Abstract
This study aimed to explore the value of long non-coding RNA nuclear enriched abundant transcript 1 (lnc-NEAT1) in predicting chronic obstructive pulmonary disease (COPD) susceptibility and acute exacerbation risk, and to investigate the correlation of lnc-NEAT1 with disease severity, inflammation level, and miR-193a in COPD patients. 90 AECOPD patients, 90 stable COPD patients and 90 healthy controls were consecutively recruited. Severity of airflow obstruction in COPD patients was defined by GOLD guidelines. Plasma samples were collected from all participants, then lnc-NEAT1 and miR-193a expressions were measured by qPCR, and TNF-α, IL-1β, IL-6, and IL-17 were measured by ELISA. Lnc-NEAT1 expression was elevated in AECOPD patients and stable COPD patients compared to healthy controls, as well as in AECOPD patients compared to stable COPD patients; moreover, ROC curves showed that lnc-NEAT1 predicted increased COPD susceptibility and acute exacerbation risk of COPD. Also, lnc-NEAT1 expression positively correlated with GOLD stage and levels of TNF-α, IL-1β, IL-6 and IL-17 in both AECOPD and stable COPD patients. Furthermore, lnc-NEAT1 expression negatively correlated with miR-193a expression, and miR-193a could predict decreased COPD susceptibility and acute exacerbation risk, and negatively correlated with GOLD stage and levels of TNF-α, IL-1β, IL-6 and IL-17 in both AECOPD and stable COPD patients. lnc-NEAT1 predicts elevated COPD susceptibility and increased acute exacerbation risk, and positively correlates with disease severity as well as inflammation, but negatively associates with miR-193a in COPD patients.
Keywords: Acute exacerbation of COPD, lncRNA NEAT1, miR-193a, severity, inflammation
Introduction
Chronic obstructive pulmonary disease (COPD), mainly characterized by airway limitation and abnormal inflammatory response in lungs, affects around 328 million individuals and causes 3.5-4 million deaths worldwide annually, and has been estimated to rank as the third leading cause of global death by 2020 [1-4]. Acute exacerbation of COPD (AECOPD) is an aggravation in the COPD symptoms (typically dyspnea, cough, increased sputum volume and sputum purulence), which is principally caused by respiratory infection and is also triggered by other factors such as smoking, air pollution, inhaled allergens, surgery, sedative drugs, pneumothorax, pleural effusion, congestive heart failure, arrhythmia, and pulmonary embolism [5-7]. The current disease managements for AECOPD (such as bronchodilators, steroids, antibiotics, oxygen and noninvasive ventilation) focus on ameliorating the AECOPD symptoms; whereas, effective treatments for ameliorating the deterioration in lung function are limited [2,8,9]. The AECOPD patients still face poor prognosis, and one-year mortality in those requiring noninvasive ventilation and those requiring intensive care unit care are 28% and 43% respectively [6,10]. In recent years, great interest has arisen in biomarkers that predict disease risk and monitors disease progression at early stages. Thus, with the aim of preventing AECOPD and improve prognosis of COPD patients, investigation of valuable biomarkers is of great importance.
Long non-coding RNA (lncRNA), the non-protein-coding RNA longer than 200 nucleotides, regulates protein-coding genes by epigenetic, transcriptional or post-transcriptional patterns [11-14]. LncRNA nuclear enriched abundant transcript 1 (lnc-NEAT1), located on chromosome 11q13.1, is widely expressed in mammalian cells and acts as an architectural component of paraspeckle structure [15-17]. According to several previous studies, lnc-NEAT1 is involved in some inflammation-related diseases (such as COPD, lupus, diabetic nephropathy, tuberculosis as well as the response to viral infections) [16,18-21]. In COPD, lnc-NEAT1 is overexpressed in fibroblasts deriving from COPD patients, indicating that lnc-NEAT1 might be related to the pathology of COPD [20]. MicroRNAs (miRNAs) are small non-coding RNAs with 18-24 nucleotides, which regulate gene expression via binding to the 3’-untranslated region of mRNAs [22,23]. As one of the common miRNAs, miR-193a has been reported to be downregulated in staphylococcal enterotoxin B-induced acute inflammatory lung injury according to an in vitro experiment [24]. Furthermore, miR-193a has been confirmed to be a target miRNA of lnc-NEAT1 in several cancers, including lung cancer [19,25-28].
Based on the premises that lnc-NEAT1 might participate in pathology of COPD and miR-193a has been identified as target miRNA of lnc-NEAT1 in various diseases, we hypothesized that lnc-NEAT1 might impact COPD progression and inflammation response through targeting miR-193a. However, related evidence is limited. Hence, we conducted this study to explore the value of lnc-NEAT1 in predicting COPD susceptibility and acute exacerbation risk, and investigated the correlation of lnc-NEAT1 with disease severity, inflammation level, and miR-193a in COPD patients.
Methods
Patients and healthy controls
Between January 2017 and June 2018, 90 AECOPD patients, 90 stable COPD patients, and 90 healthy controls from Tongji Hospital were consecutively recruited to the current study. For the enrolled AECOPD patients, the inclusion criteria were: (1) diagnosed as COPD confirmed by spirometric evidence of airflow obstruction (a postbronchodilator forced expiratory volume in 1 s (FEV1)/forced vital capacity (FVC) < 0.70) according to the criteria of Global Initiative for Chronic Obstructive Pulmonary disease (GOLD) [29]; (2) accompanied by acute exacerbations of symptoms in accordance with the definitions of the GOLD; (3) age ≥ 40 years. For stable COPD patients, the inclusion criteria were as follows: (1) diagnosed as COPD according to the criteria of the GOLD; (2) clinically stable for at least 3 months without acute exacerbations; (3) age above 40 years old. Both AECOPD patients and stable COPD patients were excluded if they had following conditions: (1) asthma, pneumonia, interstitial pulmonary disease, pneumothorax, pleural effusion or pulmonary thromboembolism; (2) congestive heart failure or arrhythmia; (3) concurrent diseases such as sepsis, malignant hematological diseases, autoimmune diseases or tumors; (4) pregnant or lactating woman. As for the healthy controls, the inclusion criteria were: (1) age- and gender- matched to the enrolled COPD patients; (2) had no history of COPD, asthma or other respiratory diseases (such as interstitial pulmonary disease, bronchiectasis, pneumonia, pulmonary thromboembolism and so on). The exclusion criteria were: (1) complicated by active inflammatory diseases, acute infections, hematological diseases, autoimmune diseases or malignancies; (2) taking medication that can potentially interfere with level of RNA and inflammatory cytokines.
Ethics
The present study was approved by the Institutional Review Board of Tongji Hospital, and written informed consents were provided by all participants or their guardians before enrollment.
Collection of data and blood samples
After signed the informed consents, characteristics of all participants were collected, which included age, gender, body mass index (BMI), family history of COPD, history of smoking and lung function test (FEV1, FEV1 (% predicted) and FEV1/FVC ratio). For the AECOPD patients and stable COPD patients, severity of airflow obstruction was defined by GOLD guidelines as follows: GOLD 1: FEV1 (% predicted) ≥ 80%, GOLD 2: 50%-79%, GOLD 3: 30%-49%, GOLD 4: < 30%. On the first day of enrollment, peripheral blood samples were collected from all participants using EDTA tubes and immediately centrifuged for 15 min at 1800 g at 4°C to separate plasma fractions, then which were aliquoted into new tubes and stored at -80°C for later determination.
Detection of lncRNA NEAT1 and miR-193a
Detection of lnc-NEAT1 and miR-193a was performed by quantitative polymerase chain reaction (qPCR) assay. Total RNA was extracted from plasma with QIAamp RNA Blood Mini Kit (Qiagen, Duesseldorf, Nordrhein-Westfalen, German). Subsequently, transcription to cDNA was conducted using PrimeScript™ RT reagent Kit (Perfect Real Time) (Takara, Kusatsu, Shiga, Japan), and then qPCR was performed using TB Green™ Fast qPCR Mix (Takara, Kusatsu, Shiga, Japan), followed by the qPCR amplification. Results of lnc-NEAT1 expression and miR-193a were calculated by the 2-ΔΔCT formula. GAPDH and U6 were used as the internal references for lnc-NEAT1 and miR-193a respectively. Primers used for PCR were as follows: lncRNA NEAT1, forward (5’->3’): TGTCCCTCGGCTATGTCAGA, reverse (5’->3’): GAGGGGACGTGTTTCCTGAG; miR-193a, forward (5’->3’): ACACTCCAGCTGGGTGGGTCTTTGCGGGCGAG, reverse (5’->3’): TGTCGTGGAGTCGGCAATTC; GAPDH, forward (5’->3’): GGAGCGAGATCCCTCCAAAAT, reverse (5’->3’): GGCTGTTGTCATACTTCTCATGG; U6, forward (5’->3’): CTCGCTTCGGCAGCACATATACTA, reverse (5’->3’): ACGAATTTGCGTGTCATCCTTGC.
Determination of inflammatory cytokines
The plasma concentrations of tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), interleukin-6 (IL-6), and interleukin-17 (IL-17) were measured by use of commercially available enzyme-linked immunosorbent assay kits (ELISA) kits (R&D Systems Inc., Minneapolis, MN, USA). All the plasma sample processing, measurement, and content calculation were conducted according to kit instructions. A standard curve was made by using standards provided in the kits, and the inflammatory cytokine concentrations were determined from the standard curves by use of linear regression analysis.
Statistical analysis
Continuous variables were determined for normality by using the Shapiro-Wilk test. For the normal distributed continuous variables, ithey were presented as mean value ± standard deviation, and the comparison among three groups was determined by one-way ANOVA; as for skewed or unknown-distributed continuous variable, they were was expressed as median (25th-75th quantiles), and the comparison among groups was determined by Wilcoxon rank sum test or Kruskal-Wallis test. Categorical variables were described as count (percentage), and comparison among groups was determined by Chi-square test. Spearman’s rank correlation test was used to perform correlation analysis. Receiver operating characteristic (ROC) curve and areas under the curve (AUC) were used to investigate the value of lncRNA NEAT1 and miR-193a in COPD diagnosis. All tests were two sided. P value < 0.05 was considered significant. All statistical analyses were performed by SPSS 20.0 software (SPSS Inc., Chicago, IL, USA), and all figures were made by using GraphPad Prism 6.01 (GraphPad Software Inc., San Diego, CA, USA).
Results
Baseline characteristics
Totally 90 AECOPD patients, 90 stable COPD patients, and 90 healthy controls were enrolled in this study, with the mean age of 68.0 ± 6.5 years, 67.4 ± 7.4 years and 68.1 ± 7.1 years respectively (Table 1). There were 69 males and 21 females among AECOPD patients, 64 males and 26 females among COPD patients, and 62 males and 28 females who were healthy controls. BMI in the three groups were 22.8 ± 3.1 kg/m2, 22.0 ± 2.8 kg/m2 and 22.8 ± 2.6 kg/m2 respectively. No difference of age (P = 0.784), gender (P = 0.487), or BMI (P = 0.125) was observed among AECOPD patients, COPD patients, and healthy controls. Whereas, significant difference of history of smoking (P = 0.007), FEV1/FVC (%) (P < 0.001), FEV1 (predicted, %) (P < 0.001), TNF-α (pg/mL) (P < 0.001), IL-1β (pg/mL) (P < 0.001), IL-6 (pg/mL) (P < 0.001) and IL-17 (pg/mL) (P < 0.001) was observed among AECOPD patients, COPD patients and healthy controls, and number of family history of COPD was numerically higher in AECOPD patients and stable COPD patients compared to healthy controls (P = 0.057).
Table 1.
Characteristics of AECOPD patients, stable COPD patients and healthy controls
| Characteristic | AECOPD patients (N = 90) | Stable COPD patients (N = 90) | Healthy controls (N = 90) | P value |
|---|---|---|---|---|
| Age (years) | 68.0 ± 6.5 | 67.4 ± 7.4 | 68.1 ± 7.1 | 0.784 |
| Gender (male/female) | 69/21 | 64/26 | 62/28 | 0.487 |
| BMI (kg/m2) | 22.8 ± 3.1 | 22.0 ± 2.8 | 22.8 ± 2.6 | 0.125 |
| Family history of COPD | 26 (28.9) | 28 (31.1) | 15 (16.7) | 0.057 |
| History of smoking | 46 (51.1) | 44 (49.8) | 27 (30.0) | 0.007 |
| FEV1/FVC (%) | 59.80 (54.53-65.05) | 61.50 (57.20-64.80) | 82.30 (79.60-84.03) | < 0.001 |
| FEV1 (predicted, %) | 56.35 (45.33-81.68) | 68.40 (56.00-82.63) | 99.15 (96.25-100.90) | < 0.001 |
| TNF-α (pg/mL) | 57.99 (34.31-85.86) | 19.60 (9.80-32.95) | 14.00 (7.67-21.45) | < 0.001 |
| IL-1β (pg/mL) | 3.87 (2.05-6.00) | 1.32 (0.64-2.14) | 0.93 (0.53-1.33) | < 0.001 |
| IL-6 (pg/mL) | 37.27 (15.46-54.76) | 9.00 (4.98-18.96) | 7.42 (3.85-11.67) | < 0.001 |
| IL-17 (pg/mL) | 51.13 (22.12-107.86) | 16.63 (6.36-33.78) | 9.77 (5.86-16.99) | < 0.001 |
Data are presented as mean value ± standard deviation, count (percentage) or median (25th-75th quantiles). Comparison between three groups was determined by one-way ANOVA, Chi-Square test, or Kruskal-Wallis test. P value < 0.05 was considered significant. AECOPD: acute exacerbation of chronic obstructive pulmonary disease; COPD: chronic obstructive pulmonary disease; FEV1/FVC: a forced expiratory volume in the first second; FVC: forced vital capacity; TNF-α: tumor necrosis factor-α; IL: interleukin.
Correlation of lnc-NEAT1 expression with AECOPD risk and stable COPD risk
Lnc-NEAT1 expression was elevated in the AECOPD group (P < 0.001) and stable COPD group (P = 0.001) compared to healthy controls group (Figure 1A). lnc-NEAT1 could distinguish AECOPD from stable COPD (AUC = 0.779, 95% CI: 0.711-0.848), and the sensitivity and specificity were 87.8% and 62.2% respectively at the best cut-off point (lnc-NEAT1 expression: 1.749) (Figure 1B). Also, it was increased in AECOPD group compared to stable COPD group (P < 0.001). ROC curve shows that lnc-NEAT1 was able to distinguish AECOPD from healthy controls with AUC of 0.869 (95% CI: 0.817-0.921) (Figure 1C). Sensitivity and specificity were 93.3% and 68.9% respectively at the best cut-off point where the AUC reached a maximum value of 1.521. Furthermore, it could distinguish stable COPD from healthy controls (AUC = 0.642, 95% CI: 0.561-0.722), with the sensitivity of 77.8% and specificity of 48.9% at the best cut-off point (lnc-NEAT1 expression: 0.946) (Figure 1D). These data indicated that lnc-NEAT1 was able to predict COPD susceptibility and acute exacerbation risk of COPD.
Figure 1.

lnc-NEAT1 expression in AECOPD, stable COPD, and healthy controls as well as ROC curves. Lnc-NEAT1 expression was higher in AECOPD patients thanin stable COPD patients or healthy controls, and it was also increased in stable COPD and healthy controls (A). According to ROC curve, lnc-NEAT1 distinguished AECOPD patients from stable COPD patients (B). ROC curves also showed that lnc-NEAT1 could distinguish AECOPD from healthy controls (C) and distinguish stable COPD from healthy controls (D). ROC curve and areas under the curve (AUC) were used to investigate the value of lncRNA NEAT1 in COPD diagnosis. P < 0.05 was considered significant. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; ROC curve, receiver operating characteristic curve. Comparison between two groups was determined by Wilcoxon rank sum test.
Correlation of lnc-NEAT1 expression with disease severity in AECOPD patients and stable COPD patients
According to the disease severity, both AECOPD patients and stable COPD patients were classified into GOLD 1 group, GOLD 2 group, and GOLD 3 group. In AECOPD patients, lnc-NEAT1 expression in the GOLD 2 group (3.312 (2.567-3.916)) was increased compared to GOLD 1 group (1.774 (1.494-3.184)) (P < 0.001), and it was also elevated in the GOLD 3 group (4.333 (2.844-5.745)) compared to GOLD 2 group (P = 0.022) and GOLD 1 group (P < 0.001) (Figure 2A). In stable COPD patients, lnc-NEAT1 expression was higher in the GOLD 2 group (1.673 (1.417-2.842)) than that in the GOLD 1 group (0.962 (0.559-1.305)) (P < 0.001), and it was elevated in the GOLD 3 group (3.413 (1.728-4.761)) compared to GOLD 2 group (P = 0.007) and GOLD 1 group (P < 0.001) (Figure 2B). These results indicated that lnc-NEAT1 expression was positively correlated with disease severity in both AECOPD and stable COPD patients.
Figure 2.
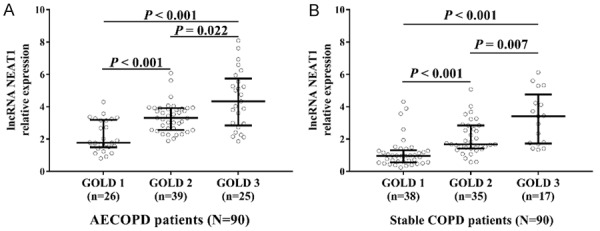
Lnc-NEAT1 expression is positively correlated with disease severity. Lnc-NEAT1 was positively correlated with GOLD stage in AECOPD patients (A). Lnc-NEAT1 was positively correlated with GOLD stage in stable COPD patients (B). Comparison between two groups was determined by Wilcoxon rank sum test. P < 0.05 was considered significant. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1; GOLD, Global Initiative for Chronic Obstructive Pulmonary disease; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease.
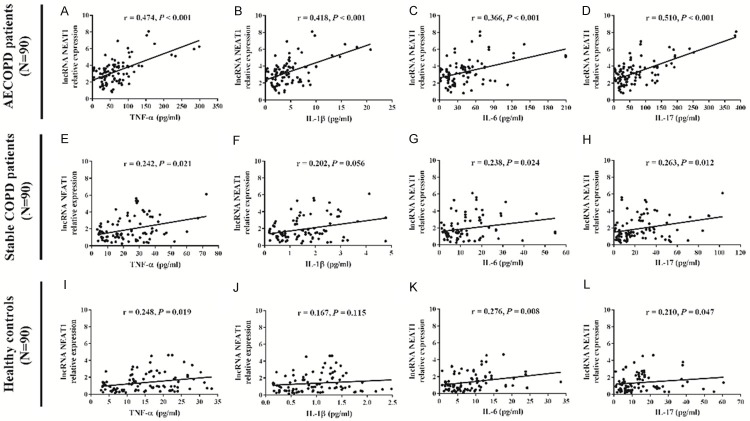
Correlation of lnc-NEAT1 expression with inflammatory cytokines in AECOPD patients, stable COPD patients, and healthy controls
In AECOPD patients, lnc-NEAT1 expression was positively correlated with TNF-α (r = 0.474, P < 0.001) (Figure 3A), IL-1β (r = 0.418, P < 0.001) (Figure 3B), IL-6 (r = 0.366, P < 0.001) (Figure 3C) and IL-17 (r = 0.510, P < 0.001) (Figure 3D). In stable COPD patients, lnc-NEAT1 expression was positively correlated with TNF-α (r = 0.242, P = 0.021) (Figure 3E), IL-6 (r = 0.238, P = 0.024) (Figure 3G), and IL-17 (r = 0.263, P = 0.012) (Figure 3H). lnc-NEAT1 high expression was numerically correlated with increased IL-1β level (r = 0.202, P = 0.056) (Figure 3F). In healthy controls, lnc-NEAT1 expression was positively correlated with TNF-α (r = 0.248, P = 0.019) (Figure 3I), IL-6 (r = 0.276, P = 0.008) (Figure 3K) and IL-17 (r = 0.210, P = 0.047) (Figure 3L). These results suggested that lnc-NEAT1 positively correlated with inflammation in both AECOPD and stable COPD patients; moreover, positive correlation of lnc-NEAT1 with inflammation level was also observed in heathy controls, while the correlation was not that close.
Figure 3.
Lnc-NEAT1 expression is positively correlated with inflammatory cytokines in AECOPD patients, stable COPD patients and healthy controls. Increased lnc-NEAT1 expression was associated with higher TNF-α, IL-1β, IL-6 and IL-17 levels in AECOPD patients (A-D). Higher lnc-NEAT1 expression correlated with raised TNF-α, IL-6 and IL-17 levels in stable COPD patients (E-H). Lnc-NEAT1 expression was positively correlated with TNF-α, IL-6 and IL-17 levels in healthy controls (I-L). Correlation of lnc-NEAT1 expression with inflammatory cytokines levels was determined by Spearman correlation analysis. P < 0.05 was considered significant. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease; TNF-α, tumor necrosis factor-α; IL-1β, interleukin-1β; IL-6, interleukin-6; IL-17, interleukin-17.
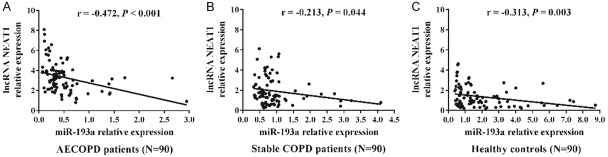
Correlation of lnc-NEAT1 expression with miR-193a in AECOPD patients, stable COPD patients, and healthy controls
There was a negative correlation between lnc-NEAT1 expression and miR-193a expression in AECOPD patients (r = -0.472, P < 0.001) (Figure 4A), stable COPD patients (r = -0.213, P = 0.044) (Figure 4B), and healthy controls (r = -0.313, P = 0.003) (Figure 4C).
Figure 4.
Lnc-NEAT1 expression negatively correlates with miR-193a expression in AECOPD patients, stable COPD patients and healthy controls. Negative correlation of lnc-NEAT1 expression with miR-193a expression was observed in AECOPD patients (A), stable COPD patients (B) and healthy controls (C). Comparison of lnc-NEAT1 expression with miR-193a expression was determined by Spearman correlation analysis. P < 0.05 was considered significant. Lnc-NEAT1, long non-coding RNA nuclear enriched abundant transcript 1; AECOPD. acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease.
Correlation of miR-193a expression with AECOPD risk and stable COPD risk
miR-193a expression was decreased in the AECOPD group (P < 0.001) and stable COPD (P < 0.001) group compared to healthy controls group. It was also lower in the AECOPD group compared to the stable COPD group (P < 0.001) (Figure 5A). ROC curve disclosed that miR-193a had the ability to distinguish AECOPD from stable COPD (AUC: 0.821, 95% CI: 0.757-0.885), and miR-193a expression at the best cut-off point was 0.566 (sensitivity: 75.6%; specificity: 80.0%) (Figure 5B). Also, miR-193a could distinguish AECOPD from healthy controls (AUC: 0.893, 95% CI: 0.847-0.938), with miR-193a expression of 0.505 as the best cut-off point (sensitivity: 70.0% and specificity: 93.3%) (Figure 5C). Moreover, miR-193a could distinguish stable COPD from healthy controls (AUC: 0.690, 95% CI: 0.612-0.769), and the miR-193a expression was 1.088 at the best cut-off point (sensitivity: 85.6%; specificity: 58.9%) (Figure 5D). These results suggested that miR-193a had a good predictive value for COPD susceptibility as well as acute exacerbation risk of COPD.
Figure 5.
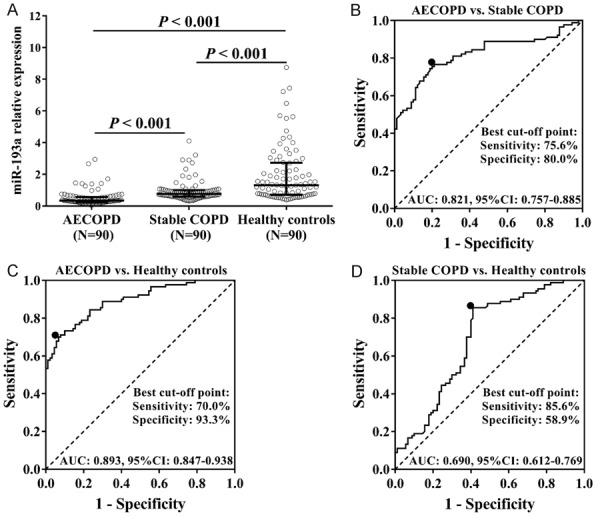
miR-193a expression in AECOPD patients, stable COPD patients, and healthy controls, as well as ROC curves. miR-193a expression was lower in AECOPD patients compared to stable COPD patients and healthy controls, and it was also decreased in stable COPD patients compared to healthy controls (A). According to ROC curves, miR-193a could distinguish AECOPD from stable COPD (B) or healthy controls (C). Also, miR-193a could distinguish stable COPD from healthy controls (D). Comparison between two groups was determined by Wilcoxon rank sum test. ROC curve and areas under the curve (AUC) were used to investigate the value of miR-193a in COPD diagnosis. P < 0.05 was considered significant. AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease.
Correlation of miR-193a expression with disease severity in AECOPD patients and stable COPD patients
In AECOPD patients, miR-193a expression was lower in the GOLD2 group compared to GOLD1 group (P < 0.001), and it was also decreased in the GOLD 3 group compared to GOLD 1 group (P < 0.001) and GOLD 2 group (P = 0.001) (Figure 6A). Furthermore, in stable COPD patients, miR-193a expression was reduced in the GOLD2 group compared to GOLD1 group (P = 0.018), while no difference of miR-193a expression was found between the GOLD3 group and GOLD1 group (P = 0.164) or GOLD2 group (P = 0.507) (Figure 6B). These data indicated that miR-193a had a negative correlation with disease severity in AECOPD patients and stable COPD patients.
Figure 6.
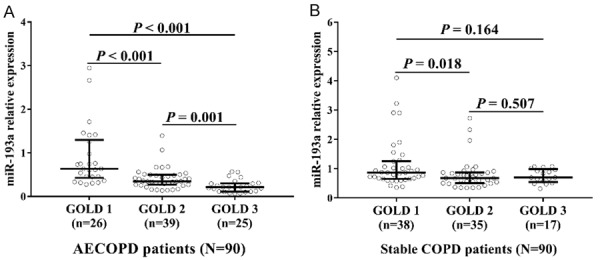
miR-193a expression negatively correlated with disease severity. miR-193a expression was negatively associated with GOLD stage in AECOPD patients (A) and stable COPD patients (B). Comparison between two groups was determined by Wilcoxon rank sum test. P < 0.05 was considered significant. GOLD, Global Initiative for Chronic Obstructive Pulmonary disease; AECOPD, acute exacerbation of chronic obstructive pulmonary disease; COPD, chronic obstructive pulmonary disease.
Correlation of miR-193a relative expression with inflammatory cytokines in AECOPD patients, stable COPD patients, and healthy controls
The correlation of miR-193a expression with inflammatory cytokine levels is assessed and displayed in Table 2. In AECOPD patients, miR-193a expression was negatively correlated with cytokines including TNF-α (P < 0.001), IL-1β (P = 0.001), IL-6 (P = 0.035) and IL-17 (P = 0.002). As to the correlation of miR-193a expression with inflammatory cytokines levels in stable COPD patients, miR-193a expression was negatively correlated with TNF-α (P = 0.007), IL-1β (P = 0.034), IL-6 (P = 0.025) and IL-17 (P < 0.001). In addition, there was a negative correlation of miR-193a expression with TNF-α (P = 0.022), IL-1β (P = 0.038), IL-6 (P = 0.002) and IL-17 (P = 0.012) in healthy controls. These results suggested that miR-193a negatively correlated with inflammation level in AECOPD, stable COPD patients, and healthy controls. Considering the strong correlation of lnc-NEAT1 with miR-193a in our data, the identification of miR-193a as a target for lnc-NEAT1 as reported, as well as the closely negative association of miR-193a with disease severity and inflammation level in COPD patients, we speculated that lnc-NEAT1 might predict susceptibility and acute exacerbation risk of COPD, as well as correlate with disease severity and inflammation level in COPD patients by regulating miR-193a.
Table 2.
Correlations of miR-193a relative expression with inflammatory cytokines
| Item | miR-193a relative expression | |
|---|---|---|
|
| ||
| P value | Correlation coefficient (r) | |
| AECOPD patients (N = 90) | ||
| TNF-α | < 0.001 | -0.381 |
| IL-1β | 0.001 | -0.344 |
| IL-6 | 0.035 | -0.223 |
| IL-17 | 0.002 | -0.324 |
| Stable COPD patients (N = 90) | ||
| TNF-α | 0.007 | -0.282 |
| IL-1β | 0.034 | -0.223 |
| IL-6 | 0.025 | -0.237 |
| IL-17 | < 0.001 | -0.383 |
| Healthy controls (N = 90) | ||
| TNF-α | 0.022 | -0.241 |
| IL-1β | 0.038 | -0.219 |
| IL-6 | 0.002 | -0.317 |
| IL-17 | 0.012 | -0.264 |
Correlations were determined by Spearman’s rank correlation test. P value < 0.05 was considered significant. AECOPD: acute exacerbation of chronic obstructive pulmonary disease; COPD: chronic obstructive pulmonary disease; TNF-α: tumor necrosis factor-α; IL: interleukin.
Discussion
Our results indicated that: (1) lnc-NEAT1 could predict the COPD susceptibility and acute exacerbation risk of COPD; (2) lnc-NEAT1 expression positively correlated with disease severity and inflammation levels in AECOPD patients and stable COPD patients; (3) lnc-NEAT1 was negatively correlated with miR-193a, and miR-193a could predict COPD susceptibility and its acute exacerbation risk, and negatively correlated with disease severity as well as inflammation levels in COPD patients. Thus we speculated that lnc-NEAT1 might predict susceptibility and acute exacerbation risk of COPD, and correlate with disease severity as well as inflammation level in COPD patients through targeting miR-193a.
LncRNAs are involved in a variety of biologic processes such as X-chromosome inactivation, neurodegenerative disorder, stem cell specification as well as carcinogenesis, and their dysregulated expressions are associated with elevated risk or progression in a wide variety of diseases [30,31]. Regarding lncRNAs in COPD, a microarray analysis displays that 120 lncRNAs are overexpressed and 43 lncRNAs are under-expressed in lung resection tissues from smokers with COPD compared to smokers without COPD, and highlights the importance of investigating diagnostic or therapeutic value of specific lncRNA as well as the underlying mechanisms in COPD [32]. lnc-NEAT1, which is mainly reported to be essential for paraspeckle structural formation by interacting with proteins of the drosophila behavior human splicing family, is also reported to participate in inflammatory responses in some experiments [17,20,33]. For instance, a study displays that lnc-NEAT1 contributes to oxidized low-density lipoprotein induced inflammation in macrophages through sponging miR-128, and further facilitates the release of IL-6, IL-1β and TNF-α [33]. Also, another study discloses that lnc-NEAT1 downregulation remarkably reduces the levels of TNF-α, IL-6, IL-8 as well as IL-1β in a lipopolysaccharide (LPS)-stimulated rat mesangial cell line and suppresses LPS-induced kidney injury by targeting miR-204 and inactivating the nuclear factor-κB (NF-κB) pathway [17]. These data reveal that lnc-NEAT1 might be involved in the pathology of some inflammation-related diseases.
Apart from the in vivo or in vitro experiments that investigate the mechanism of lnc-NEAT1, some clinical practices have also been performed and disclose the dysregulation of lnc-NEAT1 in inflammation-related diseases [15,16]. A study displays that lnc-NEAT1 is dramatically overexpressed in sepsis patients compared to healthy controls, and the ROC curves show that lnc-NEAT1 possesses a good predictive value for sepsis risk [15]. Another study shows that lnc-NEAT1 is overexpressed in Systemic Lupus Erythematosus (SLE) patients compared to normal controls [16]. As to COPD, only one study showed that lnc-NEAT1 is overexpressed in fibroblasts derived from smokers with COPD compared to smokers without COPD; whereas, further investigation about predictive value of lnc-NEAT1 for COPD risk is rarely seen [20]. In order to explore the value of lnc-NEAT1 in predicting COPD susceptibility and acute exacerbation risk, we investigated lnc-NEAT1 expression in AECOPD patients, stable COPD patients and healthy controls. We found that lnc-NEAT1 was elevated in AECOPD patients and stable COPD patients compared to healthy controls, as well as in AECOPD patients compared to stable COPD patients, moreover, ROC curves show that lnc-NEAT1 was able to predict COPD susceptibility and its acute exacerbation risk. These results might be due to: (1) lnc-NEAT1 high expression induced macrophage inflammation as well as oxidative stress, which led to the pathologic changes in AECOPD or stable COPD, therefore, lnc-NEAT1 high expression predicted increased disease susceptibility for COPD (including AECOPD and stable COPD) [33]; (2) lnc-NEAT1 high expression might activate the inflammatory cascades and reactive oxygen species that result in raised oxidative stress in lung and severer damage in cellular components, thus increased lnc-NEAT1 expression was correlated with severe symptoms in COPD and it could predict acute exacerbation risk.
Lnc-NEAT1 has been demonstrated to correlate with disease severity in some inflammation-related diseases [15,16]. For instance, a study shows that lnc-NEAT1 expression is positively correlated with Acute Physiology and Chronic Health Evaluation II score in sepsis patients [15]. Another study displays that lnc-NEAT1 expression is elevated in active SLE patients than that in inactive SLE patients, and lnc-NEAT1 is also positively correlated with Systemic Lupus Erythematosus Disease Activity Index (SLEDAI) score in SLE patients [16]. As for inflammation, several studies also display that lnc-NEAT1 expression is positively correlated with inflammatory cytokines levels (including TNF-α, IL-1β, IL-6 and IL-8) in inflammatory diseases (such as sepsis and SLE) [15,16]. Nevertheless, limited information about the role of lnc-NEAT1 in COPD patients has been found. In the present study, we observed that lnc-NEAT1 expression was positively correlated with disease severity in both AECOPD and stable COPD patients, and lnc-NEAT1 high expression correlated with raised inflammation levels in AECOPD patients, stable COPD patients, and healthy controls. The possible reasons might be: (1) lnc-NEAT1 might increase the release of inflammatory cytokines and further caused excess production of mucus as well as aggravated the obstruction in lung, thereby decreasing the values of FEV1/FVC (%) and FEV1 (predicted, %), which correlated with increased GOLD stage [2,17]; (2) lnc-NEAT1 might promote oxidative stress in COPD, which amplifies the inflammatory response and impairs the function of protease inhibitor (like secretory leukocyte protease inhibitor (SLPI)), thus the breakdown of elastin was enhanced in lung parenchyma and the airflow limitation was promoted, which resulted in a worse GOLD stage [2,33]; (3) lnc-NEAT1 high expression might enhance the level of reactive oxygen species (ROS), which initiates inflammatory cascades through multiple mechanisms (such as protein kinase pathways, transcription factors and genomic expression of pro-inflammatory regulators), thereby leading to an “over-activated” immune system and facilitating the release of inflammatory cytokines including IL-6, IL-1β and TNF-α. Thus, a positive correlation of lnc-NEAT1 expression with inflammation level was observed in AECOPD patients and stable COPD patients [34].
According to previous studies, miRNAs can participate in pathology of several diseases including malignancies, cardiovascular diseases, endocrine diseases and neurological diseases [19]. Recently, miRNAs have been found to be dysregulated in some inflammation-related diseases, including COPD [19]. As one of the common miRNAs, miR-193a has been reported to be under-expressed in lung infiltrating mononuclear cells after exposing to staphylococcal enterotoxin B, and it suppresses intestinal inflammation in murine colitis models [35,36]. These data indicate that miR-193a shows anti-inflammatory effect in these inflammatory diseases, while the role of miR-193a in COPD is largely unknown. Considering the likely participation of miR-193a in some inflammation-related diseases and the fact that miR-193a has been confirmed as a target miRNA of lnc-NEAT1 in several diseases (such as lung cancer, colorectal cancer and multiple myeloma), we inferred that miR-193a might also be a target miRNA of lnc-NEAT1 in COPD [19,25-28] We investigated the correlation of lnc-NEAT1 expression and miR-193a expression, and explored the correlation of miR-193a with disease severity of COPD as well as inflammation in COPD patients and healthy controls. We found that lnc-NEAT1 expression was negatively correlated with miR-193a expression, and miR-193a was able to predict the COPD susceptibility and acute exacerbation risk. Moreover, miR-193a low expression was correlated with increased disease severity in COPD patients and enhanced inflammation levels in COPD patients as well as healthy controls. These results indicated that lnc-NEAT1 might exert good predictive value for disease susceptibility and acute exacerbation risk of COPD, and positively correlate with disease severity as well as inflammation level in COPD patients by regulating miR-193a.
Some limitations existed in our study: (1) sample size of AECOPD patients (N = 90) and COPD patients (N = 90) was relatively small, thus the statistical power of our study might be relatively poor; (2) this was a single-center study, in which the representativeness was limited; (3) exact regulatory function of lnc-NEAT1 in COPD as well as the detailed mechanisms underlying the regulation crosstalk between lnc-NEAT1 and miR-193a were largely unknown. Further study is needed to investigate the pathology of lnc-NEAT1 and miR-193a in COPD.
In conclusion, lnc-NEAT1 predicts elevated COPD susceptibility and increased acute exacerbation risk, and positively correlates with disease severity as well as inflammation while negatively associating with miR-193a in COPD patients.
Disclosure of conflict of interest
None.
References
- 1.Eapen MS, Hansbro PM, Larsson-Callerfelt AK, Jolly MK, Myers S, Sharma P, Jones B, Rahman MA, Markos J, Chia C, Larby J, Haug G, Hardikar A, Weber HC, Mabeza G, Cavalheri V, Khor YH, McDonald CF, Sohal SS. Chronic obstructive pulmonary disease and lung cancer: underlying pathophysiology and new therapeutic modalities. Drugs. 2018;78:1717–1740. doi: 10.1007/s40265-018-1001-8. [DOI] [PubMed] [Google Scholar]
- 2.Sun Z, Li F, Zhou X, Chung KF, Wang W, Wang J. Stem cell therapies for chronic obstructive pulmonary disease: current status of pre-clinical studies and clinical trials. J Thorac Dis. 2018;10:1084–1098. doi: 10.21037/jtd.2018.01.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker-Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez-Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O’Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez-Ruiz F, Perico N, Phillips D, Pierce K, Pope CA 3rd, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui-Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman KR, Mannino DM, Soriano JB, Vermeire PA, Buist AS, Thun MJ, Connell C, Jemal A, Lee TA, Miravitlles M, Aldington S, Beasley R. Epidemiology and costs of chronic obstructive pulmonary disease. Eur Respir J. 2006;27:188–207. doi: 10.1183/09031936.06.00024505. [DOI] [PubMed] [Google Scholar]
- 5.Xie S, Wang K, Zhang W, Xiao K, Yan P, Li Y, He W, Zhang Y, Xie L. Immunodeficiency in patients with acute exacerbation of chronic obstructive pulmonary disease. Inflammation. 2018;41:1582–1589. doi: 10.1007/s10753-018-0830-7. [DOI] [PubMed] [Google Scholar]
- 6.Ko FW, Chan KP, Hui DS, Goddard JR, Shaw JG, Reid DW, Yang IA. Acute exacerbation of COPD. Respirology. 2016;21:1152–1165. doi: 10.1111/resp.12780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cai BQ, Cai SX, Chen RC, Cui LY, Feng YL, Gu YT, Huang SG, Liu RY, Liu GN, Shi HZ, Shi Y, Song YL, Sun TY, Wang CZ, Wang JL, Wen FQ, Xiao W, Xu YJ, Yan XX, Yao WZ, Yu Q, Zhang J, Zheng JP, Liu J, Bai CX. Expert consensus on acute exacerbation of chronic obstructive pulmonary disease in the People’s Republic of China. Int J Chron Obstruct Pulmon Dis. 2014;9:381–395. doi: 10.2147/COPD.S58454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, Anzueto A, Barnes PJ, Fabbri LM, Martinez FJ, Nishimura M, Stockley RA, Sin DD, Rodriguez-Roisin R. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007;187:347–65. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 9.Pauwels RA, Buist AS, Ma P, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: National Heart, Lung, and Blood Institute and World Health Organization Global Initiative for Chronic Obstructive Lung Disease (GOLD): executive summary. Respir Care. 2001;46:798–825. [PubMed] [Google Scholar]
- 10.Kim DK, Lee J, Park JH, Yoo KH. What can we apply to manage acute exacerbation of chronic obstructive pulmonary disease with acute respiratory failure? Tuberc Respir Dis (Seoul) 2018;81:99–105. doi: 10.4046/trd.2017.0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Sun D, Li D, Zheng Z, Xu J, Liang X, Zhang C, Wang S, Wang J, Lu W. Long non-coding RNA expression patterns in lung tissues of chronic cigarette smoke induced COPD mouse model. Sci Rep. 2018;8:7609. doi: 10.1038/s41598-018-25702-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li Z, Hou P, Fan D, Dong M, Ma M, Li H, Yao R, Li Y, Wang G, Geng P, Mihretab A, Liu D, Zhang Y, Huang B, Lu J. The degradation of EZH2 mediated by lncRNA ANCR attenuated the invasion and metastasis of breast cancer. Cell Death Differ. 2017;24:59–71. doi: 10.1038/cdd.2016.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang B, Song JH, Cheng Y, Abraham JM, Ibrahim S, Sun Z, Ke X, Meltzer SJ. Long non-coding antisense RNA KRT7-AS is activated in gastric cancers and supports cancer cell progression by increasing KRT7 expression. Oncogene. 2016;35:4927–4936. doi: 10.1038/onc.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu B, Sun L, Liu Q, Gong C, Yao Y, Lv X, Lin L, Yao H, Su F, Li D, Zeng M, Song E. A cytoplasmic NF-kappaB interacting long noncoding RNA blocks IkappaB phosphorylation and suppresses breast cancer metastasis. Cancer Cell. 2015;27:370–381. doi: 10.1016/j.ccell.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Huang Q, Huang C, Luo Y, He F, Zhang R. Circulating lncRNA NEAT1 correlates with increased risk, elevated severity and unfavorable prognosis in sepsis patients. Am J Emerg Med. 2018;36:1659–1663. doi: 10.1016/j.ajem.2018.06.008. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Wu L, Qian J, Qu B, Xia S, La T, Wu Y, Ma J, Zeng J, Guo Q, Cui Y, Yang W, Huang J, Zhu W, Yao Y, Shen N, Tang Y. Identification of the long noncoding RNA NEAT1 as a novel inflammatory regulator acting through MAPK pathway in human lupus. J Autoimmun. 2016;75:96–104. doi: 10.1016/j.jaut.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 17.Chen Y, Qiu J, Chen B, Lin Y, Chen Y, Xie G, Qiu J, Tong H, Jiang D. Long non-coding RNA NEAT1 plays an important role in sepsis-induced acute kidney injury by targeting miR-204 and modulating the NF-kappaB pathway. Int Immunopharmacol. 2018;59:252–260. doi: 10.1016/j.intimp.2018.03.023. [DOI] [PubMed] [Google Scholar]
- 18.Geng H, Tan XD. Functional diversity of long non-coding RNAs in immune regulation. Genes Dis. 2016;3:72–81. doi: 10.1016/j.gendis.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Molina-Pinelo S, Pastor MD, Suarez R, Romero-Romero B, Gonzalez De la Pena M, Salinas A, Garcia-Carbonero R, De Miguel MJ, Rodriguez-Panadero F, Carnero A, Paz-Ares L. MicroRNA clusters: dysregulation in lung adenocarcinoma and COPD. Eur Respir J. 2014;43:1740–1749. doi: 10.1183/09031936.00091513. [DOI] [PubMed] [Google Scholar]
- 20.N Ijiri AP, Mogas A, Nair P, Hamid Q, Martin J, Baglole C. LncRNA NEAT1 promotes IL-8 expression in fibroblasts derived from COPD patients and in response to cigarette smoking. 2017;195:A7676. [Google Scholar]
- 21.Huang S, Huang Z, Luo Q, Qing C. The expression of lncRNA NEAT1 in human tuberculosis and its antituberculosis effect. Biomed Res Int. 2018;2018:9529072. doi: 10.1155/2018/9529072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang L, Xia JW, Ke ZP, Zhang BH. Blockade of NEAT1 represses inflammation response and lipid uptake via modulating miR-342-3p in human macrophages THP-1 cells. J Cell Physiol. 2019;234:5319–5326. doi: 10.1002/jcp.27340. [DOI] [PubMed] [Google Scholar]
- 23.Almeida MI, Reis RM, Calin GA. MicroRNA history: discovery, recent applications, and next frontiers. Mutat Res. 2011;717:1–8. doi: 10.1016/j.mrfmmm.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Alghetaa H, Mohammed A, Sultan M, Busbee P, Murphy A, Chatterjee S, Nagarkatti M, Nagarkatti P. Resveratrol protects mice against SEB-induced acute lung injury and mortality by miR-193a modulation that targets TGF-beta signalling. J Cell Mol Med. 2018;22:2644–2655. doi: 10.1111/jcmm.13542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu HM, Wang C, Yuan Z, Chen GL, Ye T, Yang BW. LncRNA NEAT1 promotes the tumorigenesis of colorectal cancer by sponging miR-193a-3p. Cell Prolif. 2019;25:e12526. doi: 10.1111/cpr.12526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wu Y, Wang H. LncRNA NEAT1 promotes dexamethasone resistance in multiple myeloma by targeting miR-193a/MCL1 pathway. J Biochem Mol Toxicol. 2018;32 doi: 10.1002/jbt.22008. [DOI] [PubMed] [Google Scholar]
- 27.Xiong DD, Li ZY, Liang L, He RQ, Ma FC, Luo DZ, Hu XH, Chen G. The LncRNA NEAT1 accelerates lung adenocarcinoma deterioration and binds to mir-193a-3p as a competitive endogenous RNA. Cell Physiol Biochem. 2018;48:905–918. doi: 10.1159/000491958. [DOI] [PubMed] [Google Scholar]
- 28.Young RP, Hopkins RJ. COPD and lung cancer linked at a molecular genetic level. Chest. 2011;140:266–267. doi: 10.1378/chest.11-0220. [DOI] [PubMed] [Google Scholar]
- 29.Pauwels RA, Buist AS, Calverley PM, Jenkins CR, Hurd SS, Committee GS. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease. NHLBI/WHO Global Initiative for Chronic Obstructive Lung Disease (GOLD) Workshop summary. Am J Respir Crit Care Med. 2001;163:1256–1276. doi: 10.1164/ajrccm.163.5.2101039. [DOI] [PubMed] [Google Scholar]
- 30.Guttman M, Rinn JL. Modular regulatory principles of large non-coding RNAs. Nature. 2012;482:339–346. doi: 10.1038/nature10887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1:391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bi H, Zhou J, Wu D, Gao W, Li L, Yu L, Liu F, Huang M, Adcock IM, Barnes PJ, Yao X. Microarray analysis of long non-coding RNAs in COPD lung tissue. Inflamm Res. 2015;64:119–126. doi: 10.1007/s00011-014-0790-9. [DOI] [PubMed] [Google Scholar]
- 33.Chen DD, Hui LL, Zhang XC, Chang Q. NEAT1 contributes to ox-LDL-induced inflammation and oxidative stress in macrophages through inhibiting miR-128. J Cell Biochem. 2018 doi: 10.1002/jcb.27541. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 34.Dua K, Malyla V, Singhvi G, Wadhwa R, Krishna RV, Shukla SD, Shastri MD, Chellappan DK, Maurya PK, Satija S, Mehta M, Gulati M, Hansbro N, Collet T, Awasthi R, Gupta G, Hsu A, Hansbro PM. Increasing complexity and interactions of oxidative stress in chronic respiratory diseases: an emerging need for novel drug delivery systems. Chem Biol Interact. 2018;299:168–178. doi: 10.1016/j.cbi.2018.12.009. [DOI] [PubMed] [Google Scholar]
- 35.Rao R, Nagarkatti P, Nagarkatti M. Role of miRNA in the regulation of inflammatory genes in staphylococcal enterotoxin B-induced acute inflammatory lung injury and mortality. Toxicol Sci. 2015;144:284–297. doi: 10.1093/toxsci/kfu315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dai X, Chen X, Chen Q, Shi L, Liang H, Zhou Z, Liu Q, Pang W, Hou D, Wang C, Zen K, Yuan Y, Zhang CY, Xia L. MicroRNA-193a-3p reduces intestinal inflammation in response to microbiota via down-regulation of colonic PepT1. J Biol Chem. 2015;290:16099–16115. doi: 10.1074/jbc.M115.659318. [DOI] [PMC free article] [PubMed] [Google Scholar]