Abstract
Prostate cancer is one of the most common malignancies in men worldwide. Altered expression of ARHGAP10, a member of the Rho GTPase activating protein (RhoGAP) family, has been found in several human cancers. However, its clinical significance in prostate cancer remains unknown. In the current study, we found that mRNA levels of ARHGAP10 were significantly higher in prostate cancer tissues than in the non-cancerous controls. Gene set enrichment analysis (GSEA) revealed that ARHGAP10 expression was negatively correlated with the Wnt signaling pathway. Immunohistochemical staining results showed that 62.2% (56/90) and 65.5% (59/90) of prostate cancer tissues displayed low expression of ARHGAP10 and high expression of β-catenin, respectively. ARHGAP10 protein expression was significantly correlated with histologic grade (P < 0.0001), tumor stage (P = 0.0298), preoperative prostate specific antigen level (P = 0.0261), vital status (P = 0.0017) and β-catenin expression (P < 0.0001). Kaplan-Meier survival analysis indicated that patients with low levels of ARHGAP10 and high levels of β-catenin had poor overall survival. Multivariate analyses revealed that ARHGAP10 and β-catenin expression was independent prognostic factor for prostate cancer. In summary, the current study suggests that ARHGAP10 in association with β-catenin may play a role in the development of prostate cancer and serve as a prognostic factor for this disease.
Keywords: ARHGAP10, β-catenin, prognosis, prostate cancer
Introduction
Prostate cancer, as one of the most commonly diagnosed cancers, is the second leading cause of cancer mortality in men globally. Its incidence is predicted to increase in the next few years based on current trends [1]. Prostate specific antigen (PSA) testing is widely used method for screening prostate cancer. However, several limitations exist, including the lack of a valid threshold to distinguish between prostate cancer and benign lesions, or between aggressive prostate cancer and indolent disease [2,3]. Therefore, novel prognostic factors for prostate cancer are needed.
ARHGAP10 (also named as ARHGAP21), belonging to the Rho GTPase activating protein (RhoGAP) family, contains a RhoGAP domain, a PDZ (PSD-95/Dlg/ZO-1) domain, and a PH (pleckstrin homology) domain [4]. ARHGAP10 can terminate Rho GTPases (including Cdc42 [5], RhoA and RhoC [6])-mediated signaling pathways by stimulating the conversion of active (GTP-bound) Rho GTPases to the inactive (GDP-bound) form. Previous studies have suggested that ARHGAP10 plays important roles in vesicular trafficking of Golgi membranes, cell junction formation and stress fiber formation trough binding to ARF1 (ADP-ribosylation factor 1) [5], α-catenin [7], FAK (focal adhesion kinase) and β-arrestin 1 [8], respectively. Recent studies have revealed an association of ARHGAP10 to various human malignancies. ARHGAP10 is overexpressed in head and neck squamous cell carcinoma [9]. In contrast, ARHGAP10 expression is reduced in ovarian cancer, and its decreased expression was associated with poor survival of ovarian cancer patients [10]. Reduced expression of ARHGAP10 was also observed in lung cancer [11]. As for prostate cancer, ARHGAP10 is reported to affect the proliferation and migration of PC3, an aggressive prostate cancer cell line [6]. However, little is known about its clinical significance in prostate cancer.
The Wnt signaling pathway plays significant roles in normal embryonic development and disease pathogenesis [12]. β-catenin a downstream transcription factor of the Wnt signaling pathway, has been demonstrated to be involved in the development and progression of human prostate cancer [13-15]. A recent study suggested that the Wnt/β-catenin signaling pathway was negatively correlated with ARHGAP10 expression in lung cancer [11], yet no study has focused on the association between ARHGAP10 and the Wnt/β-catenin signaling pathway in prostate cancer.
In the current study, we used microarray data from Gene Expression Omnibus (GEO) and The Cancer Genome Atlas (TCGA) and found that the mRNA expression of ARHGAP10 was significantly decreased in prostate cancer after reanalysis. The negative correlation between ARHGAP10 expression and the Wnt signaling pathway was revealed by Gene set enrichment analysis (GSEA). We also tested the association between protein expression of ARHGAP10 and other clinical findings, and evaluated the potential prognostic value of ARHGAP10 and β-catenin expression.
Materials and methods
Bioinformatic analysis
Gene expression data in prostate cancer and non-cancerous tissues were downloaded from Gene Expression Omnibus (GEO) (Access id: GSE55945 [16]) and The Cancer Genome Atlas (TCGA). Student t test was employed to analyze the statistical significance of ARHGAP10 expression between prostate cancer and non-cancerous tissues. To explore pathways associated with the different ARHGAP10 expression levels in prostate cancer, Gene set enrichment analysis (GSEA) was conducted on TCGA dataset with the software GSEA v2.2.2 (www.broadinstitute.org/gsea) as previously described [11].
Patients and clinicopathologic data
Ninety patients with prostate cancer admitted between 2011 and 2014 were enrolled in the current study. Each patient had written informed consent and the use of materials and clinical information was approved by the Ethics Committee of Tongji University (code: 2011DF21). Tumors were graded according to Gleason score [16] and staged according to American Joint Committee on Cancer (AJCC) tumor-node-metastasis (TNM) system [17]. The clinical and pathologic characteristics of these patients are provided in Table 1. Tumor tissues and adjacent non-tumorous tissues were collected from all these patients were formalin-fixed and paraffin-embedded. Eight pairs of tumor tissues and adjacent non-tumorous tissues were frozen immediately and available for western blot analysis.
Table 1.
Clinicopathologic characteristics and ARHGAP10 expression (n = 90)
| Characteristic | Cases | % |
|---|---|---|
| Age (years) | ||
| < 65 | 22 | 24.4 |
| ≥ 65 | 68 | 75.6 |
| Histologic grade | ||
| Gleason score < 7 | 42 | 46.7 |
| Gleason score ≥ 7 | 48 | 53.3 |
| Tumor stage | ||
| I/II | 47 | 52.2 |
| III | 43 | 47.8 |
| Preoperative PSA (ng/mL) | ||
| ≤ 10 | 34 | 37.8 |
| > 10 | 56 | 62.2 |
| Vital status (at follow-up) | ||
| Alive | 36 | 40.0 |
| Dead | 54 | 60.0 |
| ARHGAP10 expression | ||
| Low | 56 | 62.2 |
| High | 34 | 37.8 |
| β-catenin expression | ||
| Low | 31 | 34.4 |
| High | 59 | 65.6 |
Western blot analysis
RIPA lysis buffer containing protease and phosphatase inhibitors (Beyotime, Shanghai, China) was used to extracted total protein from collected specimens. Samples with equal protein amounts were resolved by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), electroblotted onto nitrocellulose membranes (Millipore, Boston, MA, USA) and subjected to western blot analysis. The following primary antibodies were used: ARHGAP10 (1:1000 dilution, PA5-24188; Thermo Fisher Scientific, Rockford, IL, USA), β-catenin (1:5000 dilution, ab32572; Abcam, Cambridge, MA, USA) and GAPDH (1:2000 dilution, #5174; Cell Signaling, Danvers, MA, USA). The membranes were then incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (1:0000 dilution, Beyotime). Signals were visualized using the Enhanced Chemiluminescence Kit (Millipore Corp., Boston, MA) following the manufacturer’s instructions. Relative signal intensities for each band were quantified using Image J software (http://rsb.info.nih.gov/ij/), adjusted to loading control, GAPDH.
Immunohistochemical analysis
Immunohistochemical staining was performed on formalin-fixed, paraffin-embedded specimen sections (4 µm-thick). Briefly, the sections were deparaffinized in xylene and hydrated in a graded series of ethanols. Antigen retrieval was then performed using 0.01 M sodium citrate buffer (pH 6.0) in the microwave for 10 min. After cooling, the slides were treated with 3% H2O2 at room temperature for 10 minutes to quench endogenous peroxidase activity. The sections were then blocked with 5% normal blocking serum for 1 h before reacting with ARHAGP10 (1:100 dilution, ab222805; Abcam) and β-catenin antibodies (1:100 dilution, 51067-2-AP; Proteintech, Chicago, IL, USA) at 4°C overnight. After probing with HRP-conjugated secondary antibody (D-3004; Long Island Biotech., Shanghai, China), the slides were stained with diaminobenzidine tetrahydrochloride (DAB, Long Island Biotech.) and counterstained with hematoxylin. The stained tissues were scored blindly by two independent investigators. The specimens were considered as low expression if less than 30% of cells were positively stained, otherwise they were highly expression.
Statistical analysis
The statistical analyses were conducted with the Statistical Package for the Social Sciences software version 16.0 (SPSS Inc., Chicago, IL, USA). The Fisher exact test was used to analyze the significance of the associations between ARHGAP10 expression and the clinical measures. The measures with P value < 0.05 were then assessed by Multivariate Cox regression analysis. Significance level was set at 0.05.
Results
Decreased expression of ARHGAP10 mRNA in prostate cancer tissues
To define the expression of ARHGAP10 in prostate cancer, we reanalyzed microarray data from publically available datasets. Student t test with GEO (Access id: GSE55945 [18]) (Figure 1A, P < 0.05) and TCGA datasets (Figure 1B, P < 0.0001) showed a significant decrease of ARHGAP10 expression in prostate cancer tissues compared to normal prostate tissues. These results indicated that the expression of ARHGAP10 mRNA is down-regulated in prostate cancer.
Figure 1.
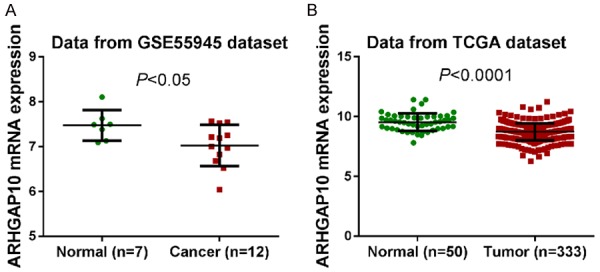
Decreased expression of ARHGAP10 mRNA in prostate cancer tissues. ARHGAP10 mRNA expression was analyzed by Student t test based on two public available datasets, GSE55945 (A) and TCGA dataset (B).
Association of ARHGAP10 and β-catenin in prostate cancer tissues
A recent study on lung cancer has shown a negative correlation between ARHGAP10 expression and the Wnt signaling pathway [11]. Similarly, we found that ARHGAP10 expression was negatively correlated with the Wnt signaling pathway by GSEA analysis in the TCGA prostate cancer dataset (Figure 2A).
Figure 2.
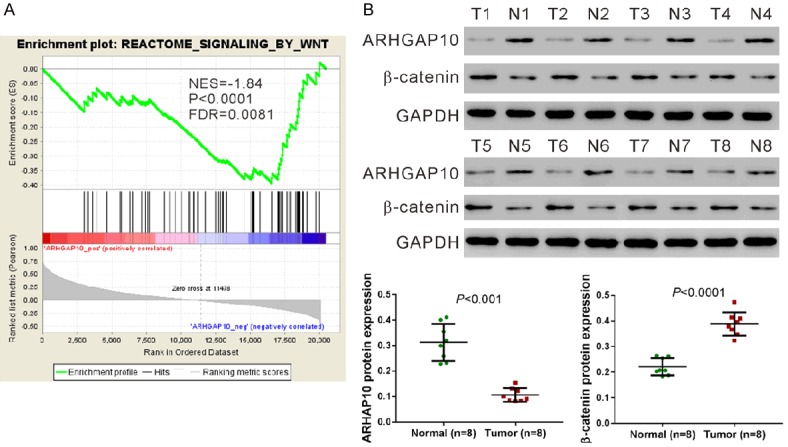
Protein expression of ARHGAP10 and β-catenin in prostate cancer tissues. A. The GSEA result showed the correlation of ARHGAP10 expression and the Wnt signaling pathways. NES, normalized enrichment score; FDR, false discovery rate. B. Protein was extracted from eight pairs of prostate cancer (T1-T8) and non-cancerous tissues (N1-N8) with ice-cold RIPA buffer. Western blotting analysis was then performed to detect the protein levels of ARHGAP10 and β-catenin, and GAPDH served as a loading control. Representative images and quantitative analysis are shown.
To validate the GSEA result at the protein level, we conducted western blot analysis to detect the protein levels of ARHGAP10 and β-catenin (an important component of the Wnt pathway) in eight pairs of prostate cancer and non-cancerous tissues (Figure 2B). ARHGAP10 protein expression was significantly lower than in the prostate cancer tissues than that in paired non-cancerous tissues (P < 0.001), while β-catenin protein expression was higher in the cancer tissues (P < 0.0001).
Down-regulation of ARHGAP10 correlates with clinical features of prostate cancer
We further detected ARHGAP10 and β-catenin expression in prostate cancer specimens from 90 patients by immunohistochemistry. Table 1 summarizes the clinical and pathologic characteristics of these patients. ARHGAP10 (Figure 3A) and β-catenin (Figure 3B) expression was observed in the cytoplasm and nucleus. Of the 90 patients, 62.2% (56 cases) and 37.8% (34 cases) showed low and high expression of ARHGAP10, respectively, while 34.4% (31 cases) and 65.5% (59 cases) showed low and high expression of β-catenin, respectively.
Figure 3.
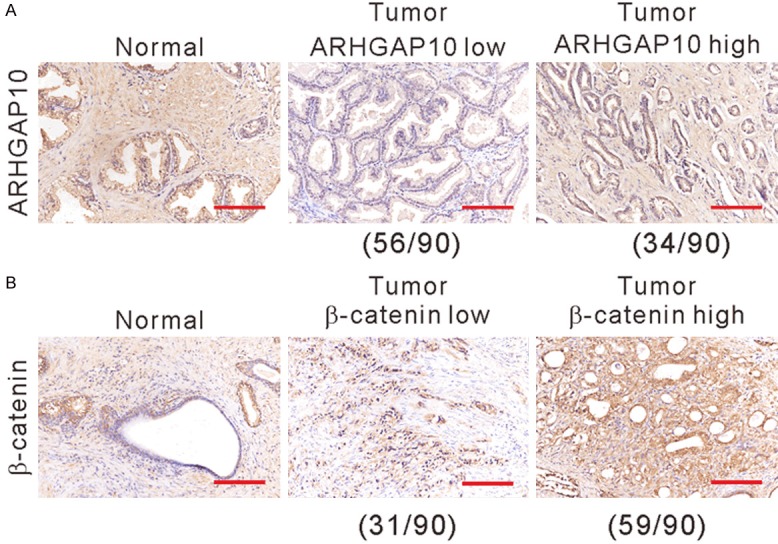
Immunohistochemical staining was conducted to detect the expression of ARHGAP10 (A) and β-catenin (B) in prostate cancer and non-cancerous tissues. Scale bars: 100 μm.
Further, Fisher exact test was carried out to analyze the correlation between ARHGAP10 expression and the clinical features of prostate cancer. As illustrated in Table 2, ARHGAP10 protein expression was significantly correlated with histologic grade (P < 0.0001), tumor stage (P = 0.0298), preoperative PSA level (P = 0.0261), vital status (P = 0.0017) and β-catenin expression (P < 0.0001), which suggested the clinical significance of ARHGAP10 in prostate cancer.
Table 2.
Correlation of ARHGAP10 expression in prostate cancer tissues with different clinicopathologic features (n = 90)
| Characteristic | ARHGAP10 | P-value | |
|---|---|---|---|
|
| |||
| Low (n = 56) | High (n = 34) | ||
| Age (years) | 0.4522 | ||
| < 65 | 12 | 10 | |
| ≥ 65 | 44 | 24 | |
| Histologic grade | < 0.0001*** | ||
| Gleason score < 7 | 17 | 25 | |
| Gleason score ≥7 | 39 | 9 | |
| Tumor stage | 0.0298* | ||
| I/II | 24 | 23 | |
| III | 32 | 11 | |
| Preoperative PSA (ng/mL) | 0.0261* | ||
| ≤ 10 | 16 | 18 | |
| > 10 | 40 | 16 | |
| Vital status (at follow-up) | 0.0017** | ||
| Alive | 15 | 21 | |
| Dead | 41 | 13 | |
| β-catenin expression | < 0.0001*** | ||
| Low | 10 | 21 | |
| High | 46 | 13 | |
Clinicopathologic features were assessed using the Fisher exact test.
P < 0.05;
P < 0.01;
P < 0.0001.
Expression of ARHGAP10 and β-catenin is closely related to poor prognosis in prostate cancer
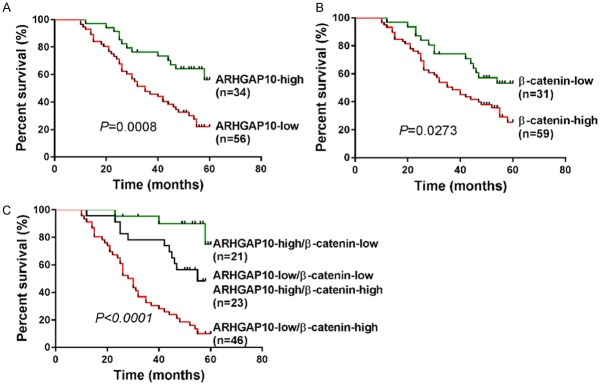
Kaplan-Meier curves followed by a log-rank test showed that low expression of ARHGAP10 (Figure 4A, P = 0.0008) and high expression of β-catenin (Figure 4B, P = 0.0273) in prostate cancer was correlated with short survival time of patients.
Figure 4.
Kaplan-Meier survival curves were generated to analyze patients in relationship to ARHGAP10 expression (A), β-catenin expression (B), and ARHGAP10 and β-catenin coexpression (C).
When both ARHGAP10 and β-catenin were analyzed (Figure 4C), patients whose tumors exhibited low expression of ARHGAP10 and high expression of β-catenin had the shortest overall survival time, whereas patients with tumors displaying high expression of ARHGAP10 and low expression of β-catenin had longest overall survival time (P < 0.0001).
Finally, a multivariate Cox regression analysis was performed. ARHGAP10 (hazard ratio, 0.393; 95% CI, 0.184-0.837; P = 0.016) and β-catenin expression (hazard ratio, 4.045; 95% CI, 1.721-9.507; P = 0.001) were independently associated with overall survival when compared with histologic grade, tumor stage, and preoperative PSA level.
Discussion
ARHGAP10 expression is increased in head and neck squamous cell carcinoma [9], but decreased in ovarian cancer [10] and lung cancer [11]. Lazarini et al. reported that ARHGAP10 knockdown increased cell migration of PC3 prostate cancer cells through its GAP activity for RhoA and RhoC, and reduced PC3 cell proliferation by the endothelin-1 pathway [6]. However, little is known about its clinical value in prostate cancer. In the current study, we found down-regulated expression of ARHGAP10 in prostate cancer specimens at both mRNA (Figure 1) and protein levels (Figure 2B), which suggests that ARHGAP10 may have diagnostic significance in prostate cancer.
Luo et al. showed that decreased expression of ARHGAP10 was correlated with poor prognosis of ovarian cancer [10]. In the current study, immunohistochemical staining was conducted to evaluate the protein expression of ARHGAP10 in 90 prostate cancer samples (Figure 3A). 62.2% of prostate cancer samples displayed low expression of ARHGAP10. By utilizing Fisher exact test, we found that ARHGAP10 expression was closely associated with histologic grade, tumor stage, preoperative PSA level, and vital status (Table 2). These data indicated the possible involvement of ARHGAP10 in the development and progression of prostate cancer.
Low frequency of mutations in the β-catenin gene (CTNNB1) has been found in prostate cancer (approximately 5%) [13]. Although controversial results have been obtained regarding altered expression of β-catenin in prostate cancer specimens, a solid link has been provided between β-catenin and prostate carcinogenesis [13-15]. In the current study, GSEA identified a negative correlation between ARHGAP10 expression and the Wnt signaling pathway in prostate cancer (Figure 2A), which was consistent with the findings in lung cancer [11]. The GSEA result was validated by western blotting (Figure 2B) and immunohistochemical staining (Figure 3B). More importantly, statistical analysis on immunohistochemical staining showed that ARHGAP10 expression negatively correlated with β-catenin expression (Table 2). Further, Kaplan-Meier analysis followed by log rank test showed a significantly worse overall survival in patients with low levels of ARHGAP10 (Figure 4A) and high levels of β-catenin (Figure 4B). Patients with low expression of ARHGAP10 and high expression of β-catenin had the worst overall survival, whereas patients with high expression of ARHGAP10 and low expression of β-catenin had the best overall survival (Figure 4C). In addition, Multivariate Cox regression analysis identified that protein levels of ARHGAP10 and β-catenin were potential prognostic factors for prostate cancer (Table 3).
Table 3.
Multivariate Cox regression of prognostic parameters for survival in 90 patients with prostate cancer
| Prognostic measure | Multivariate analysis | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | P-value | |
| ARHGAP10 expression (low vs. high) | 0.393 | 0.184-0.837 | 0.016* |
| Histologic grade (Gleason score < 7 vs. ≥ 7) | 1.499 | 0.822-2.735 | 0.187 |
| Tumor stage (I/II vs. III) | 0.823 | 0.479-1.413 | 0.480 |
| PSA level (≤ 10 ng/mL vs. > 10 ng/mL) | 1.115 | 0.622-2.00 | 0.622 |
| β-catenin expression (low vs. high) | 4.045 | 1.721-9.507 | 0.001** |
HR: Hazard ratio; CI: Confidence interval.
P < 0.05;
P < 0.01.
In sum, ARHGAP10 expression was decreased, while β-catenin expression was increased in human prostate cancer. ARHGAP10 expression negatively correlated with β-catenin expression. ARHGAP10 and β-catenin expression might be novel prognostic factors of prostate cancer although additional investigation is needed.
Acknowledgements
This work was supported by the key scientific research project of Shanghai Municipal Commission of Health and Family Planning (No. 201640014) and the project of Natural Science Foundation of Jiangxi (No. 20171BAB205019).
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.De Nunzio C, Kramer G, Marberger M, Montironi R, Nelson W, Schroder F, Sciarra A, Tubaro A. The controversial relationship between benign prostatic hyperplasia and prostate cancer: the role of inflammation. Eur Urol. 2011;60:106–117. doi: 10.1016/j.eururo.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 3.Tarhan F, Orçun A, Küçükercan I, Camursoy N, Kuyumcuoğlu U. Effect of prostatic massage on serum complexed prostate-specific antigen levels. Urology. 2005;66:1234–1238. doi: 10.1016/j.urology.2005.06.077. [DOI] [PubMed] [Google Scholar]
- 4.Basseres DS, Tizzei EV, Duarte AA, Costa FF, Saad ST. ARHGAP10, a novel human gene coding for a potentially cytoskeletal Rho-GTPase activating protein. Biochem Biophys Res Commun. 2002;294:579–585. doi: 10.1016/S0006-291X(02)00514-4. [DOI] [PubMed] [Google Scholar]
- 5.Dubois T, Paleotti O, Mironov AA, Fraisier V, Stradal TE, De Matteis MA, Franco M, Chavrier P. Golgi-localized GAP for Cdc42 functions downstream of ARF1 to control Arp2/3 complex and F-actin dynamics. Nat Cell Biol. 2005;7:353–364. doi: 10.1038/ncb1244. [DOI] [PubMed] [Google Scholar]
- 6.Lazarini M, Traina F, Machado-Neto JA, Barcellos KS, Moreira YB, Brandao MM, Verjovski-Almeida S, Ridley AJ, Saad ST. ARHGAP21 is a RhoGAP for RhoA and RhoC with a role in proliferation and migration of prostate adenocarcinoma cells. Biochim Biophys Acta. 2013;1832:365–374. doi: 10.1016/j.bbadis.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Sousa S, Cabanes D, Archambaud C, Colland F, Lemichez E, Popoff M, Boisson-Dupuis S, Gouin E, Lecuit M, Legrain P, Cossart P. ARHGAP10 is necessary for alpha-catenin recruitment at adherens junctions and for Listeria invasion. Nat Cell Biol. 2005;7:954–960. doi: 10.1038/ncb1308. [DOI] [PubMed] [Google Scholar]
- 8.Anthony DF, Sin YY, Vadrevu S, Advant N, Day JP, Byrne AM, Lynch MJ, Milligan G, Houslay MD, Baillie GS. beta-Arrestin 1 inhibits the GTPase-activating protein function of ARHGAP21, promoting activation of RhoA following angiotensin II type 1A receptor stimulation. Mol Cell Biol. 2011;31:1066–1075. doi: 10.1128/MCB.00883-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carles A, Millon R, Cromer A, Ganguli G, Lemaire F, Young J, Wasylyk C, Muller D, Schultz I, Rabouel Y, Dembele D, Zhao C, Marchal P, Ducray C, Bracco L, Abecassis J, Poch O, Wasylyk B. Head and neck squamous cell carcinoma transcriptome analysis by comprehensive validated differential display. Oncogene. 2006;25:1821–1831. doi: 10.1038/sj.onc.1209203. [DOI] [PubMed] [Google Scholar]
- 10.Luo N, Guo J, Chen L, Yang W, Qu X, Cheng Z. ARHGAP10, downregulated in ovarian cancer, suppresses tumorigenicity of ovarian cancer cells. Cell Death Dis. 2016;7:e2157. doi: 10.1038/cddis.2015.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Teng JP, Yang ZY, Zhu YM, Ni D, Zhu ZJ, Li XQ. The roles of ARHGAP10 in the proliferation, migration and invasion of lung cancer cells. Oncol Lett. 2017;14:4613–4618. doi: 10.3892/ol.2017.6729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.MacDonald BT, Tamai K, He X. Wnt/β-catenin signaling: components, mechanisms, and diseases. Dev Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chesire DR, Ewing CM, Sauvageot J, Bova GS, Isaacs WB. Detection and analysis of β-catenin mutations in prostate cancer. Prostate. 2000;45:323–334. doi: 10.1002/1097-0045(20001201)45:4<323::aid-pros7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 14.Chesire DR, Ewing CM, Gage WR, Isaacs WB. In vitro evidence for complex modes of nuclear β-catenin signaling during prostate growth and tumorigenesis. Oncogene. 2002;21:2679. doi: 10.1038/sj.onc.1205352. [DOI] [PubMed] [Google Scholar]
- 15.Verras M, Sun Z. Roles and regulation of Wnt signaling and β-catenin in prostate cancer. Cancer Lett. 2006;237:22–32. doi: 10.1016/j.canlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 16.Gleason DF, Mellinger GT. Prediction of prognosis for prostatic adenocarcinoma by combined histological grading and clinical staging. J Urol. 1974;111:58–64. doi: 10.1016/s0022-5347(17)59889-4. [DOI] [PubMed] [Google Scholar]
- 17.Edge SB, Compton CC. The American joint committee on cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–1474. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 18.Arredouani MS, Lu B, Bhasin M, Eljanne M, Yue W, Mosquera JM, Bubley GJ, Li V, Rubin MA, Libermann TA, Sanda MG. Identification of the transcription factor single-minded homologue 2 as a potential biomarker and immunotherapy target in prostate cancer. Clin Cancer Res. 2009;15:5794–5802. doi: 10.1158/1078-0432.CCR-09-0911. [DOI] [PMC free article] [PubMed] [Google Scholar]