Abstract
Non-thyroid malignancies to the thyroid gland resulting from distant metastases are extremely rare, and such cases are rarely seen in clinical settings. The question of how a tumor metastasizes to the thyroid remains unanswered. Here we report a case of lung adenocarcinoma metastasizing to the thyroid gland. The article covers the pathological features, treatments, examination reports, and the postoperative follow-up reviews of the patient. In this article, we discuss the diagnostic method, the spread route, the prognosis, the mechanism and above all, the treatment. In addition, we searched the PubMed and ISI Web of Science databases for articles published in English using the key words “lung”, “thyroid”, and “metastasis”, and we reviewed nearly all the reports about thyroid malignancies being metastasized from lung cancer. This rare case emphasizes the importance of the multifaceted comprehensiveness of the cephalometry diagnosis, pathological diagnosis, and immunohistochemical analysis to ensure that such rare cases are not missed. We declare that all cases of thyroid malignancies metastasized from the lungs shall be reported at large for further clinical research.
Keywords: Thyroid metastasis, non-small cell lung cancer, adenocarcinoma
Introduction
It is such a rare case to see thyroid metastatic carcinoma in spite of its rich vascular supply [1], 83 cases were reported in 14 years in the CKNI database between 1994 to 2008, of which 14% originated from lung cancer, and in most cases, it was covered up perfectly and mistaken as primary thyroid cancer or thyroid adenoma disease, so we can see that the diagnosis and antidiastole is rather difficult [1]. Although the occurrence of cancers metastasizing to the thyroid is uncommon in clinical practice, a high frequency of cancers metastasizing to the thyroid (1.25% to 24%) diagnosed by autopsy have been reported [2]. The prognosis for metastatic thyroid cancer is much worse than primary thyroid cancer. Metastatic thyroid cancer includes small cell, adenocarcinoma, squamous cell, and large cell carcinomas by histology, of which adenocarcinoma is the most common. Here we report a case of thyroid metastasis from non-small cell lung cancer. The aim of this report is to analyze the pathological features, treatments, examination reports and postoperative follow-up reviews of the patients to have a better understanding of the illness, contributing to a preferable therapeutic strategy to choose in the near future.
Materials and methods
A fifty-four year-old man had a physical examination in May 2012 for thyroid occupancy, and a computed tomography (CT) showed a mass in the upper lobe of the right lung. He admitted to a slight cough and expectoration but denied other symptoms such as chest tightness, shortness of breath or chest pain at that time. A transbronchial needle aspiration (TBNA) of the lymph nodes performed on him showed no evidence of a tumor. In February 2017, he underwent a pulmonary puncture, and an infiltrating adenocarcinoma of the lung was found. Then he was transferred to our hospital for further diagnosis and treatment of his disease in March 2017.
Positron emission tomography/computed tomography (PET-CT) performed on him showed a lobe mass in his right lung upper with fluorodeoxyglucose (FDG) metabolism increased (Figure 1). The increase in FDG can be seen in the lymph node located in the mediastinum, right hilar, left hilar, and left axillary metabolism. An uneven density of the nodules in the right lobe of the thyroid with increased FDG metabolism reminded us of the high possibility of malignant tumor of the thyroid. In addition, the small lymph nodes on the right clavicle and right cervical region II lymph nodes also showed increased FDG metabolism (Figure 2). Needle aspiration of nodules in the right middle neck showed a metastatic or poorly infiltrating, differentiated carcinoma. The pathology of a coarse needle puncture of the nodules in the right middle neck showed that atypical epithelial cells nests were seen in the fibers and thyroid tissues. The pathological consultation results of our hospital showed (lung puncture) adenocarcinoma. From March 29 to April 19, 2017, he received 2 cycles of a pemetrexed and cis-platinum (PC) regimen (pemetrexed 500 mg/m2 day 1, cis-platinum 75 mg/m2 day 1-3) chemotherapy every 3 weeks. Then he underwent right lobe thyroidectomy, right neck lymph node dissection, and right upper radical resection of the lung cancer on May 18, 2017. The postoperative routine pathology showed (upper right) nodular lung (2.8 × 2.2 × 1.5 cm) infiltrating adenocarcinoma (micropapilla dominant, partly solid growth), metastasizing to the right thyroid tissue and affecting the membrane. In addition, 48 lymph nodes in total were diagnosed with chronic lymphadenopathy with partial intradermal carbon deposition. Immunohistochemistry showed c-Met(partical +), ALK-NC(-), ALK(D5F3)(-), ROS1(-), CK7(+), TTF1(+), P63(-), P40(-), Napsin A(+), CK5/6(-), Napsin A(+), TG(-), Gal-3/Galectin-3(+), HBME-1(+), CK19(+), Ki-67(+, 35%) (Figure 3). No mutation was found in the EGFR gene in molecular detection. After the operation, he continued to receive 3 cycles of the pemetrexed and cis-platinum (PC) regimen (pemetrexed 500 mg/m2 day 1, cis-platinum 75 mg/m2 day 1-3) chemotherapy every 3 weeks. In November 2017, he developed a backache, radiating to both lower limbs, disturbing his sleep. Magnetic resonance imaging (MRI) of the lumbar spine showed the right transverse process of L5 and the right iliac bone were damaged, and the surrounding erector spinae and the right iliopsoas were abnormal, indicating that the tumor cells had already metastasized to the bones. From November 2, 2017 to January 3, 2018, he received 4 cycles of a gemcitabine and cis-platinum (GP) regimen (gemcitabine 1 g/m2 day 1, 8; cis-platinum 75 mg/m2 day 1-2) chemotherapy every 3 weeks. From December 12, 2017 to the present, zolephosphoric acid was used to treat the bone metastasis. The patient complained of no discomfort except fatigue. In May 2018, the patient came back to our hospital for review, and according to the chest and neck CT, there was no evidence of tumor recurrence.
Figure 1.
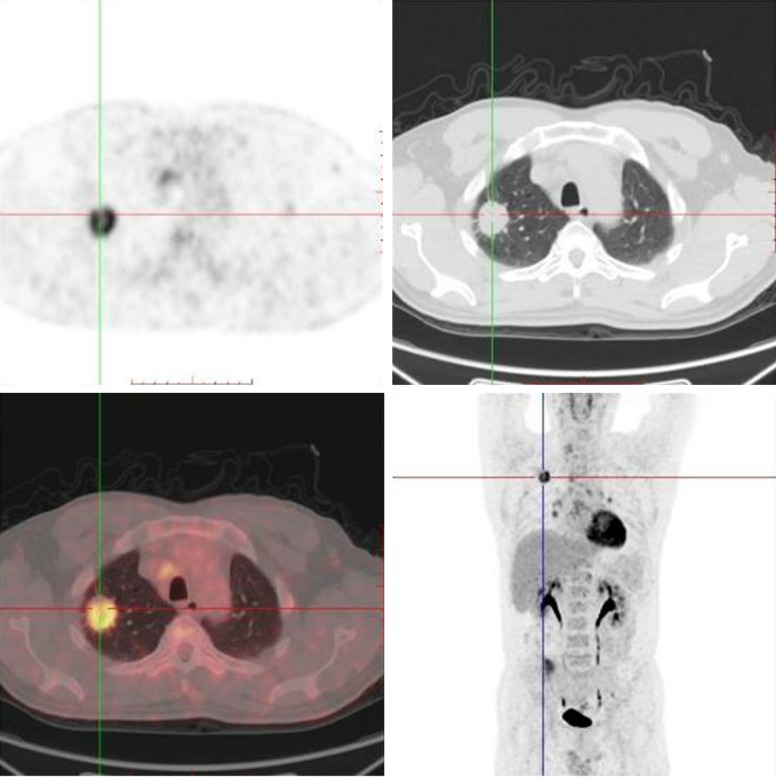
Positron emission tomography/computed tomography (PET-CT) showed a lobe mass in the right lung upper with fluorodeoxyglucose (FDG) metabolism increased.
Figure 2.
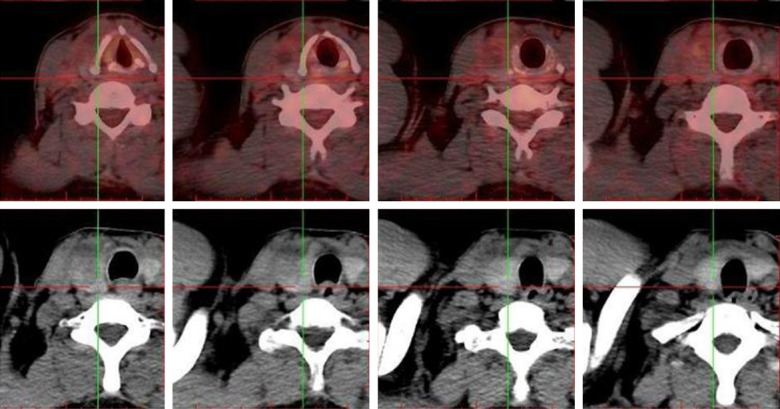
PET-CT showed an uneven density of the nodules in the right lobe of the thyroid with and increased FDG metabolism indicating a high possibility of thyroid malignant tumor.
Figure 3.
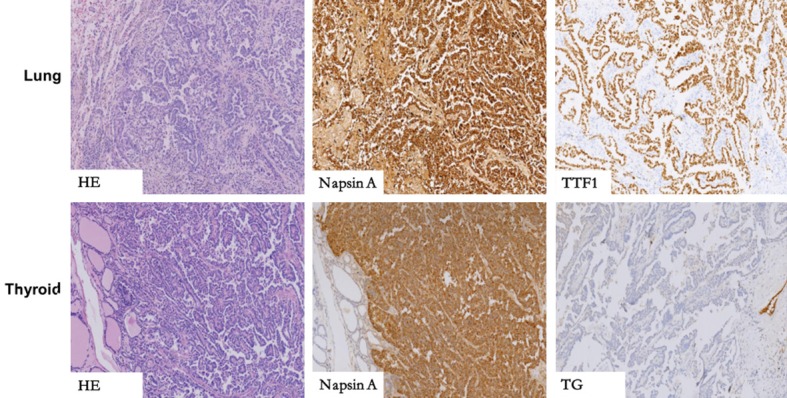
The key to distinguishing between primary thyroid papillary carcinoma and pulmonary metastasis in morphology is that the papillary thyroid carcinoma has a glassy nucleus with a nuclear overlap, sulcus, and inclusion bodies, but in lung cancer it’s invisible, in immunohistochemistry, NapsinA+, TG- can be detected in thyroid metastasis of lung cancer, TG+, Napsin A- for primary thyroid papillary carcinoma.
The above material comes from Zhejiang Cancer Hospital. The pathological specimen was reviewed by a single experienced pathologist, and the diagnoses were rendered based on the morphologic features, and the diagnoses were further confirmed by immunohistochemistry (IHC). The specimens were fixed in 10% formalin and embedded in paraffin, and 4-μm-thick slices were prepared for hematoxylin and eosin staining and IHC. The patient had signed an informed patient consent, and this study was carried out with the approval of the Ethics Committee of Zhejiang Cancer Hospital. In addition, we searched the PubMed and ISI Web of Science databases for articles published in English using the key words “lung,” “thyroid”, and “metastasis”, and we reviewed nearly all the reports about thyroid metastasis from lung cancer.
Results
We reported a 54 year-old man with a simultaneous occurrence of thyroid cancer metastasizing from lung cancer. We discovered lump in the patient’s lung when he had an examination especially for a thyroid checkup. The patient’s clinical data is summarized in Table 1. Five of the patients presented with adenocarcinoma, and there are also histological types of follicular adenoma and papillocarcinoma. A great number of patients complain of nonspecific symptoms in the lungs such as, dyspnea, dysphagia, fatigue, and cough, and the thyroid is dormant, and neurological symptoms emerged in two of the patients, including right shoulder pain and low-back pain, and the thyroid symptoms include a mass, choking and swelling pain. Surgery was the first choice for these patients, except for one who was treated with chemotherapy and radiation. The follow-up evaluation for these patients was poor.
Table 1.
Summary of prior reported cases of thyroid metastasis from non-small cell lung cancer
| Series (reference) | Patients’ age and sex | histological type | The location of thyroid metastasis | Clinical presentation | Treatment | Survival |
|---|---|---|---|---|---|---|
| Yaner Yu et al. (our study) | 54/male | adenocarcinoma | right thyroid lobe | a history of slight cough and expectoration | Drug, surgery | No recurrence in one year |
| Boukir A et al. [3] | 51/female | NA | The lower left corner of the thyroid gland, 18*21*27 mm | a persistent, limited and mild pain swelling in the left lobe of the thyroid gland. | NA | NA |
| Megumi Miyakawa. et al. [4] | 50/female | adenocarcinoma | NA | right shoulder pain | combination chemotherapy (paclitaxel:Taxol, 250 mg and CBDCA: Parapratin 525 mg, Bristol Pharmaceutical KK., Tokyo, Japan), gamma-knife radiosurgery | died of respiratory failure in 6 months |
| Narendra Hulikal et al. [5] | 42/female | NA | a 5 cm × 4 cm thyroid swelling and multiple lymph nodes on the right side of the neck levels 2 and 3, largest measuring 3 cm × 2 cm | a 1-month history of swelling in front of the neck and throat pain. | Total thyroidectomy with bilateral selective (levels 2-5) lymph node dissection and central compartment dissection+ chemotherapy. | Under therapy when reported |
| Narendra Hulikal et al. [5] | 42/female | NA | a 5 cm × 4 cm thyroid swelling and multiple lymph nodes on the right side of the neck levels 2 and 3, largest measuring 3 cm × 2 cm | a 1-month history of swelling in front of the neck and throat pain. | Total thyroidectomy with bilateral selective (levels 2-5) lymph node dissection and central compartment dissection+ chemotherapy. | Under therapy when reported |
| Tariq Namad et al. [6] | 48/female | adenocarcinoma | the largest was in the left thyroid | Diagnosed with lung cancer, progressive fatigue, dyspnea, and dysphagia | drug therapy | No recurrence in three months |
| Elliott RH et al. [7] | 64/male | NA | Hard mass in right lobe of thyroid with bilateral, small, firm supraclavicular lymph nodes. | Adyspnea and wheezing in chest for four months | Biopsy of lymph node, frozen section, right thyroid lobectomy. | NA |
| Elliott RH et al. [7] | 60/male | NA | Stony hard, 3 cm. nodule in right lobe of thyroid, not noticed by patient. | 3 month history of crippling low-back pain with radiation down legs. | Total thyroidectomy with pretracheal node and right axillary lymph node dissection and Decompression , operation of sudden atelectasis laminectomy L 2-3 and L 4-5 and palliative x-ray therapy | Die in 7 months after the operation of sudden atelectasis |
| Elliott RH et al. [7] | 67/female | adenocarcinoma | Advanced malignant disease with hard nodular thyroid and fixed left supraclavicular nodes | with gradually enlarging goiter for one year and one month of progressive dyspnea, substernal pain and cough. | Biopsy lymph node. Frozen section “carcinoma”. Total thyroidectomy. | Died 9th postoperative day. |
| Elliott RH et al. [7] | 34/male | squamous cell carcinoma | NA | enlarged lymph nodes in the right neck for one year. | Exploratory with removal of thyroid isthmus. | Died of disease elsewhere 3 months later |
| A. Dao et al. [9] | 59/male | adenocarcinoma | a right thyroid nodule. | dyspnea, dry cough, and chest pain, a smoker | The cranial palliative radiotherapy | Die |
| Wey SL et al. [15] | 64/Male | follicular adenoma | the left thyroid gland was 7.3 × 4 × 3.5 cm in size with one major well-defined nodule, and the right thyroid gland was 5.5 × 4 × 3 cm in size with several nodules. | neck mass, hoarseness, and easy choking | NA | NA |
| Wey SL et al. [15] | 71/female | papillocarcinoma | The right thyroid gland was 4.4 × 2.3 × 1.8 cm in size with one major nodule and several small nodules | NA | a radical thyroidectomy and left lung lobectomy | NA |
NA: not available.
Discussion
Thyroid malignant tumors are classified as primary or metastatic, and it is rather hard to distinguish the difference between the two using an image examination. A study [3] conducted in Korea observed over 150 patients afflicted with primary thyroid cancer. The tumor progresses slowly, and it has a good prognosis, but that is not the case for metastatic thyroid tumors, and according to Megumi Miyakawa [4], the 10-year survival rate has come up to 85.0% and the 20-year survival rate to 71.0% for primary thyroid cancer. However, when it comes to the secondary thyroid cancer, the survival rate is different - approximately 19% for the one-year survival rate and 5% for the five-year. In addition, it is difficult on the frozen sections of metastases in the lymph nodes to see the details and staining properties which differentiate the cancer from primary thyroid carcinoma or cancer from another organ, which highlights the significance of the diagnosis. It can be much more confusing when the thyroid metastasis precedes the diagnosis of the primary cancer or presents synchronously [5].
The thyroid is not a frequent organ for primary cancer. It is even less frequently the site of metastatic cancer. Most reported thyroid cancers are found at autopsy [6]. And before the thyroid metastasis appears, a disseminated disease with multiple sites of metastases has already come into being. The reported incidence of thyroid metastatic lesions among the living is 2-3% of all the thyroid malignancies as opposed to the postmortem of about 1-24% [5]. According to one study [7], 3.9 percent of all patients with malignant neoplasms have a secondary disease of the thyroid gland when examined at autopsy. Of all the patients affected by thyroid metastasis from non-small cell lung cancer (NSCLC), most are people over fifty, and the mean age of the patients is 60 [8]. Studies [9] have discovered that older female nonsmokers or Asians who are light smokers have a bigger tendency of catching the disease, which can have a genetic etiology. Statistically [4,5,10], the kidneys are responsible for the largest proportion of primary lesions in metastatic thyroid cancer, followed by the lungs and the breasts outside of China, but this is not always the case. Elliott [7] listed several cases of metastatic lesions from the breasts, lung and kidneys, and according to his study, the breasts take up the largest proportion followed up by the lungs. In China [11] the data may be different, as the lungs, breasts, and stomach are the most common primary cancer sites (in decreasing order) as reported in the majority of the oriental research studies, so we conclude this difference has something to do with the dietary habits. Among all the patients with lung cancer metastasizing to the thyroid, adenocarcinoma is the most common type in histology, and the histology type is relevant to the average life span to some extent. The balance of hormones is not disturbed in spite of the bad prognosis. Nevertheless, several cases [4,11] have seen the hypofunction of the thyroid as the cells are substituted by cells originating from the lungs, and these cells infiltrate into the thyroid, leading to follicular reduction. The mutations of the gene EGFR and EML4-ALK are associated with thyroid metastases, but the exact mechanism is not clear yet. Certainly, these two genes are conducive to therapy. The EGFR mutation is not seen in our case.
The interval between diagnosis of the primary lesions and thyroid metastasis initially is approximately 1-10 years, 24 months on average [6]. The metastasis interval is relatively long, making it a double-edged sword. Though it is a key point worthy of attention as we can control the progression of cancer to the full extent, lung cancer is often hidden, and its diagnosis is much more difficult when thyroid metastasis precedes it or occurs synchronously with another primary cancer [11], making the healing process a lot more difficult due to the long delay. Very few of the patients complain of neck pain, but under the ultrasound (US), there are some subacute thyroiditis-like changes, but only when the tumor grows so rapidly that the patient complains of much pain in the neck caused by the tumor-mass effects. Clinically, metastatic thyroid lung tumor has its obvious clinical manifestations, including a hard, fixed, rapidly growing mass of the thyroid gland, a peripheral infiltration, and a cervical lymph node metastasis. Most of the patients complain of dyspnea, dysphagia, or dysphonia, which may result from vocal cord or laryngeal paralysis [12], but unfortunately, these symptoms are uncharacteristic and may be misdiagnosed as primary thyroid cancer or as a benign tumor.
To which organ the tumor metastasizes in the study rests in two main factors: first, it depends on the organ selective membrane receptors of tumor cell, and second, the selective membrane receptors for tumors in the organs matter. We can see that the thyroid meets the requirements for the tumor cell to metastasize, and besides, the blood supply is merely inferior to the adrenal. Despite all the advantages, the thyroid is free of metastasizing in most of the cases for the following reasons [13]: first, most of the malignant tumor cells enter the venous circulation. Second, those cells successfully reaching the thyroid bed are always washed away due to the effects of high intraglandular blood flow and high intraglandular oxygen and iodine content on tumor destruction. Direct spread, blood metastasis, and lymphatic metastasis are the three main methods [7], and the hematogenous spread is the most common route among these [6]. For lung cancer to metastasize to the thyroid, a direct invasion of the malignant tumor from the adjacent organs such as the larynx, trachea, or esophagus is the most frequent route of metastasis [6], while the clinical metastasis of the menstrual lymph nodes is scarce. In our case, first we can exclude the direct diffusion as we found no tumor cells in the 11L, 4L, 7, 4R lymph glands with the aid of TBNA under a bronchoscope. The tumor cells spread mostly through the lymphatic channels, because of the metabolic enhancement of FDG in the mediastinum, hilar, left axilla, right clavicle, the area II of the right neck, revealing the tumor cells moving down the lymphatics from the lungs to the mediastinum, clavicle, and ultimately settling down in the thyroid.
An accurate diagnosis is important despite the immense difficulties and necessities in the identification between primary thyroid tumors and metastatic tumors. Here we listed a part of the methods: FNAC combined with IHC is a feasible method that can make a qualitative diagnosis for the thyroid tumor. It has a high precision when used in the diagnosis of thyroid cancer, and the false negative rate is less than 1%, and the false positive rate is about 2%-3% [5,14]. In addition, IHC can distinguish between metastatic malignant tumors and primary thyroid tumors from the histological or cytological standpoints, as well as by judging the source of the primary lesions [9]. The positive immune response to thyroglobulin suggests a diagnosis of primary thyroid tumors, but a negative immune response cannot rule out all the primary thyroid tumors in several cases [15]. However, when the FNAC findings are inconclusive, electron microscopy (EM) is an essential tool to make the distinction [1]. Megumi Miyakawa [4] found several cases in which it was difficult to make a definite diagnosis due to the lack of positive Tg or CEA staining in the thyroid FNAC specimens, but he still highly recommends thyroid biopsy and histological or IHC staining be required to make a final definitive diagnosis. According to Tariq Namad [6], integrating a fine-needle aspiration biopsy (FNA) with US guidance is a recommended method for its clarity and precision, but sufficient cells are needed to make a cell block. When the needle biopsy is not sufficient to provide sufficient cells for immunohistochemistry, then a postoperative histological consultation or intraoperative rapid freezing sections are good alternatives [6]. Using 18F-FDG [14,15] as the tracer material, combined with PET-CT is a bold innovation being conducted in clinics for the assessment of possible disease recurrence. Also, a cold nodule in a thyroid scan, a hyperechoic mass in US, or a calcification in the thyroid parenchyma or the nodules are all features of thyroid metastases.
Surgery is the first option for patients, according to Elliott RH’s research [7], and no recurrence was seen in the cervical region in those patients who underwent radical thyroid operations. Thyroxine tablets, radiotherapy, and chemotherapy are the common means taken later to reduce the recurrence of thyroid cancer. Active surgical treatment fueled by postoperative radiotherapy or chemotherapy is more effective than non-surgical treatment, and the surgery is based on the histological type of the primary tumor, the location of secondary lesions in the thyroid, the dynamics of the primary tumor, and the type of metastatic expansion. For most cases, the thyroid lump grows rapidly and invades the trachea, causing a breathing disorder. The earlier the treatment, the better the quality of life is. But what disappoints us is that the prognosis still remains poor, as surgery does not contribute to prolonging patients’ life in the majority of the cases [16,17]. Scholars are divided in their opinions regarding whether the patient should be operated on. Some recommend a thyroidectomy as it lowers the incidence of local recurrence. Others hold the view that the removal of thyroid doesn’t prolong the lifespan but reduces the amount of hormones produced by the thyroid [16,18]. The development of new targeted drugs is a current research focus for scientists. Recent research has shown that erlotinib [3] has a significant effect on targeting the lungs and the thyroid. Lenvatinib is an oral multitargeted tyrosine kinase inhibitor of vascular endothelial growth factors 1, 2, and 3, and its perfect effectiveness in the use of anaplastic thyroid cancer with lung metastasis has been reported [19]. A selected trial and a phase II trial conducted in Japan confirmed it is effective for unresectable thyroid cancer, but whether it can be applied to thyroid metastasis or not needs further clinic trials.
Due to the scarcity of literature on and the poor prognosis of this disease, close communication among clinicians, histopathologists, and radiologists, and a multidisciplinary approach and careful surveillance are important in treating the cases; moreover, a close follow-up is also needed.
Cases of thyroid cancer metastasizing from the lungs are rare, and from the above discussion we can conclude that the diagnosis is difficult because the clinical presentation is not that distinctive and can be confused with subacute thyroiditis and primary thyroid tumor. The disease is usually an incidental finding in autopsy, reminding us that sufficient attention should be paid to those patients once nodules in the thyroid are found, for the prognosis is rather poor. Surgery is the first option for thyroid metastasis. Herein, we report a case of the thyroid cancer metastasizing from lung cancer with the aim of adding to the knowledge of this rare disease.
Acknowledgements
This study was supported by two grants from the National Natural Science Foundation of China (nos. 81702653 and 81702645), and a grant from the Zhejiang Medical and Health Science and Technology Plan (no. 2018253753).
Disclosure of conflict of interest
None.
References
- 1.Katsenos S, Archondakis S, Vaias M, Skoulikaris N. Thyroid gland metastasis from small cell lung cancer: an unusual site of metastatic spread. J Thorac Dis. 2013;5:E21–24. doi: 10.3978/j.issn.2072-1439.2012.06.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lin JD, Weng HF, Ho YS. Clinical and pathological characteristics of secondary thyroid cancer. Thyroid. 1998;8:149–153. doi: 10.1089/thy.1998.8.149. [DOI] [PubMed] [Google Scholar]
- 3.Cho SW, Choi HS, Yeom GJ, Lim JA, Moon JH, Park DJ, Chung JK, Cho BY, Yi KH, Park YJ. Long-term prognosis of differentiated thyroid cancer with lung metastasis in Korea and its prognostic factors. Thyroid. 2014;24:277–286. doi: 10.1089/thy.2012.0654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miyakawa M, Sato K, Hasegawa M, Nagai A, Sawada T, Tsushima T, Takano K. Severe thyrotoxicosis induced by thyroid metastasis of lung adenocarcinoma: a case report and review of the literature. Thyroid. 2001;11:883–888. doi: 10.1089/105072501316973154. [DOI] [PubMed] [Google Scholar]
- 5.Yamazaki H, Iwasaki H, Yamashita T, Yoshida T, Suganuma N, Yamanaka T, Masudo K, Nakayama H, Kohagura K, Rino Y, Masuda M. A case of pneumothorax after treatment with lenvatinib for anaplastic thyroid cancer with lung metastasis. Case Rep Endocrinol. 2018;2018:7875929. doi: 10.1155/2018/7875929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Namad T, Wang J, Shipley R, Abdel Karim N. Thyroid metastasis from nonsmall cell lung cancer. Case Rep Oncol Med. 2013;2013:208213. doi: 10.1155/2013/208213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ELLIOTT RH Jr, FRANTZ VK. Metastatic carcinoma masquerading as primary thyroid cancer: a report of authors’ 14 cases. Ann Surg. 1960;151:551–561. doi: 10.1097/00000658-196004000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferrozzi F, Bov D, Campodonico F, Chiar FD, Conti GM, Bassi P. US and CT findings of secondary neoplasms of the thyroid--a pictorial essay. Clinical Imaging. 1998;22:157–161. doi: 10.1016/s0899-7071(97)00119-8. [DOI] [PubMed] [Google Scholar]
- 9.Dao A, Jabir H, Taleb A, Benchakroun N, Bouchbika Z, Nezha T, Jouhadi H, Sahraoui S, Benider A. Lung adenocarcinoma with thyroid metastasis: a case report. BMC Res Notes. 2017;10:130. doi: 10.1186/s13104-017-2449-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jankowska P, Teoh EM, Fisher C, Rhys Evans P, Nutting CM, Harrington KJ. Case report. Isolated intrathyroid metastasis from undifferentiated and squamous carcinoma of the head and neck: the case for surgery and re-irradiation. Br J Radiol. 2008;81:e154–161. doi: 10.1259/bjr/26919796. [DOI] [PubMed] [Google Scholar]
- 11.Lam KY, Lo CY. Metastatic tumors of the thyroid gland: a study of 79 cases in Chinese patients. Arch Pathol Lab Med. 1998;122:37–41. [PubMed] [Google Scholar]
- 12.Hulikal N, Naru RR, Gangasani R, Nandyala R, Pai A, Meenakshisundaram M. A case of synchronous isolated thyroid metastasis from a primary lung cancer presenting as thyroid primary: Diagnostic challenge! Lung India. 2016;33:326–329. doi: 10.4103/0970-2113.180912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim AY, Park SB, Choi HS, Hwang JC. Isolated thyroid metastasis from renal cell carcinoma. J Ultrasound Med. 2007;26:1799–1802. doi: 10.7863/jum.2007.26.12.1799. [DOI] [PubMed] [Google Scholar]
- 14.Corrias A, Einaudi S, Chiorboli E, Weber G, Crinò A, Andreo M, Cesaretti G, de Sanctis L, Messina MF, Segni M, Cicchetti M, Vigone M, Pasquino AM, Spera S, de Luca F, Mussa GC, Bona G. Accuracy of fine needle aspiration biopsy of thyroid nodules in detecting malignancy in childhood: comparison with conventional clinical, laboratory, and imaging approaches. J Clin Endocrinol Metab. 2001;86:4644–4648. doi: 10.1210/jcem.86.10.7950. [DOI] [PubMed] [Google Scholar]
- 15.Wey SL, Chang KM. Tumor-to-tumor metastasis: lung carcinoma metastasizing to thyroid neoplasms. Case Rep Pathol. 2015;2015:153932. doi: 10.1155/2015/153932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Papi G, Fadda G, Corsello SM, Corrado S, Rossi ED, Radighieri E, Miraglia A, Carani C, Pontecorvi A. Metastases to the thyroid gland: prevalence, clinicopathological aspects and prognosis: a 10-year experience. Clin Endocrinol (Oxf) 2007;66:565–571. doi: 10.1111/j.1365-2265.2007.02773.x. [DOI] [PubMed] [Google Scholar]
- 17.Calzolari F, Sartori PV, Talarico C, Parmeggiani D, Beretta E, Pezzullo L, Bovo G, Sperlongano P, Monacelli M, Lucchini R, Misso C, Gurrado A, D’Ajello M, Uggeri F, Puxeddu E, Nasi P, Testini M, Rosato L, Barbarisio A, Avenia N. Surgical treatment of intrathyroid metastases: preliminary results of a multicentric study. Anticancer Res. 2008;28:2885–2888. [PubMed] [Google Scholar]
- 18.Dequanter D, Lothaire P, Larsimont D, de Saint-Aubain de Somerhausen N, Andry G. [Intrathyroid metastasis: 11 cases] . Ann Endocrinol (Paris) 2004;65:205–208. doi: 10.1016/s0003-4266(04)95672-7. [DOI] [PubMed] [Google Scholar]
- 19.Passler C, Scheuba C, Prager G, Kaserer K, Flores JA, Vierhapper H, Niederle B. Anaplastic (undifferentiated) thyroid carcinoma (ATC). A retrospective analysis. Langenbecks Arch Surg. 1999;384:284–293. doi: 10.1007/s004230050205. [DOI] [PubMed] [Google Scholar]


