Abstract
The metastasis of cancer to the pituitary gland is uncommon but may mimic a typical pituitary adenoma. Such cancers often derive from the breasts or the lungs, or very rarely from thyroid carcinoma. We described the case of a 60-year-old woman who presented with headaches and pain to the right eye. CT and MRI scans of the head revealed a sellar mass compressing the optic chiasm and invading the cavernous sinus. Her serum prolactin level was 1720 mIU/L. The preoperative diagnosis was pituitary adenoma. An endoscopic endonasal transsphenoidal approach was used to remove the tumor. Immunohistochemistry revealed immunoreactivity for thyroid transcription factor-1, CK-7, TG, supporting the diagnosis of a metastatic thyroid carcinoma. The rechecked thyroid ultrasonography showed a nodus with calcification on the left side. A total thyroidectomy was performed with a final histopathological diagnosis of minimally invasive follicular thyroid carcinoma. She was advised to take 131I treatment. The relevant literature is reviewed in light of this unusual case, illustrating the problems in the diagnosis and management of this type of patient.
Keywords: Pituitary metastasis, thyroid carcinoma, minimally invasive follicular thyroid carcinoma
Introduction
Metastasis to the pituitary gland (MP) is an infrequent clinical problem reported in 0.14-28.1% of all brain metastases in autopsy series [1,2]. Breast and lung cancer are the most common primary neoplasms metastasizing to the pituitary [3]. Metastasis to the pituitary and sella region from thyroid carcinoma is very rare, with only 24 cases reported in the literature. The diagnosis is difficult, because the majority of the MPs are clinically silent. Even when symptomatic, metastatic tumors cannot be reliably distinguished from primary sellar tumors on the basis of the clinical and radiographic presentation. They may mimic a pituitary adenoma. Here, we present a case of a minimally invasive follicular thyroid carcinoma in which the absence of a known malignant background confused the diagnosis.
Case report
A 60-year-old woman presented with headaches and pain to the right eye. A CT scan of the head revealed a sellar mass (Figure 1A). An MRI showed the 2.5-cm lesion, contrast-enhanced, isointense on T1WI and T2WI (Figure 1B), compressing the optic chiasm (Figure 1C, 1D) and invading the cavernous sinus. Routine laboratory tests showed a mild elevation of serum prolactin (PRL) to 1720 mIU/ml (normal level, 131-647 mIU/ml) and thyroglobulin (TG) to 833.7 ng/ml (normal level, 1.4-78 ng/ml). The preoperative diagnosis was pituitary adenoma. The endoscopic endonasal transsphenoidal approach was used to remove the tumor. The tumor was solid and fibrous. On microscopic analysis, no pituitary adenoma cells were found. Instead, there was a follicular thyroid tumor. An immunohistochemical analysis revealed a diffuse expression of thyroglobulin (TG), cytokeratin-7 (CK-7), and thyroid transcription factor-1 (TTF-1) (Figure 2). The rechecked thyroid ultrasonography showed a nodus with calcification in the left gland. A total thyroidectomy was performed with the final histopathological diagnosis of minimally invasive follicular thyroid carcinoma. The capsule was infiltrated, and there was extensive tumor embolus in the capsule vascular (Figure 3). Postoperative routine laboratory tests showed serum PRL had decreased to 161 mIU/ml and TG had decreased to 44.1 ng/ml. The patient was advised to take 131I treatment and thyrotropin-suppressive therapy with levothyroxine.
Figure 1.
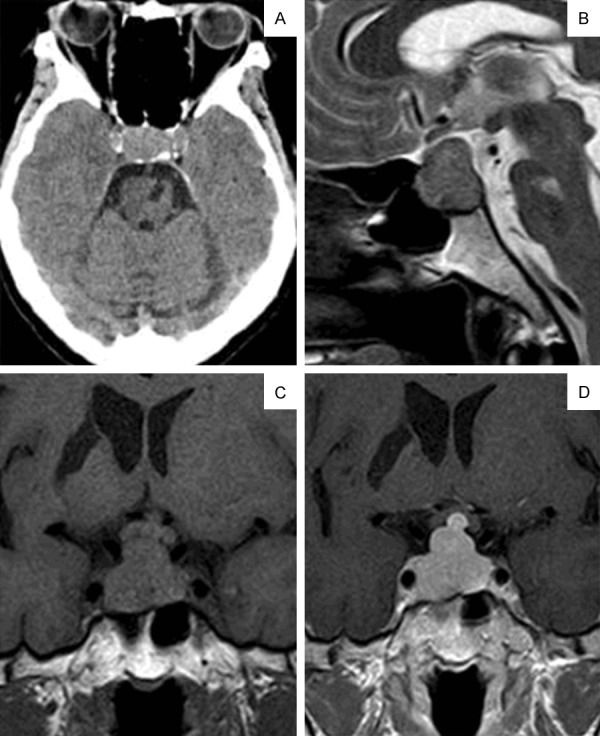
Axial CT image shows the localized pituitary lesion that eroded the bone and invaded the cavernous sinus (A). The mass extended to the suprasellar and showed up as isointense on the T2-weighted sagittal MRI image (B). Pre-(C) and post-(D) contrast images showed the mass oppressing the optic chiasm. The mass was homogeneously enhanced with Gd-DTPA.
Figure 2.
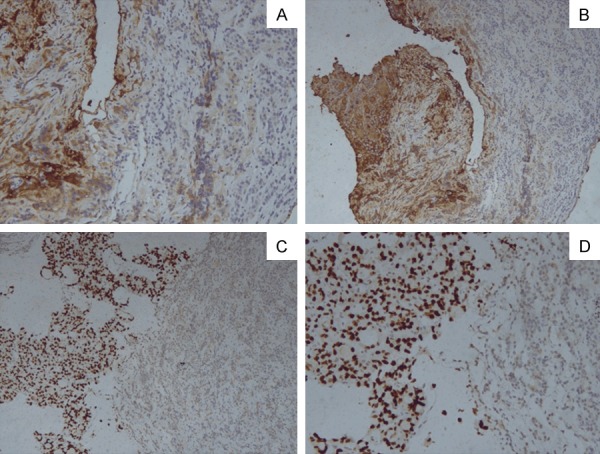
Pathological sections of immunohistochemistry of the pituitary show normal pituitary adenoma and the metastasis from thyroid carcinoma. (A -TG × 100, B -TG × 200, C -TTF1 × 100, D -TTF1 × 200).
Figure 3.
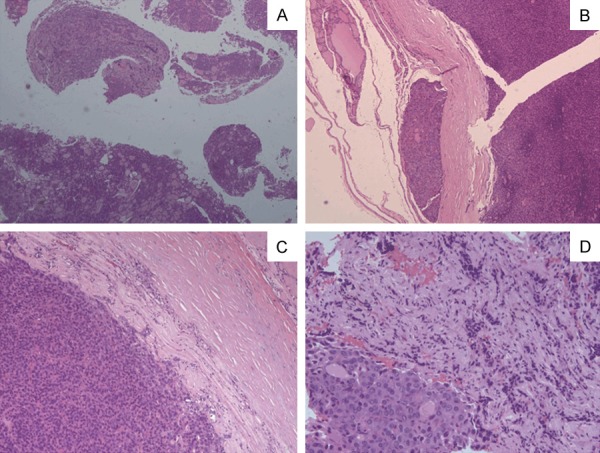
Pathological sections of HE staining of the thyroid show follicular thyroid carcinoma. The capsules and the blood vessels are invaded by the follicular epithelial tumor (B). (A × 25, B × 50, C × 100, D × 200).
Discussion
The pituitary gland is an uncommon site for metastasis. To date, only 24 cases of metastatic thyroid carcinomas involving the sella region have been reported. Many patients with this condition have a history of known primary thyroid carcinoma. However, when there is no known malignant background, the diagnosis may be confused.
The most common symptom seems to be diabetes insipidus, reflecting a predominance of metastasis to the posterior lobe. However, unlike pituitary adenomas, the metastases are likely to be invasive and often produce visual deterioration and painful ophthalmoplegia due to the involvement of chiasma and cavernous sinus, respectively [2].
Hyperprolactinemia is rare and is often attributed to stalk compression. The degree of hyperprolactinemia is important for the differential diagnosis because PRL levels above 4240 mIU/ml are generally considered as indicative of a prolactinoma. In our case, the high PRL levels due to the stalk section effect.
Radiological evaluation generally has not been fruitful in distinguishing MP of thyroid carcinoma from adenomas. The MRI may show up as isointense or hypo-intense on T1-weighted and isointense or hyper-intense on T2-weighted images, with the usual homogeneous enhancement. However, these characteristics can also occur with adenomas. Schubiger and Haller [4] reported that the invasion of the infundibular recess by a suprasellar mass favors MP, because suprasellar adenomas usually push it posteriorly.
However, the correct diagnosis was set on the histology review. The immunohistochemical analysis of the tumor should be regarded as imperative before the primary tumor is identified. The immunohistochemistry of our patient revealed diffuse staining for Tg, confirming the diagnosis of metastatic thyroid carcinoma. Diagnostic accuracy may be enhanced by combining the assessment of CK-7 and TTF-1 [5].
Treatment of these patients is still controversial. The alternatives include biopsy or extensive surgery and radioiodine or external radiotherapy and may depend on the symptoms and the extent of the systemic disease. As in our case, there was no sign of MP of thyroid carcinoma before the endoscopic transsphenoidal surgery. Total resection is the goal of the surgery. After the histology diagnosis is done, a comprehensive treatment strategy (total thyroidectomy, 131I radioiodine and thyrotropin-suppressive therapy) may be helpful.
In this case we noted that the patient initially was treated for pituitary adenoma, but then based on the histology of the resected tumor proved to be minimally invasive follicular thyroid carcinoma. Such lesions are very rare and can be misdiagnosed, so a high level of suspicion for thyroid metastasis should always be maintained.
Conclusion
MP from thyroid cancer is rare, but many patients with this condition have a history of known primary thyroid carcinoma. This case showed that a localized pituitary lesion may be metastatic thyroid cancer. Although the number of cases is small, an awareness of MP is of clinical importance, as the condition might be incorrectly diagnosed. Establishing an early diagnosis of PM is of paramount importance and represents the most significant means of offering a chance of controlling this disease.
Acknowledgements
We gratefully appreciate the kind collaboration in the Department of Pathology in Zhongshan Hospital for the identification of the pathological diagnoses. This work was supported by the Foundation of China Ministry of Science and Technology (2016YFC0106103).
Consent for publication was obtained from the patient and her legal guardian (the patient’s spouse in this case).
Disclosure of conflict of interest
None.
References
- 1.Sioutos P, Yen V, Arbit E. Pituitary gland metastases. Ann Surg Oncol. 1996;3:94–99. doi: 10.1007/BF02409058. [DOI] [PubMed] [Google Scholar]
- 2.McCormick PC, Post KD, Kandji AD, Hays AP. Metastatic carcinoma to the pituitary gland. Br J Neurosurg. 1989;3:71–79. doi: 10.3109/02688698909001028. [DOI] [PubMed] [Google Scholar]
- 3.Aaberg TM Jr, Kay M, Sternau L. Metastatic tumors to the pituitary. Am J Ophthalmol. 1995;119:779–785. doi: 10.1016/s0002-9394(14)72785-0. [DOI] [PubMed] [Google Scholar]
- 4.Schubiger O, Haller D. Metastases to the pituitary-hypothalamic axis. An MR study of 7 symptomatic patients. Neuroradiology. 1992;34:131–134. doi: 10.1007/BF00588159. [DOI] [PubMed] [Google Scholar]
- 5.Fischer SL, Asa S. Application of immunohistochemistry to thyroid neoplasms. Arch Pathol Lab Med. 2008;132:359–372. doi: 10.5858/2008-132-359-AOITTN. [DOI] [PubMed] [Google Scholar]


