Abstract
The N-myc downstream regulated gene (NDRG) protein family consists of 4 members (NDRG1, NDRG2, NDRG3, and NDRG4), that have been reported to be aberrantly expressed in human cancers. Furthermore, NDRG3 protein expression is known to promote tumor angiogenesis and cell growth. The aim of this study was to investigate the clinical significance of NDRG3 expression in invasive breast cancer (IBC). NDRG3 expression was evaluated immunohistochemically in tissue microarrays of 1339 IBC samples, and associations between NDRG3 expression and clinicopathologic parameters, including prognosis, were examined. NDRG3 protein expression was observed in 194 (14.5%) cases, and found to be associated with an age of ≥ 50 yrs (P=0.043), a high histologic grade (P < 0.001), high Ki-67 index (P < 0.001), negatively for estrogen or progesterone receptor (both P < 0.001), and positive HER2 status (P < 0.001). No significant association was found between NDRG3 expression and tumor size, lymph node status, lymphovascular invasion, or androgen receptor status. NDRG3-positive tumors were found to be associated with poorer overall survival (OS, P=0.035), and multivariate analyses showed NDRG3 expression independently predicted OS (P=0.011) and disease-free survival (P=0.051). This study shows NDRG3 protein expression is a promising prognostic marker in IBC.
Keywords: NDRG3 protein, breast cancer, prognosis
Introduction
Breast cancer (BC) is the leading cause of death in women worldwide. According to the statistics of the Korea Central Cancer Registry, breast cancer was the second-most common cancer in women after thyroid cancer, and 22,550 patients were newly diagnosed and 2353 succumbed to the disease in 2015 [1]. The identification of biologic markers predictive of prognosis or therapeutic response in cancer patients is essential in this era of tailored therapy.
The N-myc downstream regulated gene (NDRG) protein family consists of NDRG1, NDRG2, NDRG3, and NDRG4, which have genes located at 8q24.3, 14q11.2, 20q11.21-11.23, and 16q21-q22.1, respectively [2]. NDRG proteins are differentiated based on sequence homology, as NDRG1 and NDRG3 or NDRG2 and NDRG4, which have homologies of 67% and 58% respectively [3]. Although the functions of NDRG family proteins have not been clearly elucidated, emerging evidence suggests they contribute to cell proliferation, differentiation, development, and stress response [2]. However, it is known their tissue distributions differ. That is, NDRG1 is expressed ubiquitously, NDRG2 is expressed predominantly in brain, liver, and kidneys, NDRG3 is highly expressed in prostate, ovaries, and testes, and NDRG4 is expressed almost exclusively in brain and heart [4-6]. Aberrant expressions of NDRG proteins have been reported in several human cancers. In prostatic, colorectal, breast, esophageal, and pancreatic cancer and brain glioma, NDRG1 expression in tumor cells has been associated with good prognosis [7-14], whereas high NDRG1 expression has been reported to be associated with poor prognosis in hepatic and cervical cancers [15-18]. NDRG2 has also been reported to act as a tumor suppressor gene, and its downregulation has been observed in various human cancers. In particular, loss of NDRG2 expression has been associated with poor prognosis in glioma, and in colorectal, gastric, pancreatic, and renal cancer [19-25].
Few reports have been issued on the expressions of NDRG3 and NDRG4 in cancer. Recently, Lee et al. reported NDRG3 protein expression is induced under oxygen-limited conditions in diverse cell types [26]. NDRG3 protein was found to be degraded in normoxia but to be protected from proteolytic destruction by binding to lactate, and thus, to accumulate in hypoxia. It was also observed NDRG3 mediated activation of the Raf-ERK pathway promoted angiogenesis and cell growth during prolonged hypoxia. Lactate is produced in large quantities by glycolysis under hypoxic conditions, which are common in cancer cells with high proliferative activity. Furthermore, intratumoral hypoxia has been correlated with poor prognosis and poor treatment outcome in different cancers [27,28]. In an in vitro study, NDRG3 expression was induced at the mRNA and protein levels by synthetic androgen in prostate cancer cells [29], and elevated NDRG3 expression has been reported to be associated with aggressive biologic behavior and unfavorable prognosis in prostatic, laryngeal, lung, and hepatic cancer [30-33]. However, no study has yet addressed the prognostic significance of NDRG3 protein expression in breast cancer.
Accordingly, we investigated the expression of NDRG3 protein immunohistochemically in a large invasive breast cancer (IBC) cohort to clarify its prognostic significance.
Materials and methods
Case selection and collection of clinicopathological data
A total of 1518 surgical specimens of IBC that had been routinely processed in the Department of Pathology, Yeungnam University Hospital, Daegu, South Korea between December 1996 and December 2007 for pathologic diagnosis were considered for the study. Patients received standard radiotherapy or adjuvant systemic therapy (hormone therapy or chemotherapy) after surgery. Those that received neoadjuvant chemotherapy and those with inadequate immunohistochemical results or clinicopathologic information were excluded. Accordingly, the study was conducted using 1339 specimens.
Clinicopathologic characteristics, including age, tumor size, lymph node (LN) status, histologic subtype, lymphovascular invasion, histologic grade, Ki-67 labelling index (LI) (percentage of positive cells among at least 500 tumor cells), and the presence of recurrence or metastasis, were retrospectively collected by reviewing pathology reports and medical records. Information on cause of death was obtained from medical records and the microdata service system provided by Statistics Korea (http://mdis.kostat.go.kr). Overall survival (OS) was defined as time from surgical resection to date of death or last follow-up. Disease-free survival (DFS) was defined as time from surgical resection to locoregional recurrence, distant metastasis, death or last follow-up. This study was approved by the Institutional Review Board of Yeungnam University Hospital (YUMC 2017-09-038), which waived the requirement for informed consent.
Tissue microarray construction and immunohistochemical evaluation
Tissue microarray (TMA) blocks were constructed using a Quick-Ray® Manual Tissue Microarrayer (Unitma, Seoul, Korea) and Quick-Ray® recipient blocks of 1.5 mm cores (Unitma). A pair of 1.5-mm-diameter tissue cores was retrieved from a representative tumor block in each case and transferred to a recipient block. Thirty-eight TMA blocks were created from the tumor samples of the initially considered 1518 cases. Immunohistochemical stainings for NDRG3, estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2) were performed using the automated Benchmark® platform (Ventana Medical Systems, Tucson, AZ, USA) using 4 μm tissue sections obtained from the TMA blocks. Staining for androgen receptor (AR) was performed manually, as described previously [34]. A summary of the antibodies and staining conditions used is provided in Table 1.
Table 1.
Antibodies and staining conditions used in this study
| Antibody | Source | Clone | Dilution | Antigen retrieval | Incubation time | Detection kit |
|---|---|---|---|---|---|---|
| NDRG3 | Sigma-Aldrich | Polyclonal | 1:70 | Standard* | 40 min | OptiView™ DAB |
| ER | Ventana | SP1 | Predilution | Standard* | 16 min | UltraView™ DAB |
| PR | Ventana | 1E2 | Predilution | Standard* | 16 min | UltraView™ DAB |
| HER2 | Ventana | 4B5 | Predilution | Mild† | 16 min | OptiView™ DAB |
| AR | Epitomics | ER179 (2) | 1:200 | Autoclave (citrate buffer, pH 6.0) | 60 min | EnVision™ (Dako) |
NDRG3, N-myc downstream regulated gene 3; ER, estrogen receptor; PR, progesterone receptor; HER2, human epidermal growth factor receptor 2; AR, androgen receptor.
The standard antigen retrieval condition used was 60 min at 100°C in cell conditioning solution 1 or 2;
mild condition was 30 min at 100°C in either cell conditioning solution.
ER, PR, and AR were considered positive if there was nuclear immunoreactivity in at least 1% of tumor cells [35]. HER2 positivity was defined as the presence of protein overexpression (3+); however, in equivocal cases (2+), silver in situ hybridization using an INFORM® HER2 DNA probe (Ventana Medical Systems) was performed and results were interpreted according to ASCO/CAP guidelines [36]. Two pathologists (YKB and MCK) unaware of patient details, interpreted tumor cell NDRG3 staining results under a multi-headed microscope by assessing intensities and extents of staining. Staining intensity was assessed using a 0-3 scale (negative, 0; weakly positive, 1; moderately positive, 2; strongly positive, 3), and extent of staining was graded using proportions of positive tumor cells (0%, 0; 1-25%, 1; 26-50%, 2; 51-75%, 3; > 75%, 4). Final immunoreactivity scores (IRSs) were determined by multiplying intensity and extent scores (range 0 to 12). For statistical analyses, cases were dichotomized into positive (IRS ≥ 6) and negative (IRS < 6) expression groups; the cutoff was determined with respect to outcomes as determined using the Kaplan-Meier method and the log-rank test.
Statistical analysis
Statistical analysis was performed using IBM SPSS version 23.0 for Windows (IBM Co., Armonk, NY, USA). The Chi-squared test was used to evaluate associations between NDRG3 expression and clinicopathologic characteristics. Survival curves were plotted using the Kaplan-Meier method and the log-rank test was used to determine the significances of survival differences. Variables significant by univariate analyses were subjected to Cox regression proportional hazard analysis. Adjusted hazard ratio (HR) and associated 95% confidence intervals (CIs) were calculated for variables. All tests were two-sided, and p values of < 0.05 were considered significant.
Results
Patient demographics
Mean patient age at diagnosis was 48 years (range, 20-86 years). Tumor sizes ranged from 0.5 to 11 cm (mean, 2.3 cm). Six hundred and ninety-three (51.8%) patients had an invasive tumor of ≤ 2 cm (pT1), and the other 646 (48.2%) had a tumor of > 2 cm (pT2 in 595; pT3 in 46; pT4 in 5). Axillary LN metastasis was found in 625 (46.8%) patients, and lymphovascular invasion in 688 (51.4%). Sentinel LN biopsy or axillary LN dissection was not performed in three patients. Histologic grades were 1 in 232 (17.3%), 2 in 379 (28.3%), and 3 in 728 (54.4%). 809 (60.4%) patients underwent mastectomy and 530 (39.6%) breast-conserving surgery.
931 (69.5%) patients received anthracycline-based adjuvant chemotherapy, and 230 (17.2%) received non-anthracycline chemotherapeutic regimens. The remaining 178 (13.3%) patients did not receive chemotherapy. No patient with HER2-positive BC received adjuvant trastuzumab because its routine use was approved in Korea in 2010. Hormone therapy using tamoxifen or aromatase inhibitors was performed in 919 (68.6%) and radiation therapy in 641 (47.9%). During follow-up (mean, 117 months; range, 1-238 months), recurrence occurred in 211 (15.8%) patients, and at last follow-up, 174 (13%) deaths had occurred.
Correlations between NDRG3 expression and clinicopathologic variables
Non-neoplastic epithelial cells, stromal fibroblasts, and immune cells within tumor cores were all negative for NDRG3 expression. Immunoreactivity for NDRG3 in tumor cells varied from case to case (Figure 1). The distribution of NDRG3 IRSs was as follows; 0 in 630 (47.1%) cases, 1 in 16 (1.2%), 2 in 89 (6.6%), 3 in 54 (4%), 4 in 356 (26.6%), 6 in 24 (1.8%), 8 in 124 (9.3%), 9 in 1 (0.1%), and 12 in 45 (3.4%) cases. Positive NDRG3 expression (IRS ≥ 6) was observed in 194 (14.5%) cases.
Figure 1.
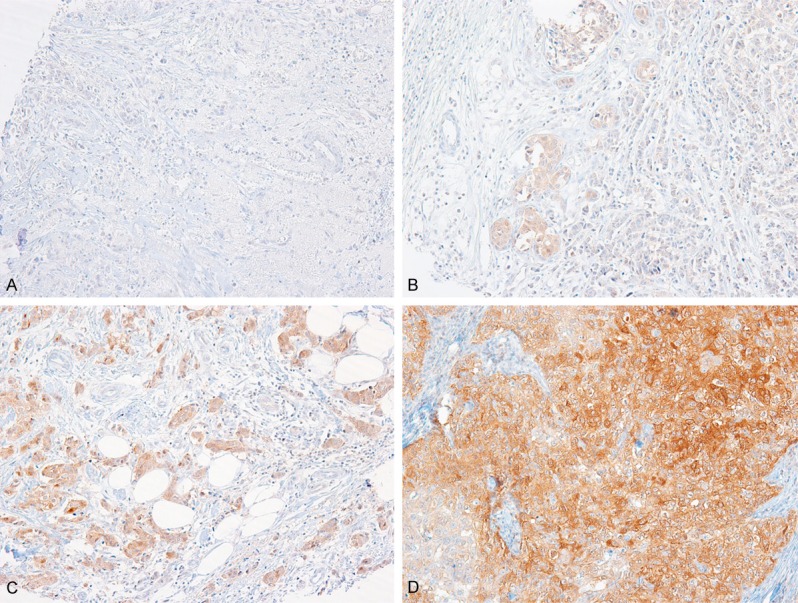
Representative immunohistochemical staining results for NDRG3 in invasive breast cancer. NDRG3 expression in tumor cells were rated as negative (A), weak (B), moderate (C) or strong (D) intensity.
NDRG3 expression was significantly associated with an age of ≥ 50 yrs (P=0.043), histologic grade 3 (P < 0.001), a negative ER (P < 0.001) or PR status (P < 0.001), HER2 positivity (P=0.003), and a high Ki-67 LI (P < 0.001). However, no significant correlation was observed between NDRG3 expression and other clinicopathologic variables including tumor size, LN metastasis, histologic subtype, lymphovascular invasion, and AR (Table 2).
Table 2.
Relations between NDRG3 protein expression and patient characteristics
| Characteristics | Cases (N=1339) | NDRG3 expression, N (%) | P value | |
|---|---|---|---|---|
|
| ||||
| Negative | Positive | |||
| Age | 0.043 | |||
| < 50 | 866 | 753 (65.8) | 113 (58.2) | |
| ≥ 50 | 473 | 392 (34.2) | 81 (41.8) | |
| Tumor size | 0.827 | |||
| ≤ 2 cm | 693 | 594 (51.9) | 99 (51) | |
| > 2 cm | 646 | 551 (48.1) | 95 (49) | |
| Lymph node metastasis* | 0.459 | |||
| Absent | 711 | 603 (52.8) | 108 (55.7) | |
| Present | 625 | 539 (47.2) | 86 (44.3) | |
| Histologic subtype | 0.589 | |||
| Invasive, NST | 1192 | 1012 (88.4) | 180 (92.8) | |
| Lobular | 38 | 35 (3.1) | 3 (1.5) | |
| Micropapillary | 28 | 25 (2.2) | 3 (1.5) | |
| Mucinous | 24 | 22 (1.9) | 2 (1) | |
| Tubular | 10 | 10 (100) | 0 (0) | |
| Medullary | 7 | 7 (100) | 0 (0) | |
| Metaplastic | 7 | 6 (0.5) | 1 (0.5) | |
| Papillary | 3 | 2 (0.2) | 1 (0.5) | |
| Mixed | 30 | 26 (2.3) | 4 (2.1) | |
| Lymphovascular invasion | 0.916 | |||
| Absent | 651 | 556 (48.6) | 95 (49) | |
| Present | 688 | 589 (51.4) | 99 (51) | |
| Histologic grade | < 0.001 | |||
| 1 & 2 | 611 | 553 (48.3) | 58 (29.9) | |
| 3 | 728 | 592 (51.7) | 136 (70.1) | |
| Estrogen receptor | < 0.001 | |||
| Negative | 434 | 349 (30.5) | 85 (43.8) | |
| Positive | 905 | 796 (69.5) | 109 (56.2) | |
| Progesterone receptor | < 0.001 | |||
| Negative | 566 | 460 (40.2) | 106 (54.6) | |
| Positive | 773 | 685 (59.8) | 88 (45.4) | |
| Androgen receptor† | 0.853 | |||
| Negative | 620 | 533 (47.5) | 87 (46.8) | |
| Positive | 688 | 589 (52.5) | 99 (53.2) | |
| HER2 status | 0.003 | |||
| Negative | 1072 | 932 (81.4) | 140 (72.2) | |
| Positive | 267 | 213 (18.6) | 54 (27.8) | |
| Ki-67 labeling index‡ | < 0.001 | |||
| ≤ 20% | 548 | 493 (43.1) | 55 (28.4) | |
| > 20% | 790 | 651 (56.9) | 139 (71.6) | |
Three patients did not undergo sentinel lymph node biopsy or axillary lymph node dissection.
Androgen receptor status was not available in 31 patients.
One patient did not have Ki-67 labeling index in her pathology report.
Prognostic significance of NDRG3 expression
Patients with NDRG3 expression had shorter OSs than those negative for NDRG3 expression (P=0.035, Figure 2). Patients with an NDRG3 expressing tumor showed a tendency to have poorer DFSs than those with a non-NDRG3 expressing tumor, but the difference was not statistically significant (P=0.132). Because NDRG3 expression was found to be associated with ER, PR, and HER2 statuses, survival analysis was performed in subgroups defined by molecular subtypes. However, no survival differences were observed (data not shown).
Figure 2.
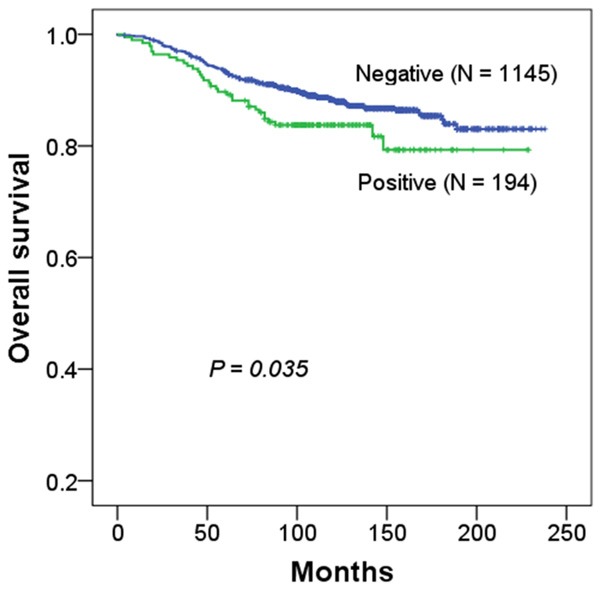
Kaplan-Meier survival curves for overall survival according to NDRG3 expression in breast cancer patients.
Multivariate analyses showed NDRG3 expression independently predicted OS (HR, 1.656; CI, 1.125-2.437; P=0.011), along with tumor size, LN status, histologic grade, and lymphovascular invasion (Table 3).
Table 3.
Multivariate analyses of clinicopathological variables affecting overall and disease-free survivals
| Clinicopathological variables | Overall survival | Disease-free survival | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P value | HR (95% CI) | P value | |
| NDRG3 expression, positive vs negative | 1.656 (1.125-2.437) | 0.011 | 1.432 (0.999-2.054) | 0.051 |
| Tumor size, > 2 cm vs ≤ 2 cm | 2.067 (1.464-2.919) | < 0.001 | 1.327 (0.992-1.774) | 0.057 |
| Histological grade, 3 vs 1&2 | 1.905 (1.342-2.703) | < 0.001 | 1.603 (1.186-2.166) | 0.002 |
| Lymph node metastasis, present vs absent | 1.754 (1.215-2.53) | 0.003 | 1.632 (1.182-2.253) | 0.003 |
| Lymphovascular invasion, present vs absent | 1.755 (1.2-2.567) | 0.004 | 1.938 (1.378-2.726) | < 0.001 |
HR, hazard ratio; CI, confidence interval.
Discussion
In the present study, NDRG3 protein was observed to be differentially expressed in tumor cells of IBC, and its expression was found to be significantly associated with clinicopathologic features of aggressive behavior, that is, high histologic grade, negative ER and PR statuses, HER2 positivity, and high Ki-67 LI. Furthermore, NDRG3 expression was associated with unfavorable outcomes and observed to be an independent prognostic marker of OS in IBC. This is the first study to address the prognostic value of NDRG3 protein expression in IBC tumor samples.
Several studies support our results. Wang et al. reported NDRG3 expression in prostate cancer cell lines (LNCaP, CL-1, DU145 and PC-3) and in the stromal cell line (WPMY-1) at the mRNA and protein levels [29]. Overexpression of NDRG3 was observed to increase the growth rate and migration of PC-3 prostatic cancer cells transfected with an NDRG3 expression construct in vitro, and to promote xenograft tumor growth in a nude mouse model. It was also reported NDRG3 overexpression upregulated the expression of angiogenic chemokines (i.e., chemokine ligand (CXCL)1, CXCL3, and CXCL5) in prostatic cancer cells, which could increase tumor angiogenesis and growth. Furthermore, Lee et al. reported NDRG3 knockdown suppressed angiogenic activity and tumor growth in BALB/c-nu mice xenografted with human hepatoma cells [26]. In this previous study, the expressions of markers of angiogenesis (IL8 and CD31) and cell proliferation (Ki-67) were effectively downregulated in NDRG3-depleted tumors, whereas, the ectopic expression of NDRG3 enhanced colony formation by human hepatoma cells in vitro, and their tumorigenic activities in BALB/c-nu mice. Li et al. also reported NDRG3 overexpression increased the proliferation, migration, and invasion of colorectal cancer (CRC) cells (SW1116), and that its depletion reduced the proliferation rate of CRC cells in vitro [37]. The same authors observed tumor xenografts were larger and heavier in BALB/c nude mice injected with SW1116/NDRG3 (SW1116 cells exogenously expressing NDRG3), and that there were more visible metastatic nodules in livers in these mice than in those transfected with SW1116/Vector. Furthermore, SW11-16/NDRG3 tumors had higher Ki-67 indices than SW1116/Vector tumors. The authors concluded NDRG3 promotes CRC proliferation, migration, invasion, and metastasis, and suggested that NDRG3 acts as an oncogene in CRC by activating Src phosphorylation.
Recently, several studies have demonstrated the prognostic value of the immunohistochemical detection of NDRG3 protein in several human cancers. In prostatic cancer, NDRG3 expression was significantly correlated with advanced stage, LN metastasis, distant metastasis and poor clinical outcome [32], and in laryngeal squamous cell carcinoma, high NDRG3 expression was associated with LN metastasis and poor OS [33]. In non-small cell lung cancer, NDRG3 expression was significantly associated with high grade, positive LN status, advanced stage, and unfavourable OS [31]; and in hepatocellular carcinoma, it was significantly associated with larger tumor size, high grade, and poor prognosis [30]. In the present study, NDRG3 expression was significantly associated with high histologic grade, high Ki-67 LI, and poor OS, but no significant association was observed between its expression and tumor size or LN metastasis. NDRG3 is an androgen-regulated gene [29], but in the present study, no relationship was evident between the expression of AR and NDRG3. These results are consistent with the notion that oncogenic functions of NDRG3 protein differ between tumor types, and indicate that NDRG3 might be a novel biomarker of prognosis in selected human cancers. In a previous study, NDRG3 was found to play a tumor-suppressive role in BC. Estiar et al. showed NDRG3 mRNA expression was downregulated in BC patients, especially in advanced stage and triple-negative BC patients [38]. In this study, low NDRG3 expression showed poorer event-free survival than normal or high NDRG3 expression. However, this is the only study to date to have evaluated NDRG3 expression in IBC, the study population was relatively small (n=88), and nature of the relation between the mRNA and protein levels of NDRG3 was not explored.
In the present study, we found NDRG3 protein expression was significantly associated with poor survival and other unfavourable clinicopathologic factors in patients with IBC. We suggest additional studies be conducted to determine the functional consequences of NDRG3 protein expression in BC.
Acknowledgements
This study was supported by the Medical Research Center Program (2015R1A5A2009124) through the National Research Foundation of Korea (NRF), Ministry of Science, ICT and Future Planning.
Disclosure of conflict of interest
None.
References
- 1.Kang SY, Kim YS, Kim Z, Kim HY, Lee SK, Jung KW, Youn HJ Korean Breast Cancer Society. Basic findings regarding breast cancer in korea in 2015: data from a breast cancer registry. J Breast Cancer. 2018;21:1–10. doi: 10.4048/jbc.2018.21.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Melotte V, Qu X, Ongenaert M, van Criekinge W, de Bruine AP, Baldwin HS, van Engeland M. The N-myc downstream regulated gene (NDRG) family: diverse functions, multiple applications. FASEB J. 2010;24:4153–4166. doi: 10.1096/fj.09-151464. [DOI] [PubMed] [Google Scholar]
- 3.Qu X, Zhai Y, Wei H, Zhang C, Xing G, Yu Y, He F. Characterization and expression of three novel differentiation-related genes belong to the human NDRG gene family. Mol Cell Biochem. 2002;229:35–44. doi: 10.1023/a:1017934810825. [DOI] [PubMed] [Google Scholar]
- 4.Zhou RH, Kokame K, Tsukamoto Y, Yutani C, Kato H, Miyata T. Characterization of the human NDRG gene family: a newly identified member, NDRG4, is specifically expressed in brain and heart. Genomics. 2001;73:86–97. doi: 10.1006/geno.2000.6496. [DOI] [PubMed] [Google Scholar]
- 5.Zhao W, Tang R, Huang Y, Wang W, Zhou Z, Gu S, Dai J, Ying K, Xie Y, Mao Y. Cloning and expression pattern of the human NDRG3 gene. Biochim Biophys Acta. 2001;1519:134–138. doi: 10.1016/s0167-4781(01)00210-x. [DOI] [PubMed] [Google Scholar]
- 6.Lee GY, Chun YS, Shin HW, Park JW. Potential role of the N-MYC downstream-regulated gene family in reprogramming cancer metabolism under hypoxia. Oncotarget. 2016;7:57442–57451. doi: 10.18632/oncotarget.10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun B, Chu D, Li W, Chu X, Li Y, Wei D, Li H. Decreased expression of NDRG1 in glioma is related to tumor progression and survival of patients. J Neurooncol. 2009;94:213–219. doi: 10.1007/s11060-009-9859-7. [DOI] [PubMed] [Google Scholar]
- 8.Maruyama Y, Ono M, Kawahara A, Yokoyama T, Basaki Y, Kage M, Aoyagi S, Kinoshita H, Kuwano M. Tumor growth suppression in pancreatic cancer by a putative metastasis suppressor gene Cap43/NDRG1/Drg-1 through modulation of angiogenesis. Cancer Res. 2006;66:6233–6242. doi: 10.1158/0008-5472.CAN-06-0183. [DOI] [PubMed] [Google Scholar]
- 9.Ando T, Ishiguro H, Kimura M, Mitsui A, Kurehara H, Sugito N, Tomoda K, Mori R, Takashima N, Ogawa R, Fujii Y, Kuwabara Y. Decreased expression of NDRG1 is correlated with tumor progression and poor prognosis in patients with esophageal squamous cell carcinoma. Dis Esophagus. 2006;19:454–458. doi: 10.1111/j.1442-2050.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- 10.Guan RJ, Ford HL, Fu Y, Li Y, Shaw LM, Pardee AB. Drg-1 as a differentiation-related, putative metastatic suppressor gene in human colon cancer. Cancer Res. 2000;60:749–755. [PubMed] [Google Scholar]
- 11.Bandyopadhyay S, Pai SK, Gross SC, Hirota S, Hosobe S, Miura K, Saito K, Commes T, Hayashi S, Watabe M, Watabe K. The Drg-1 gene suppresses tumor metastasis in prostate cancer. Cancer Res. 2003;63:1731–1736. [PubMed] [Google Scholar]
- 12.Koshiji M, Kumamoto K, Morimura K, Utsumi Y, Aizawa M, Hoshino M, Ohki S, Takenoshita S, Costa M, Commes T, Piquemal D, Harris CC, Tchou-Wong KM. Correlation of N-myc downstream-regulated gene 1 expression with clinical outcomes of colorectal cancer patients of different race/ethnicity. World J Gastroenterol. 2007;13:2803–2810. doi: 10.3748/wjg.v13.i20.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah MA, Kemeny N, Hummer A, Drobnjak M, Motwani M, Cordon-Cardo C, Gonen M, Schwartz GK. Drg1 expression in 131 colorectal liver metastases: correlation with clinical variables and patient outcomes. Clin Cancer Res. 2005;11:3296–3302. doi: 10.1158/1078-0432.CCR-04-2417. [DOI] [PubMed] [Google Scholar]
- 14.Bandyopadhyay S, Pai SK, Hirota S, Hosobe S, Takano Y, Saito K, Piquemal D, Commes T, Watabe M, Gross SC, Wang Y, Ran S, Watabe K. Role of the putative tumor metastasis suppressor gene Drg-1 in breast cancer progression. Oncogene. 2004;23:5675–5681. doi: 10.1038/sj.onc.1207734. [DOI] [PubMed] [Google Scholar]
- 15.Nishio S, Ushijima K, Tsuda N, Takemoto S, Kawano K, Yamaguchi T, Nishida N, Kakuma T, Tsuda H, Kasamatsu T, Sasajima Y, Kage M, Kuwano M, Kamura T. Cap43/NDRG1/Drg-1 is a molecular target for angiogenesis and a prognostic indicator in cervical adenocarcinoma. Cancer Lett. 2008;264:36–43. doi: 10.1016/j.canlet.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 16.Chua MS, Sun H, Cheung ST, Mason V, Higgins J, Ross DT, Fan ST, So S. Overexpression of NDRG1 is an indicator of poor prognosis in hepatocellular carcinoma. Mod Pathol. 2007;20:76–83. doi: 10.1038/modpathol.3800711. [DOI] [PubMed] [Google Scholar]
- 17.Yan X, Chua MS, Sun H, So S. N-Myc down-regulated gene 1 mediates proliferation, invasion, and apoptosis of hepatocellular carcinoma cells. Cancer Lett. 2008;262:133–142. doi: 10.1016/j.canlet.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 18.Akiba J, Ogasawara S, Kawahara A, Nishida N, Sanada S, Moriya F, Kuwano M, Nakashima O, Yano H. N-myc downstream regulated gene 1 (NDRG1)/Cap43 enhances portal vein invasion and intrahepatic metastasis in human hepatocellular carcinoma. Oncol Rep. 2008;20:1329–1335. [PubMed] [Google Scholar]
- 19.Chu D, Zhang Z, Li Y, Wu L, Zhang J, Wang W, Zhang J. Prediction of colorectal cancer relapse and prognosis by tissue mRNA levels of NDRG2. Mol Cancer Ther. 2011;10:47–56. doi: 10.1158/1535-7163.MCT-10-0614. [DOI] [PubMed] [Google Scholar]
- 20.Yamamura A, Miura K, Karasawa H, Morishita K, Abe K, Mizuguchi Y, Saiki Y, Fukushige S, Kaneko N, Sase T, Nagase H, Sunamura M, Motoi F, Egawa S, Shibata C, Unno M, Sasaki I, Horii A. Suppressed expression of NDRG2 correlates with poor prognosis in pancreatic cancer. Biochem Biophys Res Commun. 2013;441:102–107. doi: 10.1016/j.bbrc.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 21.Lorentzen A, Vogel LK, Lewinsky RH, Saebo M, Skjelbred CF, Godiksen S, Hoff G, Tveit KM, Lothe IM, Ikdahl T, Kure EH, Mitchelmore C. Expression of NDRG2 is down-regulated in high-risk adenomas and colorectal carcinoma. BMC Cancer. 2007;7:192. doi: 10.1186/1471-2407-7-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi SC, Yoon SR, Park YP, Song EY, Kim JW, Kim WH, Yang Y, Lim JS, Lee HG. Expression of NDRG2 is related to tumor progression and survival of gastric cancer patients through Fas-mediated cell death. Exp Mol Med. 2007;39:705–714. doi: 10.1038/emm.2007.77. [DOI] [PubMed] [Google Scholar]
- 23.Li W, Chu D, Chu X, Meng F, Wei D, Li H, Sun B. Decreased expression of NDRG2 is related to poor overall survival in patients with glioma. J Clin Neurosci. 2011;18:1534–1537. doi: 10.1016/j.jocn.2010.12.032. [DOI] [PubMed] [Google Scholar]
- 24.Liang ZL, Kang K, Yoon S, Huang SM, Lim JS, Kim JM, Lim JS, Lee HJ. NDRG2 is involved in the oncogenic properties of renal cell carcinoma and its loss is a novel independent poor prognostic factor after nephrectomy. Ann Surg Oncol. 2012;19:2763–2772. doi: 10.1245/s10434-011-2204-3. [DOI] [PubMed] [Google Scholar]
- 25.Ling ZQ, Ge MH, Lu XX, Han J, Wu YC, Liu X, Zhu X, Hong LL. Ndrg2 promoter hypermethylation triggered by helicobacter pylori infection correlates with poor patients survival in human gastric carcinoma. Oncotarget. 2015;6:8210–8225. doi: 10.18632/oncotarget.3601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee DC, Sohn HA, Park ZY, Oh S, Kang YK, Lee KM, Kang M, Jang YJ, Yang SJ, Hong YK, Noh H, Kim JA, Kim DJ, Bae KH, Kim DM, Chung SJ, Yoo HS, Yu DY, Park KC, Yeom YI. A lactate-induced response to hypoxia. Cell. 2015;161:595–609. doi: 10.1016/j.cell.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 27.Jubb AM, Buffa FM, Harris AL. Assessment of tumor hypoxia for prediction of response to therapy and cancer prognosis. J Cell Mol Med. 2010;14:18–29. doi: 10.1111/j.1582-4934.2009.00944.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Semenza GL. Intratumoral hypoxia, radiation resistance, and HIF-1. Cancer Cell. 2004;5:405–406. doi: 10.1016/s1535-6108(04)00118-7. [DOI] [PubMed] [Google Scholar]
- 29.Wang W, Li Y, Li Y, Hong A, Wang J, Lin B, Li R. NDRG3 is an androgen regulated and prostate enriched gene that promotes in vitro and in vivo prostate cancer cell growth. Int J Cancer. 2009;124:521–530. doi: 10.1002/ijc.23961. [DOI] [PubMed] [Google Scholar]
- 30.Jing JS, Li H, Wang SC, Ma JM, Yu LQ, Zhou H. NDRG3 overexpression is associated with a poor prognosis in patients with hepatocellular carcinoma. Biosci Rep. 2018;38 doi: 10.1042/BSR20180907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Luo X, Hou N, Chen X, Xu Z, Xu J, Wang L, Yang S, Liu S, Xu L, Chen Y, Xiong L, Wang J, Fan W, Xu J. High expression of NDRG3 associates with unfavorable overall survival in non-small cell lung cancer. Cancer Biomark. 2018;21:461–469. doi: 10.3233/CBM-170711. [DOI] [PubMed] [Google Scholar]
- 32.Ren GF, Tang L, Yang AQ, Jiang WW, Huang YM. Prognostic impact of NDRG2 and NDRG3 in prostate cancer patients undergoing radical prostatectomy. Histol Histopathol. 2014;29:535–542. doi: 10.14670/HH-29.10.535. [DOI] [PubMed] [Google Scholar]
- 33.Ma J, Liu S, Zhang W, Zhang F, Wang S, Wu L, Yan R, Wu L, Wang C, Zha Z, Sun J. High expression of NDRG3 associates with positive lymph node metastasis and unfavourable overall survival in laryngeal squamous cell carcinoma. Pathology. 2016;48:691–696. doi: 10.1016/j.pathol.2016.08.005. [DOI] [PubMed] [Google Scholar]
- 34.Choi JE, Kang SH, Lee SJ, Bae YK. Androgen receptor expression predicts decreased survival in early stage triple-negative breast cancer. Ann Surg Oncol. 2015;22:82–89. doi: 10.1245/s10434-014-3984-z. [DOI] [PubMed] [Google Scholar]
- 35.Hammond ME, Hayes DF, Dowsett M, Allred DC, Hagerty KL, Badve S, Fitzgibbons PL, Francis G, Goldstein NS, Hayes M, Hicks DG, Lester S, Love R, Mangu PB, McShane L, Miller K, Osborne CK, Paik S, Perlmutter J, Rhodes A, Sasano H, Schwartz JN, Sweep FC, Taube S, Torlakovic EE, Valenstein P, Viale G, Visscher D, Wheeler T, Williams RB, Wittliff JL, Wolff AC. American society of clinical oncology/college of American pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010;28:2784–2795. doi: 10.1200/JCO.2009.25.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, Bartlett JM, Bilous M, Fitzgibbons P, Hanna W, Jenkins RB, Mangu PB, Paik S, Perez EA, Press MF, Spears PA, Vance GH, Viale G, Hayes DF American Society of Clinical Oncology; College of American Pathologists. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of clinical oncology/college of American pathologists clinical practice guideline update. J. Clin. Oncol. 2013;31:3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 37.Li T, Sun R, Lu M, Chang J, Meng X, Wu H. NDRG3 facilitates colorectal cancer metastasis through activating Src phosphorylation. Onco Targets Ther. 2018;11:2843–2852. doi: 10.2147/OTT.S156814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Estiar MA, Zare AA, Esmaeili R, Farahmand L, Fazilaty H, Jafari D, Samadi T, Majidzadeh-A K. Clinical significance of NDRG3 in patients with breast cancer. Future Oncol. 2017;13:961–969. doi: 10.2217/fon-2016-0457. [DOI] [PubMed] [Google Scholar]


