Abstract
Osteosarcoma is one of the most common tumors of the bone in children and adolescents worldwide. The relapse and metastasis of osteosarcoma are a major therapeutic challenge. Recently, several metastasis regulators, including miRNAs, kinases, and lncRNAs, were reported in osteosarcoma. Identifying novel regulators of metastasis will be useful to explore novel biomarkers for osteosarcoma. The present study showed miR-29a overexpression significantly inhibited HOS and MG-63 cell adhesion, invasion, and migration. About 70% of the wound area was repaired by migrating cells after 24 h in the control group, and only 50% of the wound area was repaired in the miR-29a overexpression group. The numbers of invading cells were decreased by 40% and 50% in HOS and MG-63 cells transfected with miR-29a, respectively, compared with the negative control group. Moreover, the present study validated that CDC42 was a direct target of miR-29a in OS cells. In conclusion, miR-29a may serve as a therapeutic target for osteosarcoma.
Keywords: miRNA, osteosarcoma, bioinformatics analysis, expression profiling
Introduction
Osteosarcoma is one of the most common tumors of the bone in children and adolescents worldwide. The relapse and metastases of osteosarcoma are a major therapeutic challenge. Osteosarcoma often results in lung metastasis, which usually causes a low 5-year survival rate. Metastasis involves multiple steps, including migration into systemic or lymphatic vasculature, extravasion, colonization to distal sites, adaptation to the microenvironment, and proliferation [1,2]. Recently, several metastasis regulators, including miRNAs, kinases, and lncRNAs, were reported in osteosarcoma [3-7]. For example, LncRNA NEAT1 promotes osteosarcoma metastasis by binding to the G9a-DNMT1-Snail complex [8]. Identifying novel regulators of metastasis will be useful to explore novel biomarkers for osteosarcoma.
MicroRNAs, a type of small non-coding RNAs with 18-25 bps in length, have been involved in the progression of various cancers by regulating cell proliferation, apoptosis, and metastasis. miR-29a was observed to be down-regulated in prostate cancer [9], hepatocellular carcinoma [10], Burkitt lymphoma [11], and non-small cell lung cancer [12]. miR-29a functioned as a tumor suppressor by inhibiting a series of targets, such as SIRT1, IGF1R [13], CDK6 [14], and CDC42 [15]. Interestingly, several studies had reported that miR-29a was involved in cancer metastasis regulation. For example, miR-29a suppressed metastasis in papillary thyroid carcinoma by targeting AKT3 [16], inhibited cell migration and invasion by targeting Robo1 in gastric cancer [17], and inhibited prostate cancer migration and invasion by targeting LAMC1 [18]. However, the roles of miR-29a in osteosarcoma remained largely unclear.
The present study aimed to explore the roles of miR-29a in regulating osteosarcoma metastasis by using a gain of function assay. The present study hopes to provide useful information to validate whether miR-29a could serve as a therapeutic target for osteosarcoma.
Material and methods
Cell culture and transfection
HOS and MG-63 were obtained from Cell Bank of Chinese Academy of Sciences (Shanghai, China). The cell lines were confirmed by short tandem repeat (STR) analysis. The cell lines were cultured in RPMI-1640 medium (Corning, USA) supplemented with 10% FBS (PAN, German) at 37°C with 5% CO2. Mimics for miR-29a and negative control (NC) were supplied by HuaGene (China). The cell lines were seeded in a 6-well plate and cultured for 24 h. Then cells were subsequently transfected using HiPerFect (Qiagen) following the manufacturer’s instructions.
RNA extraction and quantitative RT-PCR
Total RNA for RT-qPCR was extracted using TRIeasy Total RNA Extraction Reagent TRIeasy (YeaSen, China). FastKing gDNA Dispelling RT SuperMix (TIANGEN, China) was used to Reverse transcription (RT). miR-29a-specific RT primer was supplied by GenePharma (Shanghai, China). For analysis of microRNA expression, RT-qPCR was performed using BeyoFast SYBR Green qPCR Mix (Beyotime, China) on the LightCyclerR480. The expression level of miR-29a was normalized to U6. The PCR primers for miR-29a and U6 were designed and purchased from HuaGene. The 2-ΔΔCt method was used to calculated data. The experiment was repeated at least three times.
Cell adhesion assay
Matrigel was formulated as a 0.04 ug/ul artificial basement membrane gel using serum-free medium RPMI-1640. 2 ug Matrigel/well was placed in a 96-well plate and then air-dried overnight in a super-clean bench; 100 ul serum-free RPMI-1640 and 5000 cells were mixed, then the cells were seeded in 96-well plates and incubated for 2 hours, then the cells adhered to Matrigel were washed twice in PBS, fixed in methanol for 10 minutes, then stained with DAPI, and observed under a fluorescent microscope (Olympus, Japan) to count the number of adherent cells. Each experiment was repeated at least three times.
Wound scratch assay
A wound scratch assay was used to detect cell migration. Briefly, HOS and MG-63 cells were plated on 6-well plates at a density of 2×105 cells/well. Scratches were performed with a sterile 10-μl pipette tip. Then, the cells were washed twice with PBS. The images of the wells were captured after 0, 12, and 24 hours using an inverted microscope. All experiments were repeated three times.
Cell invasion assay
A cell invasion assay was performed using Transwell plates (8-mm pore size, Corning, USA) with Matrigel (BD, USA). Briefly, 200 μl Matrigel was mixed with 1 mL RPMI-1640, then 100 μl mixture was added into each upper chamber of the system. Then the upper chamber of the system was placed in a cell culture incubator overnight to solidify Matrigel. Cells (6,000 cells/well) were placed in the upper chamber of the system incubated with RPMI-1640. The bottom hole was filled with RPMI-1640 supplemented with 10% FBS. Then the transwell system was incubated at 37°C with 5% CO2 for 24 hours. Then the uninvaded cells in the upper chamber were removed, and invaded cells were stained with DAPI and observed by a fluorescent microscope (Olympus, Japan). Each experiment was repeated at least three times.
Western blot analysis
We placed the cell culture dish on ice 48 hours after transfection and washed the cells with ice-cold PBS. PBS was aspirated, after which was added 0.5 mL ice-cold lysis buffer, and the cell suspension was collected. Suspension was centrifuged at 12000 rpm for 10 minutes at 4°C, and the supernatant was removed. The protein content was measured by the Bradford method. Then the protein detection mixture was prepared. We boiled the mixture in boiling water for 5 minutes, immediately transferred it to ice for 5 minutes, and then centrifuged at 12,000 rpm for 1 minute at room temperature. 30 μg of protein were loaded into the wells of the 15% SDS-PAGE gel, and the gel was run for 1.5 h at 100 V. Antibody staining was done following the manufacturer’s instructions. CDC42 Antibody was purchased from Proteintech (Chicago, USA. Catalog number: 10155-1-AP).
Dual-Luciferase reporter assay
Luciferase reporter assay was performed using the Dual-Luciferase Reporter Assay System (Promega, Madison, USA) following the manufacturer’s instructions. In brief, 3’UTR of CDC42 was cloned into the vector psiCHECK2 (Promega, USA). All plasmids were verified by DNA sequencing. After 48 h transfection, GloMax 96 Microplate Luminometer was used to detect the Dual-Luciferase.
Statistical analysis
The data were presented as the mean ± standard deviation. Based on the test conditions, statistical comparisons between standardized data sets were performed using either the T-test or the Mann-Whitney U test. P<0.05 was considered significant. Each experiment was repeated at least three times.
Results
MiR-29a inhibits HOS and MG-63 cell adhesion ability
The present study evaluated the effect of miR-29a on OS metastasis. Metastasis is a complex process involved in cancer cell adhesion, migration, and invasion. We overexpressed miR-29a in HOS and MG-63 cell lines and performed cell adhesion assays. The results showed that miR-29a overexpression significantly inhibited HOS (Figure 1A and 1C) and MG-63 (Figure 1B and 1D) cell adhesion to the plates.
Figure 1.
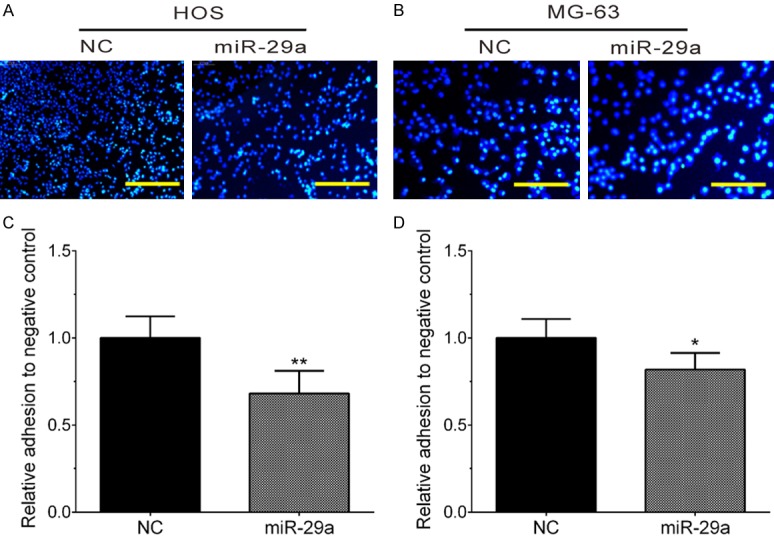
Overexpression of miR-29a overexpression significantly inhibited HOS and MG-63 cell adhesion. A, C. Overexpression of miR-29a significantly inhibited HOS adhesion. B, D. Overexpression of miR-29a significantly inhibited MG-63 adhesion. Data are presented as the mean ± SD (n=3). *, P<0.05; **, P<0.01.
MiR-29a inhibits HOS and MG-63 cell migration
Furthermore, the present study explored the role of miR-29a in regulating OS migration by using a wound-healing assay in MG-63 cell lines. As shown in Figure 2, we found about 70% of the wound area was repaired by migrating cells after 24 h in the control group, and only 50% of the wound area was repaired in miR-29a overexpression group.
Figure 2.
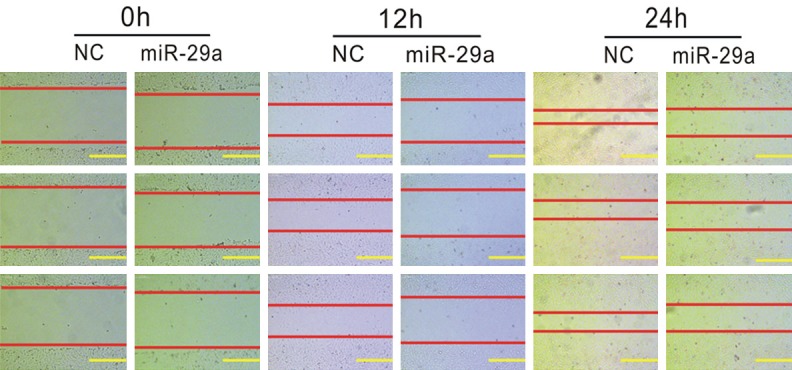
Overexpression of miR-29a inhibits MG-63 cell wound healing ability. The wound healing assay indicated that overexpression of miR-29a inhibits MG-63 cell migration ability, in contrast with the negative control group.
Next, the present study performed a transwell assay to detect the effect of miR-29a on cell migration. Our results showed that migrating cells in the miR-29a overexpression group were significantly decreased in the control group in the HOS cell line (Figure 3A and 3C). Of note, the present study observed a similar result in the MG-63 cell line (Figure 3B and 3D). These results suggested miR-29a inhibited HOS and MG-63 cell migration.
Figure 3.
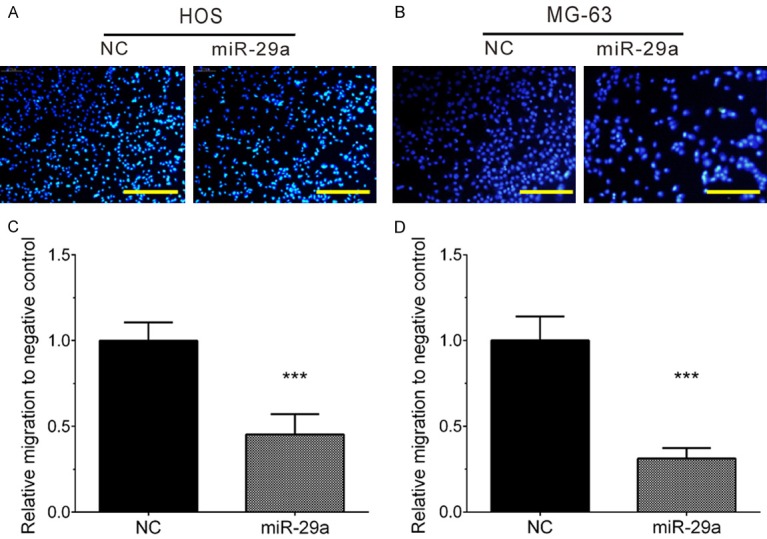
Overexpression of miR-29a inhibits HOS and MG-63 cell migration. A, C. Overexpression of miR-29a significantly inhibited HOS migration. B, D. Overexpression of miR-29a significantly inhibited MG-63 migration. Data are presented as the mean ± SD (n=3). ***, P<0.001.
MiR-29a inhibits HOS and MG-63 cell invasion
The present study also detected the invasion ability of HOS and MG-63 cells by estimating the penetration of cells through Matrigel in a transwell chamber. As illustrated in Figure 4, the present study found miR-29a significantly inhibited cell invasion in HOS (Figure 4A and 4C) and MG-63 cells (Figure 4B and 4D). The numbers of invading cells were decreased by 40% and 50% in HOS and MG-63 cells transfecting with miR-29a, respectively, compared with the negative control group.
Figure 4.
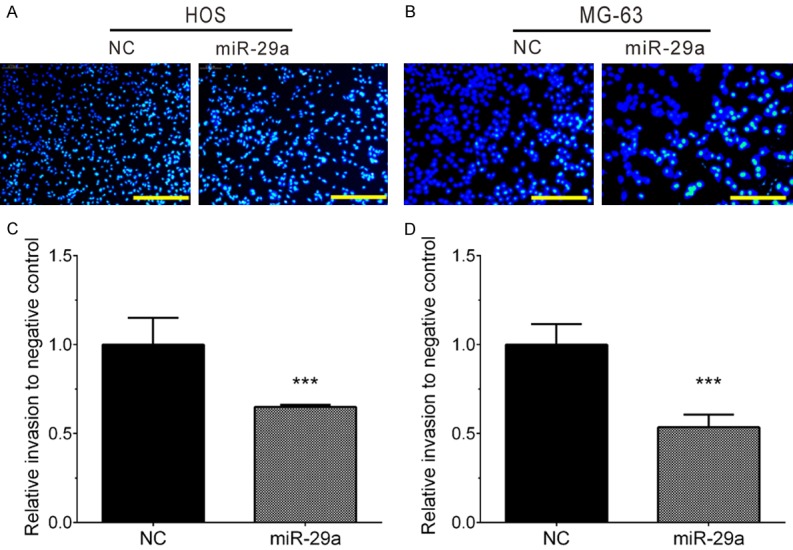
Overexpression of miR-29a significantly inhibited HOS and MG-63 cell invasion. A, C. Overexpression of miR-29a significantly inhibited HOS invasion. B, D. Overexpression of miR-29a significantly inhibited MG-63 invasion. Data are presented as the mean ± SD (n=3). ***, P<0.001.
Bioinformatics analysis revealed potential targets of miR-29a
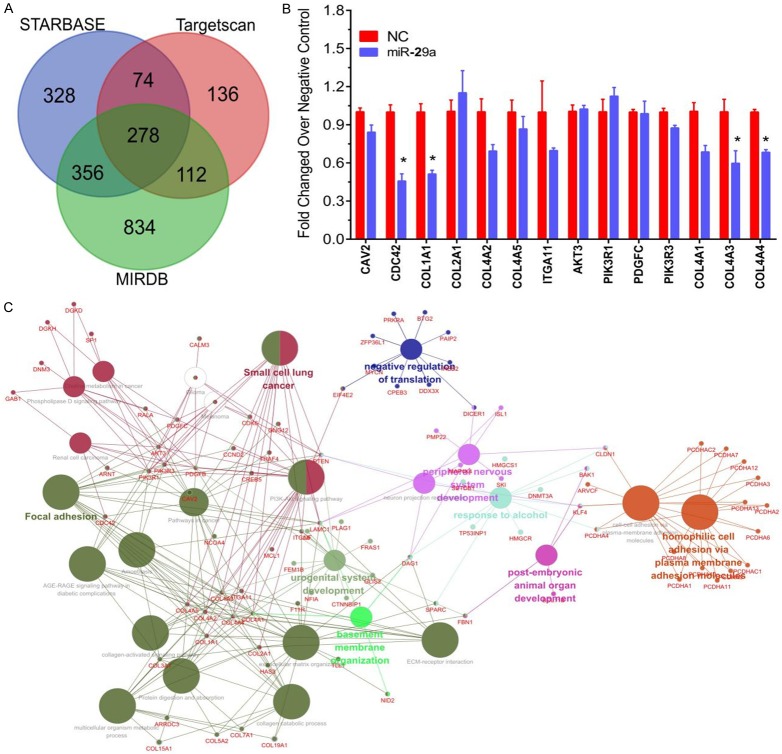
To explore the mechanisms of miR-29a underlying osteosarcoma metastasis, bioinformatics analysis was done using three online public softwares, including TARGETSCAN (http://www.targetscan.org/), STARBASE [19], and MIRDB [20,21]. A total of 278 targets were identified as the potential targets of miR-29a (Figure 5A).
Figure 5.
Analysis and identification of potential targets of miR-29a. A. The potential targets of miR-29a revealed by TARGETSCAN, STARBASE, and MIRDB. B. The molecular function of miR-29a targets predicted by ClueGO plugin in Cytoscape software. C. Overexpression of miR-29a significantly suppressed the mRNA expression of CDC42, COL1A1, COL4A3, and COL4A4.
Then ClueGO plugin in Cytoscape software was used to predict the molecular function of miR-29a targets. As shown in Figure 5B, the present study found that miR-29a was mainly involved in regulating focal adhesion, negative regulation of translation, and homophilic cell adhesion through plasma membrane adhesion molecules. The focal adhesion pathway was reported to be linked to cancer metastasis. A total of 19 genes were associated with the regulation of focal adhesion, including PDGFB, CCND2, PTEN, CAV2, CDC42, COL1A1, COL2A1, COL4A2, COL4A5, ITGA11, AKT3, PIK3R1, PDGFC, PIK3R3, COL4A1, COL4A3, COL4A4, ITGA6, and LAMC1.
The RT-qPCR assay was used to detect the effect of miR-29a on these genes’ expression. The present study found that overexpression of miR-29a significantly suppressed the expression of CDC42, COL1A1, COL4A3, and COL4A4 (Figure 5C).
CDC42 is a direct target of miR-29a in osteosarcoma cells
Previous studies had shown that CDC42 was involved in regulating cancer metastasis. The present study also revealed that CDC42 was regulated by miR-29a in osteosarcoma cells. Overexpression of miR-29a significantly suppressed CDC42 expression in both HOS and MG-63 cells (Figure 6A and 6B). Western blot analysis showed that CDC42 protein levels were down-regulated after overexpressing miR-29a (Figure 6C).
Figure 6.
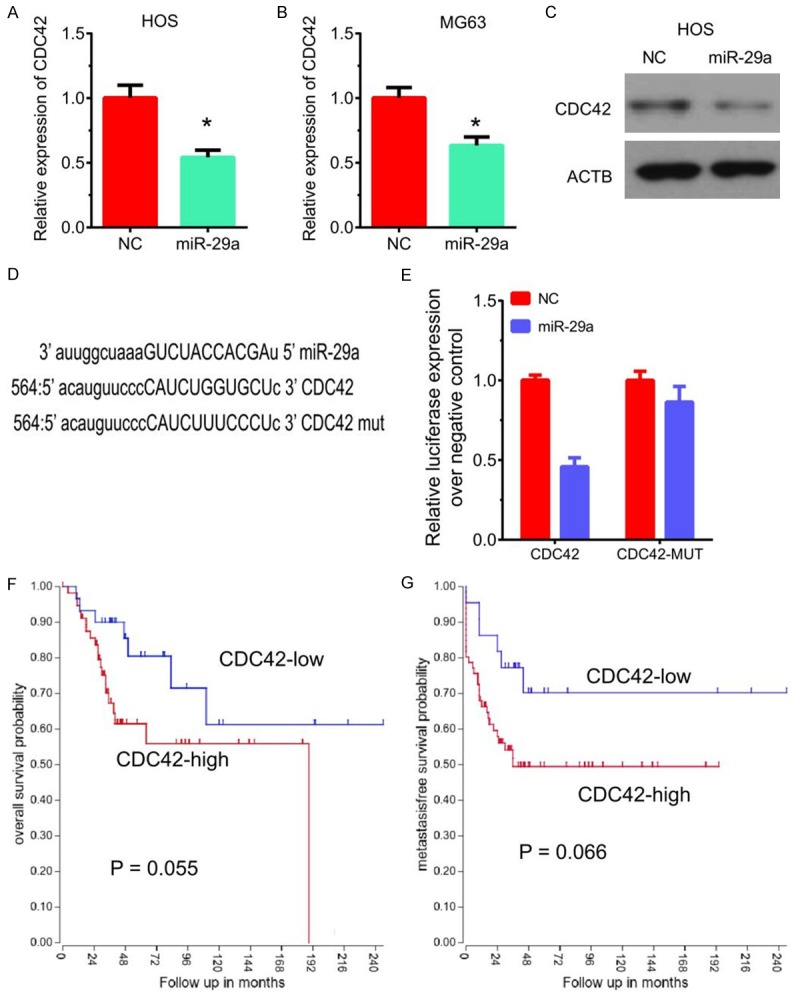
CDC42 is a direct target of miR-29a in osteosarcoma cells. (A, B) Overexpression of miR-29a significantly suppressed CDC42 mRNA expression in both HOS (A) and MG-63 (B) cells. (C) Overexpression of miR-29a significantly suppressed CDC42 protein levels in HOS cells. (D) TARGETSCAN analysis showed miR-29a could bind to 3’UTR of CDC42 at site 564. (E) Luciferase reporter assay showed that miR-29a significantly reduced the luciferase expression in HOS cells compared to NC. (F, G) Higher expression of CDC42 was weakly correlated to the shorter overall survival time (F) and metastasis-free survival time (G) in patients with osteosarcoma.
TARGETSCAN analysis showed miR-29a could bind to 3’UTR of CDC42 at site 564 (Figure 6D). Luciferase reporter assay showed that miR-29a significantly reduced the luciferase expression in HOS cells compared to NC. However, overexpression of miR-29a did not affect the luciferase expression of the CDC42 mut plasmid (Figure 6E). The results showed that miR-29a could bind to the 3’-UTR of CDC42 to downregulate its expression.
Interestingly, the present study found higher expression of CDC42 was weakly correlated to a shorter overall survival time and metastasis-free survival time in patients with osteosarcoma (Figure 6F and 6G).
Discussion
Previous studies showed that the relapse and metastases of osteosarcoma remain a therapeutic challenge. Recent reports had revealed a series of miRNAs were involved in OS metastasis regulation. For example, miR-384 [22], miR-372-3p [23] and miR-223-3p [24] were reported to inhibit cell metastasis in osteosarcoma cells. Wang et al. reported lncRNA GAS5 inhibited osteosarcoma metastasis by binding to miR-203a [25]. Of note, circular RNA circ-NT5C2 is involved in promoting osteosarcoma metastasis through sponging miR-448 [26]. The present study evaluated the roles of miR-29a in regulating osteosarcoma metastasis by using a gain of function assay and for the first time, demonstrated miR-29a was a metastasis suppressor in OS.
miRNA is an important post-transcriptional regulator of gene expression and is involved in regulating a series of biologic processes, including differentiation, proliferation, cell-cycle, apoptosis, and metabolism. MiR-29a was observed to be a tumor suppressor in many cancers, including prostate cancer, hepatocellular carcinoma, and non-small cell lung cancer by inhibiting cell proliferation, the cell cycle, and metastasis. In human gastric cancer, miR-29a inhibits cell proliferation and cell cycle progression by inhibiting p42.3 [27]. In pancreatic cancer, miR-29a was a tumor suppressor though targeting the MUC1 [28]. In prostate cancer, miR-29a promotes apoptosis by regulating KDM5B [29]. Interestingly, several studies had reported that miR-29a was involved in cancer metastasis regulation. For example, miR-29a suppressed metastasis in papillary thyroid carcinoma by targeting AKT3 [16], inhibited cell migration and invasion by targeting Robo1 in gastric cancer [17] and inhibited prostate cancer migration and invasion by targeting LAMC1 [18]. However, the role of miR-29a in osteosarcoma remained largely unclear. In this study, we explored the role of miR-29a in regulating OS adhesion, migration, and invasion. Our results showed that miR-29a overexpression significantly inhibited cell adhesion to plates. Moreover, the present study found miR-29a suppressed OS migration and invasion by using a wound-healing assay and transwell assay.
To explore the mechanism of miR-29a underlying osteosarcoma metastasis, the present study conducted bioinformatics analysis. Bioinformatics analysis revealed that miR-29a was involved in regulating focal adhesion. The focal adhesion pathway was reported to be linked to cancer metastasis. The present study found that overexpression of miR-29a significantly suppressed the expression of CDC42, COL1A1, COL4A3, and COL4A4. Moreover, CDC42 was validated as the direct target of miR-29a in osteosarcoma. Higher expression of CDC42 was correlated to the shorter overall survival time and metastasis-free survival time in patients with osteosarcoma. According to previous studies, CDC42 was suppressed by miR-29a in human gliomas, non-small cell lung cancer, and breast cancer. Here, the present study for the first time demonstrated CDC42 was also a direct target of miR-29a in osteosarcoma. CDC42 is up-regulated and correlated to the prognosis in multiple cancer types. CDC42 regulates cancer cell proliferation, cycling, and metastasis. For example, Du et al. found knockdown of CDC42 markedly inhibited the migration and invasion of gastric cancer cells [30]. These reports and our findings showed that miR-29a/CDC42 regulated osteosarcoma metastasis.
Conclusion
In conclusion, the present study demonstrates that miR-29a inhibits cell adhesion, invasion, and migration in osteosarcoma though targeting CDC42. These results provided useful information to explore whether miR-29a could serve as a target for osteosarcoma therapy. Of note, further studies are needed to clarify the mechanisms of miR-29a underlying OS progression.
Acknowledgements
This study was supported by Hubei Province Health and Family Planning Scientific Research Project (WJ2018H209).
Disclosure of conflict of interest
None.
References
- 1.Osborne TS, Khanna C. A review of the association between osteosarcoma metastasis and protein translation. J Comp Pathol. 2012;146:132–142. doi: 10.1016/j.jcpa.2011.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter J, Bielack SS. Osteosarcoma. Ann Oncol. 2010;21(Suppl 7):vii320–5. doi: 10.1093/annonc/mdq276. [DOI] [PubMed] [Google Scholar]
- 3.Kushlinskii NE, Fridman MV, Braga EA. Molecular mechanisms and microRNAs in osteosarcoma pathogenesis. Biochemistry (Mosc) 2016;81:315–328. doi: 10.1134/S0006297916040027. [DOI] [PubMed] [Google Scholar]
- 4.Ding L, Congwei L, Bei Q, Tao Y, Ruiguo W, Heze Y, Bo D, Zhihong L. mTOR: an attractive therapeutic target for osteosarcoma? Oncotarget. 2016;7:50805–50813. doi: 10.18632/oncotarget.9305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rettew AN, Getty PJ, Greenfield EM. Receptor tyrosine kinases in osteosarcoma: not just the usual suspects. Adv Exp Med Biol. 2014;804:47–66. doi: 10.1007/978-3-319-04843-7_3. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Dou P, Liu T, He S. Application of long noncoding RNAs in osteosarcoma: biomarkers and therapeutic targets. Cell Physiol Biochem. 2017;42:1407–1419. doi: 10.1159/000479205. [DOI] [PubMed] [Google Scholar]
- 7.Yang Z, Li X, Yang Y, He Z, Qu X, Zhang Y. Long noncoding RNAs in the progression, metastasis, and prognosis of osteosarcoma. Cell Death Dis. 2016;7:e2389. doi: 10.1038/cddis.2016.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Y, Cheng C. Long noncoding RNA NEAT1 promotes the metastasis of osteosarcoma via interaction with the G9a-DNMT1-Snail complex. Am J Cancer Res. 2018;8:81–90. [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Li Y, Kong D, Ahmad A, Bao B, Dyson G, Sarkar FH. Epigenetic deregulation of miR-29a and miR-1256 by isoflavone contributes to the inhibition of prostate cancer cell growth and invasion. Epigenetics. 2012;7:940–949. doi: 10.4161/epi.21236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhu XC, Dong QZ, Zhang XF, Deng B, Jia HL, Ye QH, Qin LX, Wu XZ. microRNA-29a suppresses cell proliferation by targeting SPARC in hepatocellular carcinoma. Int J Mol Med. 2012;30:1321–1326. doi: 10.3892/ijmm.2012.1140. [DOI] [PubMed] [Google Scholar]
- 11.Robaina MC, Mazzoccoli L, Arruda VO, Reis FR, Apa AG, de Rezende LM, Klumb CE. Deregulation of DNMT1, DNMT3B and miR-29s in Burkitt lymphoma suggests novel contribution for disease pathogenesis. Exp Mol Pathol. 2015;98:200–207. doi: 10.1016/j.yexmp.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 12.Fabbri M, Garzon R, Cimmino A, Liu Z, Zanesi N, Callegari E, Liu S, Alder H, Costinean S, Fernandez-Cymering C, Volinia S, Guler G, Morrison CD, Chan KK, Marcucci G, Calin GA, Huebner K, Croce CM. MicroRNA-29 family reverts aberrant methylation in lung cancer by targeting DNA methyltransferases 3A and 3B. Proc Natl Acad Sci U S A. 2007;104:15805–15810. doi: 10.1073/pnas.0707628104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang X, Zhao X, Fiskus W, Lin J, Lwin T, Rao R, Zhang Y, Chan JC, Fu K, Marquez VE, Chen-Kiang S, Moscinski LC, Seto E, Dalton WS, Wright KL, Sotomayor E, Bhalla K, Tao J. Coordinated silencing of MYC-mediated miR-29 by HDAC3 and EZH2 as a therapeutic target of histone modification in aggressive B-Cell lymphomas. Cancer Cell. 2012;22:506–523. doi: 10.1016/j.ccr.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 14.Zhao JJ, Lin J, Lwin T, Yang H, Guo J, Kong W, Dessureault S, Moscinski LC, Rezania D, Dalton WS, Sotomayor E, Tao J, Cheng JQ. microRNA expression profile and identification of miR-29 as a prognostic marker and pathogenetic factor by targeting CDK6 in mantle cell lymphoma. Blood. 2010;115:2630–2639. doi: 10.1182/blood-2009-09-243147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Park SY, Lee JH, Ha M, Nam JW, Kim VN. miR-29 miRNAs activate p53 by targeting p85 alpha and CDC42. Nat Struct Mol Biol. 2009;16:23–29. doi: 10.1038/nsmb.1533. [DOI] [PubMed] [Google Scholar]
- 16.Li R, Liu J, Li Q, Chen G, Yu X. miR-29a suppresses growth and metastasis in papillary thyroid carcinoma by targeting AKT3. Tumour Biol. 2016;37:3987–3996. doi: 10.1007/s13277-015-4165-9. [DOI] [PubMed] [Google Scholar]
- 17.Liu X, Cai J, Sun Y, Gong R, Sun D, Zhong X, Jiang S, He X, Bao E, Yang L, Li Y. MicroRNA-29a inhibits cell migration and invasion via targeting roundabout homolog 1 in gastric cancer cells. Mol Med Rep. 2015;12:3944–3950. doi: 10.3892/mmr.2015.3817. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa R, Goto Y, Kojima S, Enokida H, Chiyomaru T, Kinoshita T, Sakamoto S, Fuse M, Nakagawa M, Naya Y, Ichikawa T, Seki N. Tumor-suppressive microRNA-29s inhibit cancer cell migration and invasion via targeting LAMC1 in prostate cancer. Int J Oncol. 2014;45:401–410. doi: 10.3892/ijo.2014.2437. [DOI] [PubMed] [Google Scholar]
- 19.Li JH, Liu S, Zhou H, Qu LH, Yang JH. starBase v2.0: decoding miRNA-ceRNA, miRNA-ncRNA and protein-RNA interaction networks from large-scale CLIP-Seq data. Nucleic Acids Res. 2014;42:D92–D97. doi: 10.1093/nar/gkt1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wong N, Wang X. miRDB: an online resource for microRNA target prediction and functional annotations. Nucleic Acids Res. 2015;43:D146–D152. doi: 10.1093/nar/gku1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu W, Wang X. Prediction of functional microRNA targets by integrative modeling of microRNA binding and target expression data. Genome Biol. 2019;20:18. doi: 10.1186/s13059-019-1629-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang JF, Zhang GY, Hu XM, Luo ZP, Ma YZ. MicroRNA-384 downregulates SETD8 expression to suppress cell growth and metastasis in osteosarcoma cells. Eur Rev Med Pharmacol Sci. 2018;22:1602–1608. doi: 10.26355/eurrev_201803_14566. [DOI] [PubMed] [Google Scholar]
- 23.Xu SY, Xu PF, Gao TT. MiR-372-3p inhibits the growth and metastasis of osteosarcoma cells by targeting FXYD6. Eur Rev Med Pharmacol Sci. 2018;22:62–69. doi: 10.26355/eurrev_201801_14101. [DOI] [PubMed] [Google Scholar]
- 24.Dong J, Liu Y, Liao W, Liu R, Shi P, Wang L. miRNA-223 is a potential diagnostic and prognostic marker for osteosarcoma. J Bone Oncol. 2016;5:74–79. doi: 10.1016/j.jbo.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Kong D. LncRNA GAS5 represses osteosarcoma cells growth and metastasis via sponging MiR-203a. Cell Physiol Biochem. 2018;45:844–855. doi: 10.1159/000487178. [DOI] [PubMed] [Google Scholar]
- 26.Liu X, Zhong Y, Li J, Shan A. Circular RNA circ-NT5C2 acts as an oncogene in osteosarcoma proliferation and metastasis through targeting miR-448. Oncotarget. 2017;8:114829–114838. doi: 10.18632/oncotarget.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cui Y, Su WY, Xing J, Wang YC, Wang P, Chen XY, Shen ZY, Cao H, Lu YY, Fang JY. MiR-29a inhibits cell proliferation and induces cell cycle arrest through the downregulation of p42.3 in human gastric cancer. PLoS One. 2011;6:e25872. doi: 10.1371/journal.pone.0025872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Trehoux S, Lahdaoui F, Delpu Y, Renaud F, Leteurtre E, Torrisani J, Jonckheere N, Van Seuningen I. Micro-RNAs miR-29a and miR-330-5p function as tumor suppressors by targeting the MUC1 mucin in pancreatic cancer cells. Biochim Biophys Acta. 2015;1853:2392–2403. doi: 10.1016/j.bbamcr.2015.05.033. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Wan X, Qiang W, Li T, Huang W, Huang S, Wu D, Li Y. MiR-29a suppresses prostate cell proliferation and induces apoptosis via KDM5B protein regulation. Int J Clin Exp Med. 2015;8:5329–5339. [PMC free article] [PubMed] [Google Scholar]
- 30.Du DS, Yang XZ, Wang Q, Dai WJ, Kuai WX, Liu YL, Chu D, Tang XJ. Effects of CDC42 on the proliferation and invasion of gastric cancer cells. Mol Med Rep. 2016;13:550–554. doi: 10.3892/mmr.2015.4523. [DOI] [PubMed] [Google Scholar]