Chung WC, Tsai PS, Pak TR. Differential fibroblast growth factor 8 (FGF8)-mediated autoregulation of its cognate receptors, Fgfr1 and Fgfr3, in neuronal cell lines. PLoS One. 2010;5:e10143. doi: 10.1371/journal.pone.0010143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen F, Hristova K. The physical basis of FGFR3 response to fgf1 and fgf2. Biochemistry. 2011;50:8576–8582. doi: 10.1021/bi200986f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang K, Song MR. Diverse FGF receptor signaling controls astrocyte specification and proliferation. Biochem Biophys Res Commun. 2010;395:324–329. doi: 10.1016/j.bbrc.2010.03.174. [DOI] [PubMed] [Google Scholar]
- 15.Young KM, Mitsumori T, Pringle N, Grist M, Kessaris N, Richardson WD. An Fgfr3-iCreER (T2) transgenic mouse line for studies of neural stem cells and astrocytes. Glia. 2010;58:943–953. doi: 10.1002/glia.20976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Decosterd I, Woolf CJ. Spared nerve injury: an animal model of persistent peripheral neuropathic pain. Pain. 2000;87:149–158. doi: 10.1016/S0304-3959(00)00276-1. [DOI] [PubMed] [Google Scholar]
- 17.Morioka N, Fujii S, Kondo S, Zhang FF, Miyauchi K, Nakamura Y, Hisaoka-Nakashima K, Nakata Y. Downregulation of spinal astrocytic connexin 43 leads to upregulation of interleukin-6 and cyclooxygenase-2 and mechanical hypersensitivity in mice. Glia. 2018;66:428–444. doi: 10.1002/glia.23255. [DOI] [PubMed] [Google Scholar]
- 18.Wang Q, Jiang J, Ying G, Xie XQ, Zhang X, Xu W, Zhang X, Song E, Bu H, Ping YF, Yao XH, Wang B, Xu S, Yan ZX, Tai Y, Hu B, Qi X, Wang YX, He ZC, Wang Y, Wang JM, Cui YH, Chen F, Meng K, Wang Z, Bian XW. Tamoxifen enhances stemness and promotes metastasis of ERalpha36(+) breast cancer by upregulating ALDH1A1 in cancer cells. Cell Res. 2018;28:336–358. doi: 10.1038/cr.2018.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Grand EK, Chase AJ, Heath C, Rahemtulla A, Cross NC. Targeting FGFR3 in multiple myeloma: inhibition of t (4;14)-positive cells by SU5402 and PD173074. Leukemia. 2004;18:962–966. doi: 10.1038/sj.leu.2403347. [DOI] [PubMed] [Google Scholar]
- 20.Berta T, Poirot O, Pertin M, Ji RR, Kellenberger S, Decosterd I. Transcriptional and functional profiles of voltage-gated Na (+) channels in injured and non-injured DRG neurons in the SNI model of neuropathic pain. Mol Cell Neurosci. 2008;37:196–208. doi: 10.1016/j.mcn.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 21.Yamanaka H, Obata K, Kobayashi K, Dai Y, Fukuoka T, Noguchi K. Activation of fibroblast growth factor receptor by axotomy, through downstream p38 in dorsal root ganglion, contributes to neuropathic pain. Neuroscience. 2007;150:202–211. doi: 10.1016/j.neuroscience.2007.08.024. [DOI] [PubMed] [Google Scholar]
- 22.Pringle NP, Yu WP, Howell M, Colvin JS, Ornitz DM, Richardson WD. Fgfr3 expression by astrocytes and their precursors: evidence that astrocytes and oligodendrocytes originate in distinct neuroepithelial domains. Development. 2003;130:93–102. doi: 10.1242/dev.00184. [DOI] [PubMed] [Google Scholar]
- 23.Inglis-Broadgate SL, Thomson RE, Pellicano F, Tartaglia MA, Pontikis CC, Cooper JD, Iwata T. FGFR3 regulates brain size by controlling progenitor cell proliferation and apoptosis during embryonic development. Dev Biol. 2005;279:73–85. doi: 10.1016/j.ydbio.2004.11.035. [DOI] [PubMed] [Google Scholar]
- 24.Vavrek R, Girgis J, Tetzlaff W, Hebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- 25.Ji RR, Samad TA, Jin SX, Schmoll R, Woolf CJ. p38 MAPK activation by NGF in primary sensory neurons after inflammation increases TRPV1 levels and maintains heat hyperalgesia. Neuron. 2002;36:57–68. doi: 10.1016/s0896-6273(02)00908-x. [DOI] [PubMed] [Google Scholar]
- 26.Galvez-Contreras AY, Gonzalez-Castaneda RE, Luquin S, Gonzalez-Perez O. Role of fibroblast growth factor receptors in astrocytic stem cells. Curr Signal Transduct Ther. 2012;7:81–86. doi: 10.2174/157436212799278205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fillmore CM, Gupta PB, Rudnick JA, Caballero S, Keller PJ, Lander ES, Kuperwasser C. Estrogen expands breast cancer stem-like cells through paracrine FGF/Tbx3 signaling. Proc Natl Acad Sci U S A. 2010;107:21737–21742. doi: 10.1073/pnas.1007863107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Okamoto K, Martin DP, Schmelzer JD, Mitsui Y, Low PA. Pro- and anti-inflammatory cytokine gene expression in rat sciatic nerve chronic constriction injury model of neuropathic pain. Exp Neurol. 2001;169:386–391. doi: 10.1006/exnr.2001.7677. [DOI] [PubMed] [Google Scholar]
- 29.Myers RR, Shubayev VI. The ology of neuropathy: an integrative review of the role of neuroinflammation and TNF-alpha axonal transport in neuropathic pain. J Peripher Nerv Syst. 2011;16:277–286. doi: 10.1111/j.1529-8027.2011.00362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gudernova I, Vesela I, Balek L, Buchtova M, Dosedelova H, Kunova M, Pivnicka J, Jelinkova I, Roubalova L, Kozubik A, Krejci P. Multikinase activity of fibroblast growth factor receptor (FGFR) inhibitors SU5402, PD173074, AZD1480, AZD4547, and BGJ398 compromises the use of small chemicals targeting FGFR catalytic activity for therapy of short-stature syndromes. Hum Mol Genet. 2016;25:9–23. doi: 10.1093/hmg/ddv441. [DOI] [PubMed] [Google Scholar]