Abstract
PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2) belongs to the phosphokinase family, that has been reported to play an important role in several cancers. However, the expression of PHLPP2 and its correlation with clinicopathologic characteristics in colorectal cancer (CRC) have yet to be determined. The aim of this study is to investigate the expression of PHLPP2 and explore its role in CRC. The expression of PHLPP2, PTEN, PI3KCA, and PI3KCB in 130 cases of CRC and normal tissues was assessed by immunohistochemistry. In addition, the expression of PHLPP2, PTEN, PI3KCA, and PI3KCB in 32 pairs of CRC tissues and their corresponding normal tissues was determined by RT-PCR and western blotting, respectively. PHLPP2 expression in CRC was significantly lower than that of normal tissues. However, PHLPP2 mRNA shows no significant difference between CRC and normal tissue. PTEN expression in left colorectal cancer (LCC) was absent, while PI3KCA and PI3KCB in right colorectal cancer (RCC) were significantly higher than those in LCC. PHLPP2 was negatively correlated with p-Akt1 in CRC. The expression of p-Akt1 in PHLPP2 (+)/PTEN (+) in CRC tissues was significantly lower than that in other groups. PHLPP2 expression was correlated with differentiation, invasion, and lymph node metastasis. Kaplan-Meier analysis and multivariate analysis reveal that PHLPP2 is closely related to prognosis; more importantly, it is an independent prognostic factor for CRC. In conclusion, PHLPP2 may play a major role in the development, metastasis, and prognosis of CRC.
Keywords: PHLPP2, PTEN, left colorectal cancer, right colorectal cancer, prognosis
Introduction
The incidence of colorectal cancer (CRC) is continuously rising in China. According to China Cancer Association [1], 60900 new cases of CRC were added in 2013. CRC ranked fourth only after lung cancer, gastric cancer, and liver cancer, and its high incidence and mortality are a public health hazard. CRC is a heterogeneous disease, and its pathogenesis is complex. Recent studies have shown that the pathogenesis and prognosis of left colorectal cancer (LCC) and right colorectal cancer (RCC) are significantly different. Therefore, further studies the different pathogenesis of RCC and LCC, searching for independent prognostic factors, and then exploring relavant individualized therapy, are needed. PH domain leucine-rich repeat protein phosphatase 2 (PHLPP2), a member of the phosphokinase family, through specific dephosphorylation of the Serine/threonine kinase of Akt1, affects the PI3K/Akt signaling pathway, and thus plays an important role in inhibiting the tumor [2,3]. In the present study, the expression of PHLPP2, PTEN, PI3KCA, and PI3KCB in RCC and LCC was detected, and the relationships between PHLPP2, PTEN, PI3KCA, and PI3KCB expression and clinicopathologic parameters in RCC and LCC were assessed.
Materials and methods
Patients and tissue samples
Formalin-fixed and paraffin-embedded (FFPE) CRC specimens and normal tissues were retrieved from the archive of Department of Pathology, Binzhou Medical University Hospital, China. A total of 130 patients with CRC who had undergone curative surgery from January 2011 to December 2012 are collected. These patients included 58 males and 72 females (male to female ratio of 0.82:1) with median age of 62 years, range 34 to 82. 65 patients had LCC and 65 patients had RCC. None of the patients underwent any type of treatments before surgery. Clinical demographic data of all the patients were collected retrospectively by reviewing medical records. The detailed information of patients is listed in Table 1.
Table 1.
Correlation between clinicopathologic characteristics and the expression of PHLPP2, PTEN, PI3KCA, and PI3KCB in CRC
| Feature | n | PHLPP2 | r | P | PTEN | r | P | PI3KCA | r | P | PI3KCB | r | P |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| + | + | + | + | ||||||||||
| Age (years) | |||||||||||||
| <60 | 67 | 16 | -0.039 | 0.660 | 18 | -0.035 | 0.692 | 47 | -0.120 | 0.174 | 52 | -0.155 | 0.078 |
| ≥60 | 63 | 13 | 15 | 39 | 40 | ||||||||
| Gender | |||||||||||||
| Male | 58 | 10 | -0.109 | 0.216 | 11 | -0.132 | 0.133 | 35 | -0.141 | 0.110 | 47 | 0.203 | 0.021 |
| Female | 72 | 19 | 22 | 53 | 45 | ||||||||
| Tumor size (cm) | |||||||||||||
| <5 | 63 | 15 | -0.035 | 0.693 | 13 | 0.106 | 0.231 | 45 | -0.077 | 0.381 | 45 | -0.048 | 0.588 |
| ≥5 | 67 | 14 | 20 | 43 | 46 | ||||||||
| Differentiation | |||||||||||||
| High | 35 | 14 | 0.236 | 0.007* | 13 | 0.111 | 0.208 | 26 | 0.161 | 0.067 | 23 | -0.020 | 0.823 |
| Middle | 71 | 12 | 14 | 50 | 53 | ||||||||
| Low | 24 | 3 | 6 | 12 | 16 | ||||||||
| Histology | |||||||||||||
| Tubular | 97 | 22 | -0.015 | 0.862 | 23 | 0.066 | 0.456 | 69 | -0.126 | 0.153 | 70 | -0.053 | 0.552 |
| Mucinous | 33 | 7 | 10 | 19 | 22 | ||||||||
| Invasion | |||||||||||||
| Mucosa and submucosa | 18 | 11 | -0.418 | <0.001* | 7 | -0.225 | 0.010* | 9 | 0.122 | 0.166 | 11 | 0.059 | 0.503 |
| Muscularis | 34 | 10 | 13 | 24 | 25 | ||||||||
| Serosa | 78 | 8 | 13 | 55 | 56 | ||||||||
| Lymph node metastasis | |||||||||||||
| No | 68 | 20 | -0.203 | 0.020* | 24 | -0.265 | 0.002* | 38 | 0.197 | 0.024* | 39 | 0.237 | 0.007* |
| Yes | 62 | 9 | 9 | 50 | 53 |
P<0.05.
Fresh-frozen tumor tissues and the corresponding normal tissues were used for western blotting and mRNA extraction. These tissues were collected from 32 patients with CRC who underwent curative surgery between May 2016 and October 2017. Tumor samples were obtained at surgery and stored at -80°C. The corresponding normal tissues were considered as the control tissues. There are 16 cases of LCC and 16 cases of RCC.
This research was approved by the Ethics Committee of Binzhou Medical University Hospital.
Follow up
All patients are accomplished by telephone or outpatient clinics.
Immunohistochemistry (IHC)
The expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, Bcl-2, VEGF, and Cyclin-D1 in CRC and normal tissues was determined through IHC. FFPE archived tissues were cut into 3-μm sections, dewaxed, rehydrated, and blocked with 3% hydrogen peroxide. The sections were subjected to heat for antigen retrieval. The sections were then incubated with rabbit polyclonal antibody against human PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, Bcl-2, VEGF and Cyclin-D1, respectively, overnight at 4°C. The sections were washed with PBS and incubated with horseradish peroxidase-labeled secondary antibody for 30 min. Subsequently, all sections were visualized with DAB kit (Zhong Shan Golden Bridge Biotechnology, Beijing, China) and the nucleus was counterstained with hematoxylin. All the primary antibodies were purchased from Abcam, USA. A positive control was supplied by Abcam, and negative controls were prepared by replacing the primary antibody with PBS.
The results of final IHC was evaluated according to the intensity and percentage of positively stained cells. The staining intensity was graded as the followings: 0 (negative), 1 (weak), 2 (moderate) and 3 (strong). The percentage of positive cells was assigned as the followings: 1 (≤25%), 2 (26%-50%), 3 (51%-75%), 4 (>76%). The final IHC score was calculated by multiplying the score of staining intensity and the score of percentage of positive cells. A score lower than 4 was regarded as low expression and a score higher than 4 was regarded as high expression. The results of IHCs were blindly evaluated by two pathologists.
Western blotting
Fresh tissue was extracted on ice. Protein concentrations were measured by BCA assay. The protein extracts were separated through SDS-PAGE and then transferred onto PVDF membranes (Millipore, USA). The membranes were blocked with 5% nonfat milk at room temperature for 90 min. The membranes were then incubated with PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, Bcl-2, VEGF, Cyclin-D1 or GAPDH, respectively, overnight at 4°C. Next, the membranes were incubated at room temperature for 1.5 hours with secondary antibodies. The results were observed with enhanced chemiluminescence (ECL; Thermo Fisher, USA) by chemiluminescence detection system.
Real-time PCR analysis (RT-PCR)
Trizol reagent (Takara, Dalian, China) was used to extract total RNA from 32 pairs of CRC tissues and corresponding normal tissues, and cDNA was obtained using revers transcription using PrimeScript Reverse Transcriptase (Takara, Dalian, China). The RT-PCR was carried on CFX96T RT-PCR Detection System C1000 using the following conditions: the first denaturation step at 95°C for 30 seconds, followed by 40 cycles at 95°C for 5 seconds and 60°C for 30 seconds. Primer sequences were summarized in Table 2. Gene expression was calculated using the comparative threshold cycles (2-ΔΔCt) method. Experiments were performed at least in triplicate, and their average is used for statistical analysis.
Table 2.
Real time-PCR primer sequence
| Gene | Sequence | Gene | Sequence |
|---|---|---|---|
| PHLPP2 | F: 5’-CCTTCCAACACTGGTAGAGCAC-3’ | VEGF | F: 5’-GGCAGAAGGAGGAGGGCAGA-3’ |
| R: 5’-CGGATGGTAAAGACTCCAGACTA-3’ | R: 5’-CCTATGTGCTGGCCTTCGTGAG-3’ | ||
| PTEN | F: 5’-TGAGTTCCCTCAGCCGTTACCT-3’ | Bcl-2 | F: 5’-AGATGTCCAGCCAGCTGCACC-3’ |
| R: 5’-GAGGTTTCCTCTGGTCCTGGTA-3’ | R: 5’-TGACCCCACCGAACTCAAAGA-3’ | ||
| PI3KCA | F: 5’-CTGTCAATCGGTGACTGTGTGG-3’ | Cyclin-D1 | F: 5’-GTGTATCGAGAGGCCAAAGG-3’ |
| R: 5’-AAACAGGTCAATGGCTGCATCATA-3’ | R: 5’-GCAACCAGAAATGCACAGAC-3’ | ||
| PI3KCB | F: 5’-CTTTATGGGTAGACTGGGCCTTTG-3’ | ||
| R: 5’-A AGCCTTAGCCTATTCCTGAGGTTG-3’ |
Statistical analysis
SPSS23.0 was used to conduct all statistical analyses. The correlation between the proteins of different groups was performed using Spearman’s correlation analysis. The correlation of protein expression with clinical parameters was analyzed by Pearson chi-square test. Overall survival (OS) was plotted using the Kaplan-Meier method. Log-Rank method was used to compare the statistical differences, and the significance of various survival related variables was assessed using a multivariate Cox regression model. P<0.05 was considered significant.
Results
The expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2, and Cyclin-D1 in CRC
The expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2, and Cyclin-D1 were assessed in 130 CRC by both IHC (Figure 1) and western blotting (Figure 2). By IHC (Figure 1), the positive rates of PHLPP2 and PTEN in CRC (22.31% and 25.38%, respectively) were lower than those in normal tissues (91.54% and 86.92%, respectively, P<0.05). The positive rates of PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2, and Cyclin-D1 in CRC were obviously higher than those in normal tissues (P<0.05, Table 3).
Figure 1.
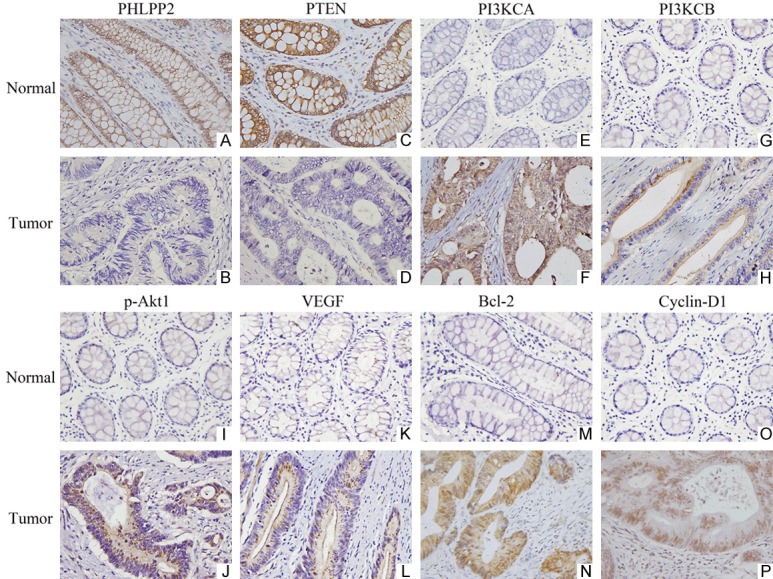
The expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC and normal tissues by IHC. (×400). A. High expression of PHLPP2 in normal tissues. B. Low expression of PHLPP2 in CRC. C. High expression of PTEN in normal tissues. D. Low expression of PTEN in CRC. E. Low expression of PI3KCA in normal tissues. F. High expression of PI3KCA in CRC. G. Low expression of PI3KCB in normal tissues. H. High expression of PI3KCB in CRC. I. Low expression of p-Akt1 in normal tissues. J. High expression of p-Akt1 in CRC. K. Low expression of VEGF in normal tissues. L. High expression of VEGF in CRC. M. Low expression of Bcl-2 in normal tissues. N. High expression of Bcl-2 in CRC. O. Low expression of Cyclin-D1 in normal tissues. P. High expression of Cyclin-D1 in CRC.
Figure 2.
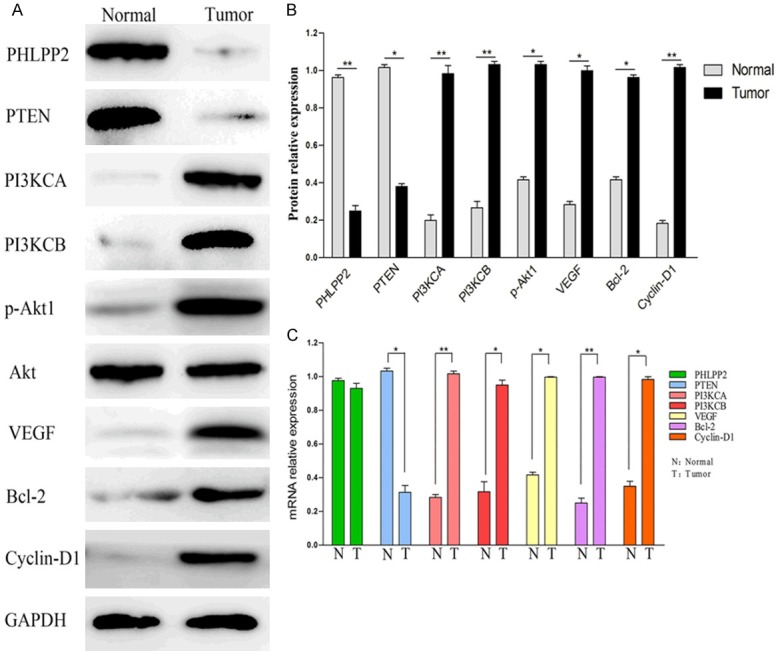
The expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC and normal tissues by Western blot and RT-PCR. A. Representative results of proteins expression in CRC (Tumor) and the normal tissues (Normal). B. All of the paired CRC and the normal tissues from 32 patients by Western blot. C. The expression of those mRNA in CRC and the normal tissues. *P<0.05, **P<0.01.
Table 3.
Expression of PHLPP2, PTEN, PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC and normal tissue [n (%)]
| n | PHLPP2 | PTEN | PI3KCA | PI3KCB | p-Akt1 | VEGF | Bcl-2 | Cyclin-D1 | |
|---|---|---|---|---|---|---|---|---|---|
| Normal tissue | 130 | 119 (91.54) | 113 (86.92) | 21 (16.15) | 24 (18.46) | 15 (11.54) | 21 (16.15) | 19 (14.62) | 25 (19.23) |
| CRC | 130 | 29 (22.31)* | 33 (25.38)* | 88 (67.69)* | 92 (70.77)* | 96 (73.85)* | 101 (77.78)* | 100 (76.92)* | 91 (70.00)* |
P<0.05.
By western blotting, the expressions of PHLPP2 and PTEN in CRC were significantly lower than those in normal. To the contrary, the expressions of PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC were significantly higher than those in normal tissues (P<0.05, Figure 2).
At the mRNA level, PTEN in CRC was higher than in normal tissue, but PI3KCA, PI3KCB, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC were significantly higher by RT-PCR (P<0.05, Figure 2C). However, there was no difference in the expression of PHLPP2 mRNA between CRC and normal (P>0.05).
The expression of PHLPP2, PTEN, PI3KCA and PI3KCB in LCC and RCC
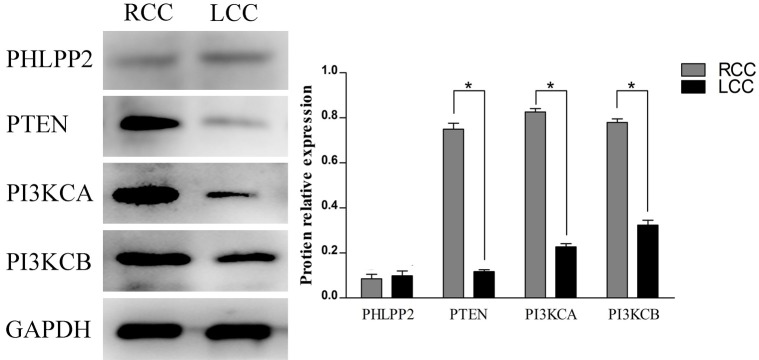
According to the tumor site, the CRCs were further divided into two groups: left colorectal cancer (LCC) and right colorectal cancer (RCC). According to the results of IHC, the expression of PTEN in LCC was significantly lower than that in RCC. To the contrary, the expression of PI3KCA and PI3KCB was significantly higher in RCC than LCC (P<0.05, Table 4). However, there was no difference in the expression of PHLPP2 between LCC and RCC.
Table 4.
Expression of PHLPP2, PTEN, PI3KCA, and PI3KCB in LCC and RCC
| n | PHLPP2 | P | PTEN | P | PI3KCA | P | PI3KCB | P | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||||||
| - | + | - | + | - | + | - | + | ||||||
| RCC | 65 | 50 | 15 | 0.835 | 43 | 22 | 0.027* | 11 | 54 | 0.001* | 11 | 54 | 0.002* |
| LCC | 65 | 51 | 14 | 54 | 11 | 31 | 34 | 27 | 38 | ||||
P<0.05.
The relative expression of PI3KCA, PI3KCB and PTEN in LCC and RCC were significantly different (P<0.05, Figure 3). There was no significant difference in the expression of PHLPP2 in LCC and RCC by western blot (P>0.05, Figure 3).
Figure 3.
The expression of PHLPP2, PTEN, PI3KCA and PI3KCB in LCC and RCC by Western blot.
Correlation between the expressions of PHLPP2 and PTEN, p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC
Spearman correlation analysis shows that PHLPP2 was negatively correlated with p-Akt1, VEGF, Bcl-2 and Cyclin-D1 in CRC, respectively (P<0.05, Table 5); however, there was no correlation between the expression of PHLPP2 and PTEN in CRC (P>0.05, Table 5).
Table 5.
Correlation between the expressions of PHLPP2 and PTEN, p-Akt1, VEGF, Bcl-2, and Cyclin-D1 in CRC
| n | PTEN | P | p-Akt1 | P | VEGF | P | Bcl-2 | P | Cyclin-D1 | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
||||||||||||
| - | + | - | + | - | + | - | + | - | + | |||||||
| PHLPP2 | ||||||||||||||||
| - | 101 | 78 | 23 | 0.150 | 19 | 82 | 0.001* | 18 | 83 | 0.022* | 19 | 82 | 0.031* | 25 | 76 | 0.015* |
| + | 29 | 19 | 10 | 15 | 14 | 11 | 18 | 11 | 18 | 14 | 15 | |||||
P<0.05.
According to the expressions of PHLPP2 and PTEN by IHC, 130 cases of CRC were divided into 4 groups as shown in Table 6. In addition, the expression of PHLPP2 and PTEN protein was negatively correlated with p-Akt1 expression (P<0.05, Table 6). Significantly more cases express (88%; 69/78) p-Akt1 in PHLPP2 (-)/PTEN (-) than in the remaining three groups. On the other hand, the expression of p-Akt1 in PHLPP2 (+)/PTEN(+) group was significantly lower than in the remaining three groups. The expression of p-Akt1 in PHLPP2 (+)/PTEN (-) and PHLPP2 (-)/PTEN (+) groups was significantly higher than the both positive groups (P<0.05, Table 6; Figure 4).
Table 6.
Correlation between the expression of PHLPP2 and PTEN and the expression of p-Akt1 in colorectal cancer
| n | PHLPP2+PTEN+ | PHLPP2+PTEN- | PHLPP2-PTEN+ | PHLPP2-PTEN- | r | P | |
|---|---|---|---|---|---|---|---|
| p-Akt1 | |||||||
| - | 34 | 6 | 9 | 10 | 9 | -0.414 | <0.001* |
| + | 96 | 4 | 10 | 13 | 69 |
P<0.05.
Figure 4.
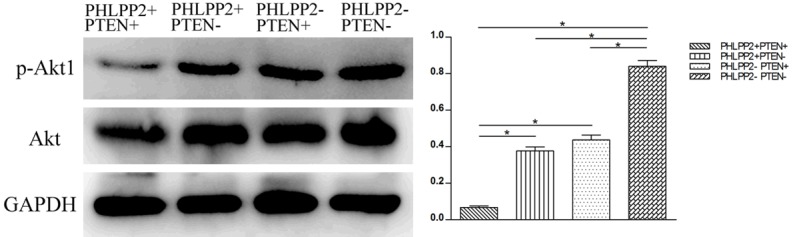
The expression of p-Akt1 in different groups in colorectal cancer by Western blot.
Low expression of PHLPP2 among others, is an independent biomarker of poor prognosis in CRC patients
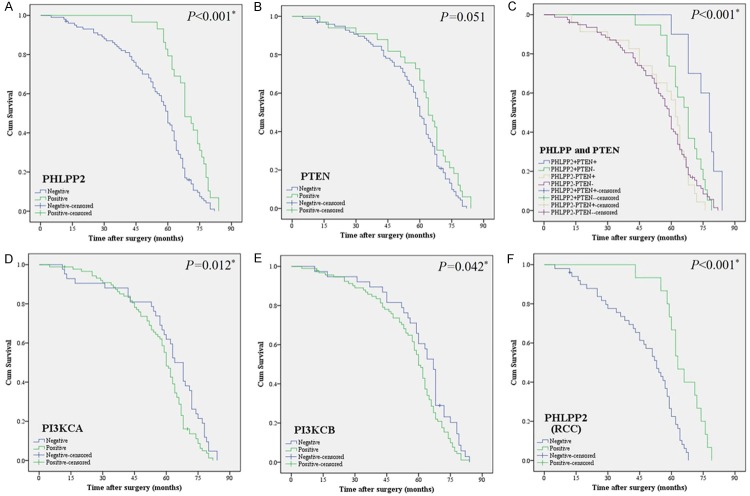
All 130 patients with CRC had complete follow-up data: the median overall survival (OS) time is 57.6 months, and the 5-year survival rate is 57.69%. Kaplan-Meier analysis showed that patients with low expression of PHLPP2 had a worse OS time than that of patients with high expression of PHLPP2. However, a worse OS was associated with high expression of PI3KCA and PI3KCB. Interestingly, patients with both high expression of PHLPP2 and PTEN had a better prognosis, with 5-year survival rate of 90% in comparison to only 47.44% (P<0.05%, Figure 5) in patients with negative expression of both PHLPP2 and PTEN. Multivariate survival analysis showed that PHLPP2, PTEN, tumor site, and lymph node metastasis were independent prognostic factors for CRC patients (Table 7).
Figure 5.
Kaplan-Meier survival analysis for PHLPP2, PTEN, PI3KCA and PI3KCB expression in CRC. A. The expression of PHLPP2 and overall survival of CRC patients: positive expression [PHLPP2 (+)], n=29, negative expression [PHLPP2 (-)], n=101. B. The expression of PTEN and the overall survival of patients with CRC: positive expression [PTEN (+)], n=33, negative expression [PTEN (-)], n=97. C. The expression of PHLPP2 and PTEN and the overall survival of patients with CRC: positive expression [PHLPP2 (+), PTEN (+)], n=10, negative expression PHLPP2 (-), PTEN (-)], n=78. D. The expression of PI3KCA and overall survival of CRC patients: positive expression [PI3KCA (+)], n=88, negative expression [PI3KCA (-)], n=42. E. The expression of PI3KCB and overall survival of CRC patients: positive expression [PI3KCB (+)], n=92, negative expression [PI3KCB (-)], n=38. F. The expression of PHLPP2 and overall survival of RCC patients: positive expression [PHLPP2(+)], n=15, negative expression [PHLPP2 (-)], n=50. The log-rank test was used to calculate P value.
Table 7.
Multivariate survival analysis of clinicopathologic parameters with OS by Cox proportional hazards regression
| B | SE | Wald | df | Exp(B) | 95% CI | P | ||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Lower | Upper | |||||||
| PHLPP2 | -0.962 | 0.283 | 11.519 | 1 | 0.382 | 0.219 | 0.666 | 0.001* |
| PTEN | -0.463 | 0.236 | 3.871 | 1 | 0.629 | 0.396 | 0.998 | 0.049* |
| PI3KCA | -0.147 | 0.225 | 0.424 | 1 | 0.864 | 0.555 | 1.343 | 0.515 |
| PI3KCB | -0.133 | 0.240 | 0.306 | 1 | 0.876 | 0.547 | 1.402 | 0.580 |
| Age | -0.103 | 0.198 | 0.271 | 1 | 0.902 | 0.612 | 1.330 | 0.603 |
| Sex | 0.216 | 0.233 | 0.864 | 1 | 1.241 | 0.787 | 1.959 | 0.353 |
| Tumor size | -0.260 | 0.204 | 1.624 | 1 | 0.771 | 0.517 | 1.150 | 0.203 |
| Tumor site | -1.500 | 0.253 | 35.295 | 1 | 0.223 | 0.136 | 0.366 | 0.000* |
| Differentiation | -0.106 | 0.158 | 0.451 | 1 | 0.899 | 0.660 | 1.226 | 0.502 |
| Histology | -0.142 | 0.234 | 0.369 | 1 | 0.867 | 0.548 | 1.373 | 0.543 |
| Invasion | 0.028 | 0.169 | 0.028 | 1 | 1.029 | 0.738 | 1.433 | 0.868 |
| Lymph node metastasis | 0.533 | 0.235 | 5.146 | 1 | 1.704 | 1.075 | 2.701 | 0.023* |
P<0.05.
Correlation of PHLPP2, PTEN, PIK3CA and PIK3CB with clinicopathologic features
We analyzed the correlation between the PHLPP2, PTEN, PIK3CA and PIK3CB expression levels in CRC and a set of clinicopathologic parameters including age, gender, histologic type, and tumor site (Table 1). The expression of PHLPP2 was found to be significantly correlated with tumor differentiation (P=0.007), invasion (P=0.001), and lymph node metastasis (P=0.020). Other characteristics, such as age, gender, tumor size, and histology, were not associated with PHLPP2 expression. The expression of PTEN was correlated with the depth of invasion and lymph node metastasis. High expression of PI3KCA and PI3KCB were positively correlated with lymph node metastasis (P<0.05, Table 1).
Discussion
We herein demonstrated that PHLPP2 is negatively correlated with CRC invasion, differentiation, and metastasis. PHLPP2 is closely related to prognosis, and it is an independent prognostic factor for CRC. Meanwhile, the survival time of patients with PHLPP (+)/PTEN (+) was significantly longer than that in the other three groups. Our results suggest that inactivation of PHLPP2 may lead to tumorigenesis, and the loss of expression significantly affects the prognosis of the CRC. More importantly, PHLPP2 may serve as a valuable independent prognostic marker.
PHLPP is located on chromosome 16 and 18, and its encoded proteins belong to the phosphokinase family, which affects a variety of signaling pathways, including the inhibition of the PI3K/Akt signaling pathway [4-7]. There are two isoforms in the PHLPP family due to alternative splicing: one is PHLPP1, which has two predominant splice variants: PHLPP1α and PHLPP1β; the other is PHLPP2. PHLPP is widely expressed in various human tissues. Its physiologic function is specific dephosphorylation of Akt hydrophobic group. This makes Akt lose its activity, which in turn results in inhibition of downstream targets. In a study of cerebral ischemia/reperfusion injury in rats, inhibition of PHLPP2 prevented neuronal death after I/R injury [8]. Inhibition of PHLPP1 in type II diabetes can increase the sensitivity of cells to insulin, and further promote the absorption of blood glucose and decrease the level of blood glucose [9]. Bradley et al [10] have shown that PHLPP1 deficiency can promote cartilage regeneration and regulate entochondrostosis. The research on the correlation between PHLPP and tumors has just started. Studies showed that the expression of PHLPP in tumor tissues is absent, and it was postulated that PHLPP may be a tumor suppressor. Recently, studies showed that the expression of PHLPP in lung adenocarcinoma [11], hypopharyngeal squamous cell carcinoma [12], gastric cancer [13] and other tumor tissues was significantly reduced. Liu et al [14] showed that the expression of PHLPP1 and PHLPP2 was absent in CRC, and knock-down of PHLPP in DLD1 cells could promote tumor proliferation. Our study not only showed that the expression of PHLPP2 in CRC tissue was significantly lower than the corresponding normal tissue, but the expression of VEGF, Bcl-2 and Cyclin-D1, which are closely related to tumor growth, are highly expressed in the same specimen, and their expressions are negatively correlated with the expression of PHLPP2. We propose that PHLPP2 behaves as a tumor suppressor by inhibiting tumor proliferation, angiogenesis and anti-apoptosis. The main mechanism of PHLPP2 may be through inhibition of VEGF, Bcl-2, and Cyclin-D1. Note that despite absence of PHLPP2 protein expression, there was not significantly reduced mRNA; thus post-transcriptional modification and/or protein degradation is suspected.
The PI3K/Akt signaling pathway is an important pathway involved in development of tumor [15]. Its downstream target gene is closely related to cell proliferation and apoptosis. The phosphorylation of the important molecule Akt/PKB (protein kinase B) is closely related to the activity of the PI3K/Akt signaling pathway [16]. There are many molecules that phosphorylate or dephosphorylate Akt [17-19]. Previous studies in our group showed that PI3KCA and PI3KCB activated Akt and regulated its downstream molecules, and they promoted tumor proliferation, invasion, metastasis, and tumor apoptosis [20]. PTEN, a tumor suppressor, can interact with the PI3K-PI3P pathway, and inhibits the phosphorylation of Akt by dephosphorylation of PI3P, which further inhibits the PI3K/Akt signaling pathway, and inhibits tumor growth. However, PTEN can inhibit the excessive phosphorylation of Akt, but it cannot affect the phosphorylation of Akt [18]. Therefore, searching for the factors that can inhibit phosphorylation of Akt and reverse phosphorylation of Akt, may be a potential way to inhibit the PI3K/Akt signaling pathway and inhibit the tumor.
Recently, the effect of inhibition of PHLPP2 on the PI3K/Akt signaling pathway and its mechanism have attracted attention. Studies have shown that activation of Akt is required for simultaneous phosphorylation of Ser473 and Thr308 residues. Dephosphorylation at one of the sites can directly inactivate Akt, and thus terminate its signaling [21]. Newton et al. have reported that PHLPP2 can directly interact with Ser473, and their interaction can lead to the dephosphorylation of Akt, thus inhibiting the PI3K/Akt signaling pathway [2]. Akt can be divided into three subtypes: Akt1, Akt2, and Akt3. Studies have shown that there are PHLPP-Akt substrate pathways, such as PHLPP1-Akt2-HDM2 and PHLPP2-Akt3-p27 [22]. Brognard et al. have found that PHLPP1 and PHLPP2 exert different roles on different Akt subtypes. PHLPP1 affects the phosphorylation of Akt2 and Akt3, while PHLPP2 mainly dephosphorylates Akt1 and Akt3 [2,3]. The Akt isoform expressed in colon cancer cells is Akt1, whereas only a very small amount of Akt2 (less than 5% of Akt1) and no Akt3 are detected [14]. The results show that p-Akt1 in CRC is highly expressed, and it negatively correlated with PHLPP2. We consider that the inability of dephosphorylation of Akt is due to the absence of PHLPP2 in CRC. This leads to enhanced activity of p-Akt, which in turn activates its downstream target genes and promotes tumor development and metastasis.
PHLPP and PTEN are two genes that affect the balance of Akt/p-Akt. Ghalali et al. have found that overexpression of PTEN in prostate cancer cells can inhibit PHLPP. Conversely, when PHLPP isoverexpressed, the expression of PTEN is decreased [23]. However, the relationship between PHLPP and PTEN in CRC has not been reported so far to the best of our knowledge. Our results show that there is no correlation between PHLPP2 and PTEN expression, but the expression of p-Akt1 in PHLPP2 (+)/PTEN(+) CRC patients is significantly lower than that in the other three groups (Table 6 and Figure 4). The above results suggested that PHLPP2 and PTEN may both function as tumor suppressors in CRC, which can inhibit the PI3K/Akt signaling pathway. We speculate that PHLPP2 and PTEN in CRC play a role in dephosphorylating Akt, which jointly affect the downstream factors, such as VEGF, Bcl-2, Cyclin-D1, and further affect tumor proliferation, apoptosis, and angiogenesis.
Recent studies [24] have shown that the tumor site is closely related to prognosis in CRC, and its mechanism is not only correlated with the origin of the embryo, the supply of circulation and clinical manifestations, but also that the difference in molecular biologic characteristics is the main cause of the difference between LCC and RCC. The prognosis of LCC is related to the inactivation of the tumor suppressor genes (such as APC, P53, etc.) and the KRAS mutation, while the prognosis of RCC is related to the activation of oncogenes, the BRAF mutation, the CpG island methylator phenotype (CIMP+), the inactivation of MLH1 and the positive expression of MSI. Our results show that PTEN, which functions as a suppressor, is significantly absent in LCC, while the PI3KCA and PI3KCB are activated in RCC. However, PHLPP2 in RCC and LCC were not significantly different. The prognosis of patients with PHLPP2 positive expression in RCC was better than that of those with negative expression. We hypothesize that the mechanism that affects the prognosis of RCC is related to the activation of oncogenes, such as PI3KCA and PI3KCB, and it is also closely related to the inactivation of the suppressor gene PHLPP2. It is suggested that a deficiency of PHLPP2 is closely related to the prognosis of CRC. It is important to detect PHLPP2 expression to determine the prognosis of colorectal cancer.
In conclusion, the absence of PHLPP2 in CRC may play a synergistic role with PTEN in inhibiting the phosphorylation of Akt on the PI3K/Akt signaling pathway. At the same time it is closely related to the prognosis of CRC. However, the study only discussed the correlation of proteins expression and clinical significance, and we need to increase the sample quantity to further reveal its function and mechanism. Therefore, it is important to discuss the relationship between PHLPP2 and tumor development and its molecular mechanisms. It is of great significance to assess prognosis of CRC and inhibit tumor development.
Acknowledgements
This project was supported in part by the National Natural Science Foundation of China (No. 81772637). The funder had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Disclosure of conflict of interest
None.
References
- 1.Chen W, Zheng R, Zhang S, Zeng H, Zuo T, Xia C, Yang Z, He J. Cancer incidence and mortality in China in 2013: an analysis based on urbanization level. Chin J Cancer Res. 2017;29:1–10. doi: 10.21147/j.issn.1000-9604.2017.01.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton AC, Trotman LC. Turning off AKT: PHLPP as a drug target. Annu Rev Pharmacol Toxicol. 2014;54:537–558. doi: 10.1146/annurev-pharmtox-011112-140338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brognard J, Sierecki E, Gao T, Newton AC. PHLPP and a second isoform, PHLPP2, differentially attenuate the amplitude of Akt signaling by regulating distinct Akt isoforms. Mol Cell. 2007;25:917–931. doi: 10.1016/j.molcel.2007.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Liu J, Stevens PD, Gao T. mTOR-dependent regulation of PHLPP expression controls the rapamycin sensitivity in cancer cells. J Biol Chem. 2011;286:6510–6520. doi: 10.1074/jbc.M110.183087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li X, Stevens PD, Liu J, Yang H, Wang H, Wang C, Zeng Z, Schmidt MD, Yang M, Lee EY, Gao T. PHLPP is a negative regulator of RAF1, which reduces colorectal cancer cell motility and prevents tumor progression in mice. Gastroenterology. 2014;146:1301–1312. doi: 10.1053/j.gastro.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agarwal NK, Zhu X, Gagea M, White CL, Cote G, Georgescu MM. PHLPP2 suppresses the NF-κB pathway by inactivating IKKβ kinase. Oncotarget. 2014;5:815–823. doi: 10.18632/oncotarget.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aviv Y, Kirshenbaum LA. Novel phosphatase PHLPP-1 regulates mitochondrial Akt activity and cardiac cell survival. Circ Res. 2010;107:448–450. doi: 10.1161/CIRCRESAHA.110.225896. [DOI] [PubMed] [Google Scholar]
- 8.Wei XE, Zhang FY, Wang K, Zhang QX, Rong LQ. Assembly of the FKBP51-PHLPP2-AKT signaling complex in cerebral ischemia/reperfusion injury in rats. Brain Res. 2014;1566:60–68. doi: 10.1016/j.brainres.2014.04.009. [DOI] [PubMed] [Google Scholar]
- 9.Cozzone D, Fröjdö S, Disse E, Debard C, Lacille M, Pirola L, Vidal H. Isoform-specific defects of insulin stimulation of Akt/protein kinase B (PKB) in skeletal muscle cells from type 2 diabetic patients. Diabetologia. 2008;51:512–521. doi: 10.1007/s00125-007-0913-8. [DOI] [PubMed] [Google Scholar]
- 10.Bradley EW, Carpio LR, Westendorf JJ. Histone deacetylase 3 suppression increases PH domain and leucine-rich repeat phosphatase (Phlpp)1 expression in chondrocytes to suppress Akt signaling and matrix secretion. J Biol Chem. 2013;288:9572–9582. doi: 10.1074/jbc.M112.423723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lv D, Yang H, Wang W, Xie Y, Hu W, Ye M, Chen X. High PHLPP expression is associated with better prognosis in patients with resected lung adenocarcinoma. BMC Cancer. 2015;15:687. doi: 10.1186/s12885-015-1711-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhou J, Yu X, Wang J, Li T, Jin T, Lei D, Pan X. Aberrant expression of PHLPP1 and PHLPP2 correlates with poor prognosis in patients with hypopharyngeal squamous cell carcinoma. PLoS One. 2015;10:e0119405. doi: 10.1371/journal.pone.0119405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang Z, Shu H, Wang Z, Li G, Cui J, Wu H, Cai S, He W, He Y, Zhan W. Loss expression of PHLPP1 correlates with lymph node metastasis and exhibits a poor prognosis in patients with gastric cancer. J Surg Oncol. 2013;108:427–432. doi: 10.1002/jso.23419. [DOI] [PubMed] [Google Scholar]
- 14.Liu J, Weiss HL, Rychahou P, Jackson LN, Evers BM, Gao T. Loss of PHLPP expression in colon cancer: role in proliferation and tumorigenesis. Oncogene. 2009;28:994–1004. doi: 10.1038/onc.2008.450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hemmings BA, Restuccia DF. The PI3K-PKB/Akt pathway. Cold Spring Harb Perspect Biol. 2012;4:a011189. doi: 10.1101/cshperspect.a011189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pompura SL, Dominguez-Villar M. The PI3K/AKT signaling pathway in regulatory T-cell development, stability, and function. J Leukoc Biol. 2018 doi: 10.1002/JLB.2MIR0817-349R. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 17.Sugiyama G, Takeuchi H, Kanematsu T, Gao J, Matsuda M, Hira M. Phospholipase C-related but catalytically inactive protein, PRIP as a scaffolding protein for phospho-regulation. Adv Biol Regul. 2013;53:331–340. doi: 10.1016/j.jbior.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 18.Sharma P, Arias EB, Cartee GD. Protein phosphatase 1-α regulates AS160 Ser588 and Thr642 dephosphorylation in skeletal muscle. Diabetes. 2016;65:2606–2617. doi: 10.2337/db15-0867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim DH, Suh J, Surh YJ, Na HK. Regulation of the tumor suppressor PTEN by natural anticancer compounds. Ann N Y Acad Sci. 2017;1401:136–149. doi: 10.1111/nyas.13422. [DOI] [PubMed] [Google Scholar]
- 20.Wen F, He S, Sun C, Li T, Wu S. PIK3CA and PIK3CB expression and relationship with multidrug resistance in colorectal carcinoma. Int J Clin Exp Pathol. 2014;7:8295–8303. [PMC free article] [PubMed] [Google Scholar]
- 21.Manning BD, Toker A. AKT/PKB signaling: navigating the network. Cell. 2017;169:381–405. doi: 10.1016/j.cell.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nitsche C, Edderkaoui M, Moore RM, Eibl G, Kasahara N, Treger J, Grippo PJ, Mayerle J, Lerch MM, Gukovskaya AS. The phosphatase PHLPP1 regulates Akt2, promotes pancreatic cancer cell death, and inhibits tumor formation. Gastroenterology. 2012;142:377–387. doi: 10.1053/j.gastro.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ghalali A, Ye ZW, Högberg J, Stenius U. Phosphatase and tensin homolog deleted on chromosome 10 (PTEN) and PH domain and leucine-rich repeat phosphatase cross-talk (PHLPP) in cancer cells and in transforming growth factor β-activated stem cells. J Biol Chem. 2014;289:11601–11615. doi: 10.1074/jbc.M113.537241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.NCCN clinical practice guidelines in Oncology: colon cancer (2017.V1) http://www.nccn.org/professionals/physician_gls/pdf/colon.pdf. [DOI] [PubMed]