Abstract
Background: Cervical cancer is a very serious female disease worldwide, especially in developing countries. The expression level of miRNA-145 has been revealed to be decreased in various cancers, including cervical cancer. However, the molecular mechanisms by which miR-145 inhibits tumor growth and induces apoptosis in cervical cancer remain unclarified. Methods: The mRNA expression levels of miR-145 and WNT2B were measured by quantitative real-time polymerase chain reaction (qRT-PCR). The effect of miR-145 on cervical cancer proliferation was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. Transwell assay was conducted to measure cell migration and invasion. Cell apoptosis was examined by flow cytometry assay. The target of miR-145 was predicted by online database and verified by luciferase reporter assay. In vivo experiment was employed to clarify the effect of miR-145 on tumor growth. Results: The expression level of miR-145 was down-regulated while WNT2B expression was up-regulated in cervical cancer cell tissues and cell lines. MiR-145 suppressed cell proliferation, migration and invasion, inhibited Epithelial-Mesenchymal Transition (EMT) process and induced apoptosis. We confirmed that miR-145 weakened WNT2B expression by binding with WNT2B. Furthermore, over-expression of WNT2B reversed the inhibition effects on miR-145 on proliferation and metastasis as well as promoted effects on apoptosis of cervical cells. In addition, we demonstrated that upregulation of miR-145 repressed the Wnt/β-catenin signaling pathway by repressing WNT2B expression in cervical cancer cells. Conclusion: MiR-145 inhibits cervical cancer progression and metastasis through inactivating Wnt/β-catenin pathway by targeting WNT2B.
Keywords: miR-145, WNT2B, cervical cancer, Wnt/β-catenin signaling pathway
Introduction
Cervical cancer is the second common cancer in women worldwide, with an estimated 500,000 new cases and almost 300,000 deaths per year [1,2], and it is the principal cancer in less developed countries where widespread screening is unavailable. In recent studies, the human papillomavirus (HPV) has been demonstrated to be the primary cause of infecting cervical cell [3,4]. MicroRNAs (miRNAs), a class of small single strand non-coding RNAs about 18-25 nucleotides in length, negatively regulate gene expression in the post-transcription by binding to the 3’ untranslated region (3’UTR) of target mRNA molecules [5-7]. The functions of miRNAs in cell biological behaviors such as cell proliferation, apoptosis and stem cell differentiation have been ascertained [8,9]. Previous researches indicated aberrant expression of miRNAs was associated with various human malignancies, including cervical cancer. MiR-145 was first reported to be upregulated in colorectal neoplasia [10]. It has been known as a tumor suppressor which is consistently decreased in various types of cancer, such as bladder cancer, breast cancer, lung cancer and cervical cancer [11-14]. Recent studies have suggested that miR-145 may play an important role in regulating tumor cell progression, migration, invasion, and apoptosis of several types of cancers by targeting c-Myc [15], Mucin-1 [16], p70S6 kinase [17], and insulin receptor substrate 1 [18].
The protein WNT2B which is involved in the Wnt/β-catenin signaling pathway has been found to be expressed in gastric cancer, breast cancer, and cervical cancer, except in normal tissues [19]. WNT2B has been demonstrated to exert its functions by promoting cell migration and invasion, suppressing cell apoptosis by activation of the Wnt/β-catenin signaling pathway [20].
The aim of this study was to investigate the biological functions of miR-145 on cell proliferation, apoptosis, and Epithelial-Mesenchymal Transition (EMT) process, and identify whether WNT2B is the target gene of miR-145 in cervical cancer. In addition, we further explored underlying mechanisms by which miR-145 regulating Wnt/β-catenin signaling pathway.
Materials and methods
Clinical specimens
58 pairs of cervical tumor tissues and corresponding adjacent normal tissues were obtained from cervical cancer patients undergone a surgical procedure at the Department of Pathology, Jiangxi Maternal and Child Health Hospital. Tissue samples were immediately frozen in liquid nitrogen and then stored at -80°C until further used. Written consent was achieved from all patients, and all aspects of this study were approved by the Ethics Committee of Department of Pathology, Jiangxi Maternal and Child Health Hospital.
RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
Total RNA was extracted from tissue samples and cells using TRIzol reagent (Thermo Fisher Scientific, Inc., Waltham, MA, USA) according to the manufacturer’s instructions. cDNA was synthesized using Prime Script RT Reagent kit (Takara, Dalian, China), and qRT-PCR was performed using a SYBR-Green PCR kit (Applied Biosystems, Foster City, CA, USA) with special primers on ABI Prism7500 Fast Real-Time PCR system. The relative expressions of miR-145 and WNT2B were examined using 2-ΔΔCt method. U6 small RNA and Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) served as endogenous controls. The primers were as follows: miR-145, forward, 5’-GTCCAGTTTTCCCAGGAATCCCT-3’, reverse, 5’-TGGTGTCGTGGAGTCG-3’; U6 forward, 5’-GCTCGGCAGCACATATACTAAAAT-3’, reverse, 5’-CGCTTCACGAATTTGCGTGTCAT-3’; WNT2B, forward 5’-GCTGGACCAAACCTGAAC-3’, reverse, 5’-CAAGAAGTATCGGGAAGC-3’; GAPDH forward, 5’-CGAGCCACATCGCTCAGACA-3’, reverse, 5’-GTGGTGAAGACGCCAGTGGA-3’. The primers above were purchased from GeneCopoeia (Guangzhou, China).
Cell culture and transfection
The human cervical cell line NCEC and 5 human cervical cancer lines (Hela, SiHa, SW756, CaSki, and C33A) were purchased from the American Type Culture Collection (ATCC, Rockville, MD, USA). All cells were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Carlsbad, CA, USA) containing 10% fetal bovine serum (FBS; Gibco), 1% penicillin/streptomycin (Gibco) in an atmosphere with 5% CO2 at 37°C.
The miR-145 mimic, miR-145 inhibitor, and their negative controls were purchased from RiboBio (Guangzhou, China). The Hela and SiHa cells were seeded into 6-well plates at a density of 1 × 107 cells/mL for 24 h. Then transfection was done using the Lipofectamine 2000 reagent (Thermo Fisher Scientific) following the manufacturer’s instructions. After transfection for 48 h, cells were harvested for further analysis.
Immunohistochemical analysis
The tissue samples preserved in 2.5% glutaraldehyde-polyoxymethylene solution were dehydrated and embedded in paraffin. The paraffin sections were removed of paraffin and washed with PBS. The sections were first blocked with 3% peroxide-methanol at room temperature for 30 min, next they were incubated with WNT2B antibody at room temperature for 60 min (1:500, Abcam, Cambridge, UK), and then with the secondary antibody, finishing coloration with 3,3-diaminobenzidin (DAB) and neutral gums. The negative control group was dealt with the same steps as described above, but the secondary antibody was replaced by PBS. The numbers of positive cells were observed under the microscope.
Cell proliferation assay
We used 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) to investigate proliferation of cervical cells. The Hela and SiHa cells were seeded in 96-well plates for 48 h prior to the experiment, and MTT solution (5 mg/mL) was added. Subsequently cells were incubated for 4 h at 37°C. Dimethyl sulfoxide (DMSO) was used to dissolve the formazan crystals after removing the supernatants. The absorbance at 490 nm was measured using the Thermo Scientific Evolution 300 instrument (Thermo Fisher Scientific).
Flow cytometry analysis for cell apoptosis
The Hela and SiHa cells were collected and washed with phosphate buffered saline (PBS). Cells pellets were re-suspended in binding buffer and stained using the Annexin V-fluorescein isothiocyanate (FITC) for 15 min in the dark at room temperature, and then pro-pidium iodide (PI) (BD Pharmingen, San Diego, CA, USA) was added at the above condition for 5 min. In the end, cells were stained and analyzed using flow cytometry (BD Biosciences, Franklin Lakes, NJ, USA).
The xenograft models of nude mice
All procedures were conducted aseptically referring to the institutional guidelines of Department of Pathology, Jiangxi Maternal and Child Health Hospital and approved by the Experimental Animal Ethics Committee of Department of Pathology, Jiangxi Maternal and Child Health Hospital. Xenografts were constructed by subcutaneous injection of 1 × 106 Hela cells stably transfected with miR-145 mimic or miR-NC mimic in the right flank region of each nude mouse. 5 mice were involved in each group. The tumor size was measured once a week with a caliper, and tumor volume was calculated according to the formula: volume = length × width2/2. 6 weeks later, all mice were sacrificed, and tumors were collected for weighing and qRT-PCR assay.
Western blot assay
Total protein was isolated from cervical tissue samples and cell lines using RIPA buffer (Pierce, Rockford, IL, USA). The concentrations of protein were examined using Bradford method (Pierce). Forty micrograms of lysate proteins were separated by 10% sodium dodecyl sulfate polyacrylamide gel (SDS-PAGE) and subsequently transferred on polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Next, the membranes were blocked with 5% non-fat dried milk for 2 h and incubated with the corresponding primary antibodies overnight at 4°C, washed with PBS for four times, and then incubated with horse radish peroxidase (HRP) labeled anti-rabbit secondary antibody for 1 h at room temperature. In the end, protein bands were detected by enhanced chemiluminescent method (ECL) (Pierce).
Cell migration and invasion assay
Invasion assay was performed with Transwell chamber (8 µm pore; Corning, Inc., Corning, NY, USA) with matrigel (BD Biosciences). 5 × 104 Hela and SiHa cells were placed into the upper chamber containing DMEM Medium (without serum), while Medium with 10% FBS was added in the lower chamber as nutritional attractant. After incubation for 48 h, 4% paraformaldehyde was used to fix the cells of invaded through the membrane, and then cells were stained with 0.1% crystal violet for 30 min, and Microscope was applied to count the cells. As for migration assay, procedures were similar, except the matrigel on the membrane.
Luciferase reporter assay
WNT2B was predicted to be a potential target of miR-145 using Targetscan (www.targetscan.org). The 3’UTR of WNT2B containing miR-145-binding sites was amplified by PCR and cloned into pGL3 plasmids, namely as WNT2B-WT. Concurrently, the corresponding sequence not carrying the miR-145 binding site was amplified and cloned into pGL3 plasmids, namely as WNT2B-MUT. The reporter vectors and miR-NC or miR-145 mimics were cotransfected into Hela and SiHa cells using Lipofectamine 2000 (Thermo Fisher Scientific). 36 h later, cells were lysed and luciferase activities were detected Using Dual-Luciferase Reporter Assay System (Promega, Madison, WI, USA).
Statistical analysis
The data were presented as the mean ± standard deviation (S.D.) from 3 or more repeated parallel experiments. The differences between two groups were analyzed by student’s t test and differences among multiple groups were performed using a one-way analysis of variance (ANOVA) using GraphPad Prism 7 (GraphPad Inc., La Jolla, CA, USA). The definition of statistically significance is that the P value is less than 0.05.
Results
MiR-145 was down-regulated while WNT2B was up-regulated in cervical cancer tissues and cell lines
The expression of miR-145 and WNT2B were measured by qRT-PCR in tumor tissues cell lines. The results showed that miR-145 expression was decreased in cervical cancer tissues compared with normal tissues (Figure 1A), while WNT2B expression was enhanced (Figure 1D). Also, we found cervical cancer patients with the higher expression of miR-145 had a higher overall survival (Figure 1B). In addition, the expression of miR-145 in cervical cancer patients was inversely associated with tumor group, FIGO stage and Lymph node metastasis (Table 1). The result of immunohistochemistry analysis demonstrated that WNT2B expression in cervical tumor tissues was higher than that in adjacent normal tissues (Figure 1C). Furthermore, we analyzed the relationship between miR-145 and WNT2B, and found that miR-145 expression is negatively correlated with WNT2B in cervical cancer tissues (Figure 1E). Moreover, reduced expression of miR-145 and elevated expression of WNT2B were observed in 5 cervical cancer cell lines (Hela, SiHa, SW756, CaSki, and C33A) compared with NCEC cells (Figure 1F, 1G). In general, the low miR-145 expression in cervical cancer suggested that miR-145 might be involved in the pathogenesis and development of cervical cancer.
Figure 1.
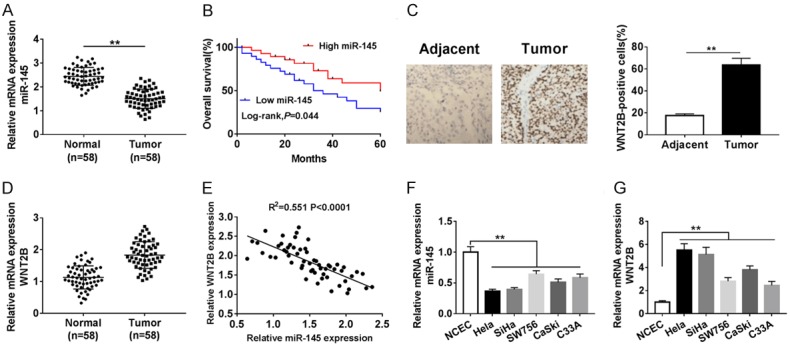
The expressions of miR-145 and WNT2B in cervical cancer tissues and cell lines. (A) The level of miR-145 in cervical cancer tissues and adjacent normal tissues was measured by qRT-PCR. (B) The relationship between the expression of miR-145 and overall survival of cervical cancer patients (P = 0.044). (C) The protein expression level of WNT2B in cervical cancer tissues and control tissues was examined using immunohistochemistry. (D) QRT-PCR was performed to measure relative expression of miR-145 in tumor tissues and non-tumor tissues. (E) The correlation between miR-145 and WNT2B in cervical cancer tissues was analyzed. (F, G) The levels of miR-145 and WNT2B (G) were detected in 6 cell lines by qRT-PCR. **P < 0.01.
Table 1.
Clinicopathological factors and miR-145 expression in patients with cervical cancer
| Parameter | Case | MiR-145 expression | P valuea | |
|---|---|---|---|---|
|
| ||||
| High (n = 29) | Low (n = 29) | |||
| Age (years) | ||||
| ≤50 | 21 | 12 | 9 | 0.305 |
| >50 | 37 | 17 | 20 | |
| Tumor size | ||||
| ≤4 cm | 28 | 18 | 10 | 0.026* |
| >4 cm | 30 | 11 | 19 | |
| Histological differentiation | ||||
| Well/Moderate | 27 | 11 | 16 | 0.058 |
| Poor | 31 | 18 | 23 | |
| Histological type | ||||
| Adenocarcinoma | 26 | 12 | 14 | 0.542 |
| Squamous cell carcinoma | 32 | 17 | 15 | |
| FIGO stage | ||||
| I-II | 25 | 18 | 7 | 0.006* |
| III-IV | 33 | 11 | 22 | |
| Lymph node metastasis | ||||
| No | 32 | 20 | 12 | 0.028* |
| Yes | 26 | 9 | 17 | |
| HR-HPV | ||||
| No | 26 | 15 | 11 | 0.125 |
| Yes | 32 | 14 | 18 | |
P < 0.05;
Chi-square test.
HR-HPV, high-risk human papilloma virus; FIGO, International Federation of Gynaecology and Obstetrics.
Over-expression of miR-145 suppressed tumor cell growth and induced apoptosis in cervical cancer cell
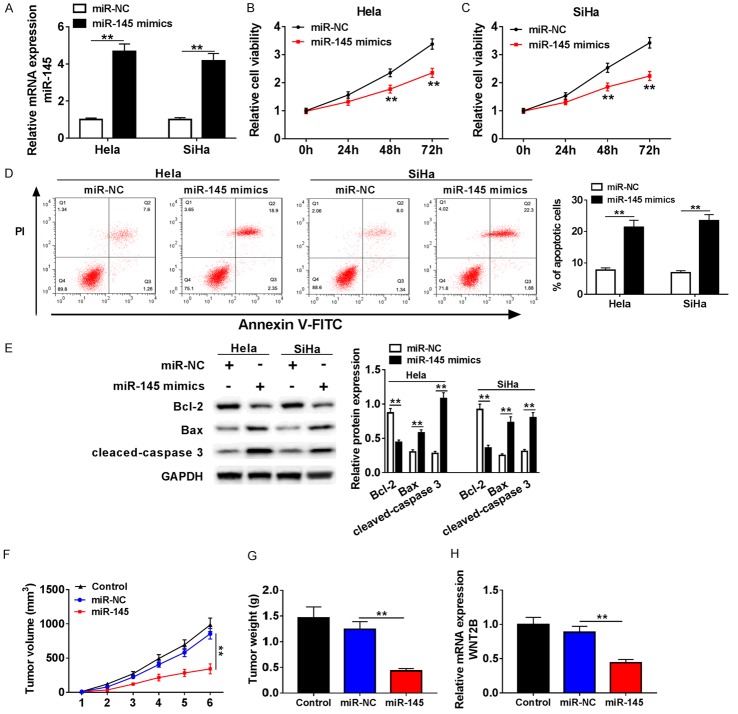
In order to investigate the effects of miR-145 on proliferation of Hela and SiHa cells, a gain-of-function approach was utilized. Hela and SiHa cells were transfected with miR-NC or miR-145 mimic. Over-expression of miR-145 was confirmed by qRT-PCR (Figure 2A). The results of cell viability assay showed that over-expression of miR-145 inhabited cell proliferation in both Hela and SiHa cells (Figure 2B, 2C). Moreover, flow cytometrynalysis showed that miR-145 over-expression induced cervical cells apoptosis (Figure 2D). As is known to all, Bcl-2 negatively regulated cell apoptosis while Bax and caspase 3 positively regulated the cell apoptosis process. Western blot was performed to examine expression levels of apoptosis-related proteins in transfected Hela and SiHa cells. As shown in Figure 2E, Bcl-2 expression was down-regulated while Bax and cleaved caspase 3 expression were up-regulated. Additionally, to confirm the effects of miR-145 on cervical cancer in vivo, we established Xenograft tumor model and found that miR-145 significantly inhibited tumor volume, weight, and WTN2B expression, compared with miR-NC-transfected group (Figure 2F-H). Collectively, these data indicated that miR-145 over-expression suppresses cervical cancer progression and induced apoptosis in vitro and in vivo.
Figure 2.
Over-expression of miR-145 inhibited proliferation and induced apoptosis in cervical cancer cells. A-E. Hela and SiHa cells were transfected with miR-NC or miR-145 mimic. A. The over-expression efficiency of miR-145 was detected by qRT-PCR. B, C. The cell ability of Hela and SiHa cells with over-expressed miR-145 was determined by MTT assay. D. The effect of miR-145 over-expression on cell apoptosis in transfected Hela and SiHa cells was confirmed by flow cytometry. E. Protein levels of apoptosis-related proteins in treated Hela and SiHa cells were analyzed using western blot. F. The tumor volume was examined every week. G. The tumor weight was measured at 6 weeks post injection. H. QRT-PCR was applied to detect WNT2B expression in Xenograft tumor model. **P < 0.01.
Over-expression of miR-145 restained tumor cell migration, invasion and EMT
The cell migration and invasion were examined using transwell assay. The data showed miR-145 over-expression inhibited cell migration and invasion in both Hela and SiHa cells (Figure 3A, 3B). Epithelial-mesenchymal transition (EMT) is a critical step in the progression of tumors metastasis. During this process, E-cadherin expression is decreased, while ICAM-1 and vimentin expression are increase. Western blot assay results revealed that E-cadherin expression was elevated while ICAM-1 and vimentin expression were declined in both Hela and SiHa cells transfected with miR-145 mimics, compared with miR-NC group (Figure 3C). These data suggested that miR-145 inhibited migration invasion of cervical cells by negatively regulating EMT process.
Figure 3.
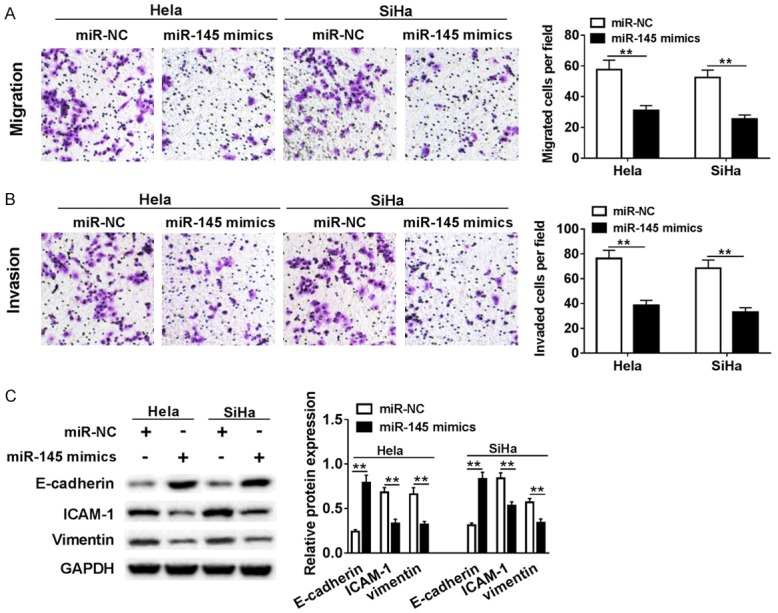
Ectopic expression of miR-145 repressed cervical cells migration, invasion and EMT. Hela and SiHa cells were transfected with miR-NC or miR-145 mimic. (A, B) The effects of over-expressions of miR-145 on migration (A) and invasion (B) were examined using transwell invasion assay. (C) The expression levels of EMT-related proteins were analyzed by western blot. **P < 0.01.
WNT2B was target of miR-145 in cervical cancer cells
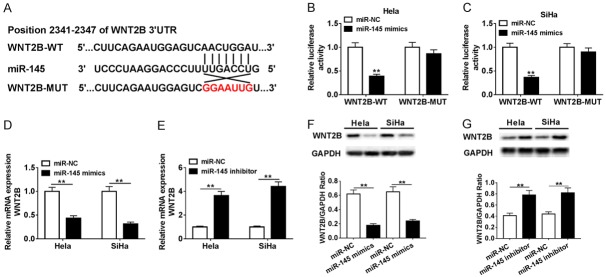
Bioinformatics software Target-Scan was employed to seek the target of miR-145, and we found that the 3’UTR of WNT2B mRNA contained the binding sequence of miR-145 (Figure 4A). To confirm the prediction, the WNT2B-WT and WNT2B-MUT luciferase reporter plasmids were constructed. Relative activities of luciferase reporters showed that WNT2B-WT significantly decreased the luciferase activity in both Hela and SiHa cells. However, luciferase expression of WNT2B-MUT remained almost unchanged (Figure 4B, 4C). In order to clarify the impact of miR-145 on WNT2B expression, Hela and SiHa cells were transfected with miR-NC, miR-145 mimics or miR-145 inhibitor. The results shown that the expression levels of WNT2B in Hela and SiHa cells transfected with miR-145 mimics were dramatically decreased, while the WNT2B expression apparently increased in cells transfected with miR-145 inhibitor (Figure 4D-G). These results implied that miR-145 negatively regulated WNT2B expression in Hela and SiHa cells by binding WNT2B.
Figure 4.
WNT2B was a target of miR-145 in cervical cancer cells. A. The predicted binding sites of miR-145 on 3’UTR of WNT2B and its mutant were shown. B, C. The luciferase activities of WNT2B-WT and WNT2B-MUT in Hela and SiHa cells transfected with miR-145 mimic or negative control were evaluated by luciferase reporter assay. D-G. Hela and SiHa cells were transfected with miR-NC, miR-145 mimic or miR-145 inhibitor. The mRNA and protein levels of WNT2B were detected in transfected Hela and SiHa cells by qRT-PCR and western blot, respectively. **P < 0.01.
Restoration of WNT2B reversed the effects of miR-145 on cell apoptosis and EMT
To further make clear how the miR-145 regulating cervical cancer cell migration, invasion and apoptosis, Hela and SiHa cell were transfected miR-NC, miR-145 mimics, miR-145 mimics + Vector or miR-145 mimics + WNT2B. QRT-PCR and western blot assay were performed to examine the mRNA and protein levels of WNT2B, respectively. Apparently, over-expression of miR-145 inhibited mRNA and protein levels of WNT2B in Hela and SiHa cells, while the introduction of WNT2B significantly recovered the inhibitory effect indiced by miR-145 (Figure 5A-C). As shown in Figure 5D, upregulation of WNT2B obviously weakened miR-145-mediated suppressed impact on WNT2B expression. We then examined the protein expression of apoptosis-related proteins using western blot assay, and found that miR-145 suppressed Bcl-2 expression, while up-regulated Bax and cleaved-caspase 3 expression (Figure 5E, 5F). Furthermore, we assessed the expression levels of three proteins E-cadherin, ICAM-1, and Vimentin involved in EMT process. The data suggested that miR-145 significantly elevated E-cadherin expression, but inhibited ICAM-1 and Vimentin expression (Figure 5G, 5H). Transwell assay was performed to measure migration and invasion of cervical cell. As displayed in Figure 5I, 5J, over-expression of miR-145 led to the reduction of migration and invasion abilities of cervical cells, while WNT2B abrogated these repressed impacts in a large proportion. Taken together, these results suggested that miR-145 played a suppressed role in metastasis and exerted promotion function in cell apoptosis in Hela and SiHa cells, while over-expression of WNT2B could reverse these effects.
Figure 5.
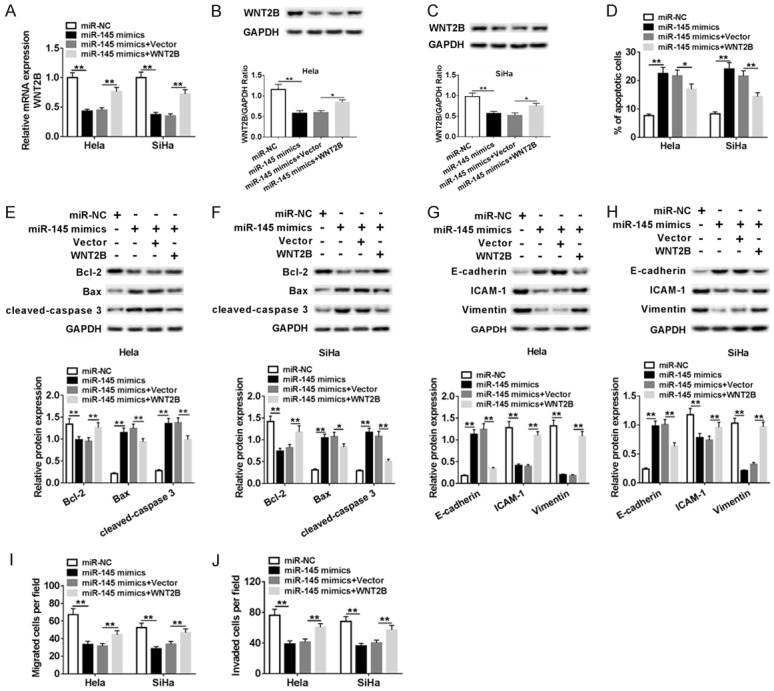
miR-145 repressed proliferation, migration, and invasion, induced apoptosis in cervical cancer cells by targeting WNT2B. Hela and SiHa cells were transfected with miR-NC, miR-145 mimic, miR-145 mimic + Vector, or miR-145 mimic + WNT2B. (A) The mRNA expression level of WNT2B in transfected Hela and SiHa cells was tested by qRT-PCR. (B, C) The m protein expression level of WNT2B was examined by western blot. (D) Flow cytometry for cell apoptosis analysis of transfected cells was conducted. (E-H) Western blot assay was employed to evaluate expression of apoptosis-related proteins (E, F) and EMT-related proteins (G, H) in transfected cervical cancer cells. (I, J) Transwell assay was conducted to measure migration and invasion abilities of treated Hela and SiHa cells. **P < 0.01.
MiR-145 suppressed the Wnt/β-catenin signaling pathway by regulating WNT2B in cervical cells
In consideration of WNT2B was involved in the Wnt/β-catenin signaling pathway, we speculated that miR-145 could regulate this pathway. Therefore, we examined the expression levels of WNT-associated proteins using western blot in SiHa cell transfected with miR-NC, miR-145 mimics, miR-145 mimics + Vector or miR-145 mimics + WNT2B. As exhibited in Figure 6, the expression levels of p-GSK-3β, active β-catenin, cyclin D1, and c-Myc were decreased in SiHa cells transfected with miR-145 mimics. Above reduction of protein expressions was recovered by over-expression of WNT2B. The data demonstrated that miR-145 inactived Wnt/β-catenin signaling pathway by suppressing WNT2B expression.
Figure 6.
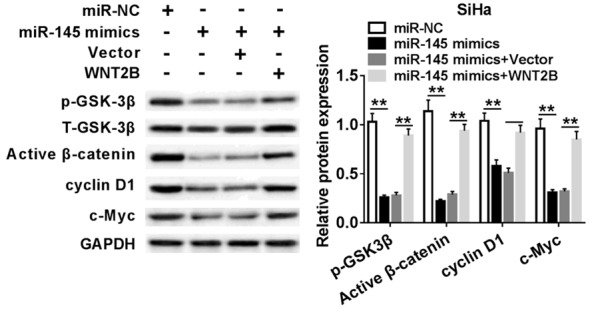
miR-145 regulated the Wnt/β-catenin pathway in SiHa by affecting WNT2B. Protein expression levels of p-GSK-3β, Active β-catenin, cyclin D1, and c-Myc were evaluated in SiHa cells transfected with miR-NC, miR-145 mimic, miR-145 mimic + Vector, or miR-145 mimic + WNT2B by western blot assay. **P < 0.01.
Discussion
In the current study, we studied the possible role of miR-145 in cervical cancer. We found that miR-145 expression was declined in cervical specimens compared with those normal tissues. However, WNT2B expression was significantly up-regulated in cervical specimens. Furthermore, over-expression of miR-145 significantly inhibited cell proliferation, migration and invasion, as well as induced apoptosis in vitro by inactivating Wnt/β-catenin pathway by directly targeting WNT2B in vitro.
A previous research suggested that miR-145 could function as potential cervical marker or therapeutic target, and was associated with aggressive progression and poor prognosis [21]. Wang et al. found that miR-145 significantly down-regulated in cervical cancer tissues, and functional studies indicated that miR-145 played a tumor suppressor role to prevent tumor cell growth [22]. In our study, functional experiments revealed that miR-145 over-expression inhibited cell growth and induced apoptosis, and expression of miR-145 was associated with EMT processes. Similarly, research conducted by Kano et al. illuminated that miR-145 was down-regulated in esophageal squamous cell carcinoma and it was a tumor suppressor candidate [23]. However, the functional mechanism of miR-145 in cervical cancer is not fully understood. Zhao and his colleagues found that miR-145 is down-regulated in MCF-7 cells when compared with MCF10A cells, and over-expression of miR-145 blocked growth and induced apoptosis of MCF-7 cell by targeting RTKN [24]. Sachdeva et al. pointed out that p53 is able to repress c-Myc by introduction of miR-145, thus miR-145 directly targeted the oncogene c-Myc [25]. In our research, we proved that miR-145 could decrease the expression level of c-Myc and the biomarkers of epithelial-mesenchymal transition, including E-cadherin and vimentin. Peng et al. disclosed that over-expression miR-145 reduced the migration and invasion abilities of PC-3 cell in vitro [26]. The low miR-145 expression in tumor tissues strongly suggested that miR-145 could be regarded as a tumor suppressor in cervical cancer.
The Wnt/β-catenin signaling pathway is critical for a variety of biological processes, including cell proliferation, differentiation, tumorigenesis, organ fibrosis, and EMT. WNT2B is a highly conserved secretory factor of WNT family and promotes cancer formation in the different forms. For example, proto-oncogene WNT2 was commonly up-regulated in primary gastric cancer and primary colorectal cancer [27]. Liu et al. uncovered that silenced WNT2B could strikingly inhibited migration and invasion of NPC cells [28]. Similarly, we also found that WNT2B was up-regulated in cervical cancer tissues and cell lines, and promoted migration and invasion capabilities of cervical cancer cells.
We all know that the 3’UTR region of non-coding RNA can bind to target mRNAs and thus exert its regulatory role. Mounting review focused on the role of the relationship between miRs and the Wnt/β-catenin signaling pathway in cancer development. The up-regulation of WNT2B expression could be observed in many human cancers. Increasing experimental results also confirmed that WNT2B plays a major role in tumorigenesis and chemo-resistance in oral cancer and provided potential therapeutic targets. Previous findings of Katoh et al. suggested that high-expression of WNT2B was induced by bHLH transcription factors [29].
The relationship between miR-145 and WNT2B and how they regulate each other is unclear. In this study, we used bioinformatics software to predict potential target genes for miR-145. Studies have shown that WNT2B is a potential target of miR-145. It is worth noting that the mRNA expression level of WNT2B Significantly decreased in cervical specimens, and negatively correlated with the expression level of miR-145 in cervical cancer tissues. The following functional experiments indicated that miR-145 directly targeted WNT2B. Moreover, MiR-145 negatively regulated both mRNA and protein expression of WNT2B. Additionally, restoration of WNT2B reversed the effects of miR-145. Therefore, we concluded that miR-145 took effect on cell proliferation and apoptosis by regulating Wnt/β-catenin pathway.
In conclusion, our results revealed that miR-145 inhibited cervical cancer progression and metastasis. We subsequently proved that miR-145 suppressed cervical cell proliferation, migration and invasion by inactivating Wnt/β-catenin pathway by targeting WNT2B in vitro. The study provided new molecular regulatory mechanism in the pathogenesis of cervical cancer and indicated miR-145 could function as a suppressor for the treatment of cervical cancer.
Conclusion
MiR-145 is down-regulated in cervical cancer tissues and cells, and miR-145 over-expression inhibits proliferation, migration, and promotes apoptosis of cervical cancer cells. WNT2B binds to miR-145 in cervical cancer cells. Introduced WNT2B remarkably attenuated the effect of miR-145 on proliferation and apoptosis of cancer cervical cells. In short, MiR-145 inhibits cervical cancer progression and metastasis by Wnt/β-catenin pathway by affecting WNT2B. This study helped to better understand the molecular mechanisms of cervical cancer and a promising target for the treatment of cervical disease.
Disclosure of conflict of interest
None.
References
- 1.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94:153–6. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 2.Catarino R, Petignat P, Dongui G, Vassilakos P. Cervical cancer screening in developing countries at a crossroad: emerging technologies and policy choices. World J Clin Oncol. 2015;6:281–90. doi: 10.5306/wjco.v6.i6.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Castellsagué X, Díaz M, De Sanjosé S, Muñoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS, Snijders PJ. Worldwide human papillomavirus etiology of cervical adenocarcinoma and its cofactors: implications for screening and prevention. J Natl Cancer Inst. 2006;98:303–315. doi: 10.1093/jnci/djj067. [DOI] [PubMed] [Google Scholar]
- 4.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, Snijders PJ, Peto J, Meijer CJ, Muñoz N. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–19. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 5.Lai EC. Micro RNAs are complementary to 3’UTR sequence motifs that mediate negative post-transcriptional regulation. Nat Genet. 2002;30:363–4. doi: 10.1038/ng865. [DOI] [PubMed] [Google Scholar]
- 6.Lee RC, Ambros V. An extensive class of small RNAs in caenorhabditis elegans. Science. 2001;294:862–864. doi: 10.1126/science.1065329. [DOI] [PubMed] [Google Scholar]
- 7.Ambros V. microRNAs: tiny regulators with great potential. Cell. 2001;107:823–826. doi: 10.1016/s0092-8674(01)00616-x. [DOI] [PubMed] [Google Scholar]
- 8.Xu N, Papagiannakopoulos T, Pan G, Thomson JA, Kosik KS. MicroRNA-145 regulates OCT4, SOX2, and KLF4 and represses pluripotency in human embryonic stem cells. Cell. 2009;137:647–658. doi: 10.1016/j.cell.2009.02.038. [DOI] [PubMed] [Google Scholar]
- 9.Le Sage C, Nagel R, Egan DA, Schrier M, Mesman E, Mangiola A, Anile C, Maira G, Mercatelli N, Ciafrè SA. Regulation of the p27Kip1 tumor suppressor by miR-221 and miR-222 promotes cancer cell proliferation. EMBO J. 2007;26:3699–3708. doi: 10.1038/sj.emboj.7601790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Michael MZ, O’Connor SM, van Holst Pellekaan NG, Young GP, James RJ. Reduced accumulation of specific microRNAs in colorectal neoplasia. Mol Cancer Res. 2003;1:882–891. [PubMed] [Google Scholar]
- 11.Chiyomaru T, Enokida H, Tatarano S, Kawahara K, Uchida Y, Nishiyama K, Fujimura L, Kikkawa N, Seki N, Nakagawa M. miR-145 and miR-133a function as tumour suppressors and directly regulate FSCN1 expression in bladder cancer. Br J Cancer. 2010;102:883–91. doi: 10.1038/sj.bjc.6605570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cho WC, Chow AS, Au JS. Restoration of tumour suppressor hsa-miR-145 inhibits cancer cell growth in lung adenocarcinoma patients with epidermal growth factor receptor mutation. Eur J Cancer. 2009;45:2197–206. doi: 10.1016/j.ejca.2009.04.039. [DOI] [PubMed] [Google Scholar]
- 13.Wu H, Xiao Z, Wang K, Liu W, Hao Q. MiR-145 is downregulated in human ovarian cancer and modulates cell growth and invasion by targeting p70S6K1 and MUC1. Biochem Biophys Res Commun. 2013;441:693–700. doi: 10.1016/j.bbrc.2013.10.053. [DOI] [PubMed] [Google Scholar]
- 14.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, Zhou H, Zhao RC. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34:1461–6. [PubMed] [Google Scholar]
- 15.Zhang W, Wang Q, Yu M, Wu N, Wang H. MicroRNA-145 function as a cell growth repressor by directly targeting c-Myc in human ovarian cancer. Technol Cancer Res Treat. 2014;13:161–8. doi: 10.7785/tcrt.2012.500367. [DOI] [PubMed] [Google Scholar]
- 16.Sachdeva M, Mo YY. MicroRNA-145 suppresses cell invasion and metastasis by directly targeting mucin 1. Cancer Res. 2010;70:378–387. doi: 10.1158/0008-5472.CAN-09-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu Q, Liu LZ, Qian X, Chen Q, Jiang Y, Li D, Lai L, Jiang BH. MiR-145 directly targets p70S6K1 in cancer cells to inhibit tumor growth and angiogenesis. Nucleic Acids Res. 2012;40:761–74. doi: 10.1093/nar/gkr730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu Z, Xu T, Wang L, Wang X, Zhong S, Xu C, Shen Z. MicroRNA-145 directly targets the insulin-like growth factor receptor I in human bladder cancer cells. FEBS Lett. 2014;588:3180–3185. doi: 10.1016/j.febslet.2014.06.059. [DOI] [PubMed] [Google Scholar]
- 19.Katoh M. Differential regulation of WNT2 and WNT2B expression in human cancer. Int J Mol Med. 2001;8:657–60. doi: 10.3892/ijmm.8.6.657. [DOI] [PubMed] [Google Scholar]
- 20.Ghahhari NM, Babashah S. Interplay between microRNAs and WNT/β-catenin signalling pathway regulates epithelial-mesenchymal transition in cancer. Eur J Cancer. 2015;51:1638–49. doi: 10.1016/j.ejca.2015.04.021. [DOI] [PubMed] [Google Scholar]
- 21.Wang Q, Qin J, Chen A, Zhou J, Liu J, Cheng J, Qiu J, Zhang J. Downregulation of microRNA-145 is associated with aggressive progression and poor prognosis in human cervical cancer. Tumour Biol. 2015;36:3703–3708. doi: 10.1007/s13277-014-3009-3. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Tang S, Le SY, Lu R, Rader JS, Meyers C, Zheng ZM. Aberrant expression of oncogenic and tumor-suppressive microRNAs in cervical cancer is required for cancer cell growth. PLoS One. 2008;3:e2557. doi: 10.1371/journal.pone.0002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kano M, Seki N, Kikkawa N, Fujimura L, Hoshino I, Akutsu Y, Chiyomaru T, Enokida H, Nakagawa M, Matsubara H. miR-145, miR-133a and miR-133b: tumor-suppressive mi-RNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127:2804–2814. doi: 10.1002/ijc.25284. [DOI] [PubMed] [Google Scholar]
- 24.Wang S, Bian C, Yang Z, Bo Y, Li J, Zeng L, Zhou H, Zhao RC. miR-145 inhibits breast cancer cell growth through RTKN. Int J Oncol. 2009;34:1461–6. [PubMed] [Google Scholar]
- 25.Sachdeva M, Zhu S, Wu F, Wu H, Walia V, Kumar S, Elble R, Watabe K, Mo YY. p53 represses c-Myc through induction of the tumor suppressor miR-145. Proc Natl Acad Sci U S A. 2009;106:3207–3212. doi: 10.1073/pnas.0808042106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peng X, Guo W, Liu T, Wang X, Tu X, Xiong D, Chen S, Lai Y, Du H, Chen G, Liu G, Tang Y, Huang S, Zou X. Identification of miRs-143 and -145 that is associated with bone metastasis of prostate cancer and involved in the regulation of EMT. PLoS One. 2011;6:e20341. doi: 10.1371/journal.pone.0020341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katoh M, Kirikoshi H, Terasaki H, Shiokawa K. WNT2B2 mRNA, up-regulated in primary gastric cancer, is a positive regulator of the WNT- beta-catenin-TCF signaling pathway. Biochem Biophys Res Commun. 2001;289:1093–1098. doi: 10.1006/bbrc.2001.6076. [DOI] [PubMed] [Google Scholar]
- 28.Liu C, Li G, Yang N, Su Z, Zhang S, Deng T, Ren S, Lu S, Tian Y, Liu Y, Qiu Y. miR-324-3p suppresses migration and invasion by targeting WNT2B in nasopharyngeal carcinoma. Cancer Cell Int. 2017;17:2. doi: 10.1186/s12935-016-0372-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Katoh M, Katoh M. Transcriptional regulation of WNT2B based on the balance of hedgehog, notch, BMP, and WNT signals. Int J Oncol. 2009;34:1411–5. [PubMed] [Google Scholar]