Abstract
MicroRNAs (miRNAs) have been revealed to be involved in dysfunction and inflammatory conditions of vascular endothelial cells (ECs). However, the role of miR-499a in inflammatory responses and apoptosis of human umbilical vein endothelial cells (HUVECs) remains unclear. The expression of miR-499a and signal transducer and activator of transcription 1 (STAT1) was analyzed using quantitative real-time polymerase chain reaction or western blot assay, respectively. Cells apoptosis was determined by Flow cytometry. Western blot was used to evaluate the protein expression of STAT1, interleukin-6 (IL-6), vascular cell adhesion molecule-1 (VCAM-1), intercellular adhesion molecule-1 (ICAM-1), B cell lymphoma (Bcl-2), Bcl-2 associated X (Bax) and Cleaved Caspase-3. The interaction between miR-499a and STAT1 was confirmed by bioinformatics analysis and luciferase reporter assay. The expression of miR-499a was significantly down-regulated, while the STAT1 level was obviously up-regulated in LPS-induced HUVECs. Overexpressed miR-499a inhibited LPS-activated expression of IL-6, VCAM-1 and ICAM-1, and protected HUVECs against LPS-induced apoptosis by suppressing the expression of Bax and cleaved caspase 3 expressions. However, STAT1 promoted LPS-induced inflammatory injury and apoptosis in HUVECs. In addition, STAT1 was predicted and confirmed to be a target of miR-499a, and rescue experiment indicated that STAT1 was involved in the miR-499a mediated protection on LPS-induced HUVECs inflammatory injury and apoptosis. MiR-499a protects HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression, which providing a novel insight to assist researchers and clinicians in developing potential therapeutic strategies for sepsis.
Keywords: miR-499a, STAT1, apoptosis, inflammatory response, HUVECs
Introduction
Sepsis is characterized by excessive host inflammatory responses and multiple organ dysfunction, which is an extremely life threatening and the leading cause of mortality in critically-ill patients worldwide [1]. Endothelial cells (ECs) are an elementary part of the heart and vasculature and form an important link between the immune system as well as cardiovascular system [2]. ECs form the vascular endothelium, which is the major factor of the maintenance of vascular homeostasis [3]. ECs dysfunction and inflammatory responses induced by various stimuli, such as pro-inflammatory cytokines, hemodynamic factors and certain bacterial endotoxins, results in local thrombosis, loss of vessel barrier function, and rapid and robust leukocyte recruitment, which may contribute to the occurrence and progression of early cardiovascular diseases, including sepsis [4-6]. Thus, elucidating the molecular mechanisms underlying inflammatory injury in EC will shed light on novel insights in sepsis treatment.
MicroRNAs (miRNAs) with approximately 22 nucleotides in sizes are small, endogenous, non-coding RNAs, which act as post-transcriptional regulators of gene expression that enmeshed in most biological processes, including cell differentiation, proliferation and apoptosis [7,8]. Accumulating evidence has revealed the notion of the implication of miRNAs in sepsis biology and the regulatory effects in the various stages of sepsis [9]. The critical role of miRNAs has been identified in fine tuning and in maintaining the physiological balance of the vascular endothelium and may sever as vital targets for current miRNA-based therapies through reprogramming of ECs with synthetic miRNA mimics or inhibitors [10,11]. MiR-499a, a member of miR-499 family, has been revealed to be related to susceptibility to cancer, thereby involving in the development of diseases [12,13]. Recently, emerging evidence also indicates the key role of miR-499a in the regulation of the immune response, cell proliferation, apoptosis in some vascular diseases, such as acute myocardial infarction (AMI) and rheumatoid arthritis [14,15]. However, the exact role of miR-499a in inflammatory responses of vascular ECs in sepsis remains vague. Inflammation is a crucial contributor for the sepsis pathogenesis. Signal transducers and activators of transcription (STAT) proteins are the major signaling proteins of many cytokines and growth factors in mammals and have an important effect on modulating immune cell homeostasis, differentiation, and cellular functions [16]. STAT1 is a member of STAT. A distinctive effect of STAT1 on cross-talk between the pro-inflammatory cytokine interferon (IFN)-γ and Toll-like receptor 4 activators (TLR4-A) has been revealed in the increasing inflammation of immune and vascular cells [17,18]. Additionally, emerging studies investigated that STAT1 can stage-manage a transcriptional platform for the cross-talk between TLR4-A as well as IFN-γ in ECs and vascular smooth muscle cells (VSMCs), which resulting in amplified pro-atherogenic responses in the vasculature [19].
Lipopolysaccharide (LPS) has been illustrated to be a main pathogenic element leading to immune response and inflammation [20]. Thus, in this work, we probed the effects of LPS treatment on miR-499a expression and explored the biological function of miR-499a in LPS-induced inflammatory injury and apoptosis in human ECs as well as the underlying mechanisms.
Materials and methods
Cell culture
Human umbilical vein endothelial cells (HUVECs) were purchased from American Tissue Culture Collection (Manassas, VA, USA) and cultured in endothelial cell basal medium (EBM-2; Lonza, Walkersville, MD, USA) containing EGM-2MV at 37°C with 5% CO2. Cells between passage 3 and 5 were utilized for the experiments.
Cell transfection
MiR-499a mimics (miR-499a), mimic negative control (miR-NC), miR-499a inhibitor (anti-miR-499a), inhibitor negative control (anti-NC), small interfering RNA (siRNA) against STAT1 (si-STAT1) or siRNA negative control (si-NC) were all purchased from Genepharma (Shanghai, China). Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) was utilized to transfect all oligonucleotides or vectors into the cells in accordance with the manufacturer’s instructions. After transfection for 48 h, cells were harvested and collected for subsequent treatment or analysis.
Quantitative real-time polymerase chain reaction
Total RNA was isolated from HUVECs using the TRIzol reagent (Invitrogen) following the manufacturer’s procedure. Reverse-transcribed complementary DNAs (cRNAs) were synthesized by using miScript Reverse Transcription Kit (Takara, Dalian, China). Then qPCR was performed using SYBR Premix Ex Taq (Takara) on a 7500 Sequence Detection System (ABI, Foster City, CA, USA) according to the standard protocol. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or U6 seversed as an internal control and the relative expression level was quantified by the 2-ΔΔCt method. The specific primer sequences were listed as follows: miR-499a, forward 5’-TGCGGTGGCAGTGTATTGTTAGC-3’, and reverse 5’-CCAGTGCAGGGTCCGAGGT-3’; STAT1, forward 5’-TGGTGAAATTGCAAGAGCTG-3’, and reverse 5’-TGTGTGCGTACCCAAGATGT-3’; U6, forward 5’-AGCCCGCACTCAGAACATC-3’, and reverse 5’-GCCACCAAGACAATCATCC-3’; GAPDH, forward 5’-AAGAAGGTGGTGAAGCAGGC-3’, and reverse 5’-GTCAAAGGTGGAGGAGTGGG-3’.
Western blot assay
After treatment or transfection, HUVECs were lysed under the help of RIPA lysis buffer (Beyotime, Shanghai, China) and the content was quantified using a bicinchoninic acid (BCA) protein assay kit (Beyotime) following the standard instructions. Equal amount of the extracted protein were separated by 10% SDS-polyacrylamide gels electrophoresis, and then transferred onto polyvinylidene fluoride (PVDF) membranes (Millipore, Billerica, MA, USA). Then incubations with primary antibodies against STA-T1, VCAM-1, ICAM-1, IL-6, Bax, Bcl-2 or Cleaved Caspase-3 as well as GAPDH at 4°C overnight after block, and followed by interaction with the appropriate horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h. Immunoreactive signals were visualized using Enhanced Chemiluminescence (ECL). GAPDH was used as an intern control.
Cell apoptosis assay
The Annexin V-FITC/PI apoptosis detection kit (BD Biosciences, San Jose, CA, USA) was used to quantify apoptotic cells following the manufacturer’s protocol. Briefly, cells were resuspended using 200 μL binding buffer and then stained with 10 μL Annexin V-FITC, followed 5 μL PI for 15 min in the dark. Finally, the apoptotic cells were analyzed on a flow cytometer (BD Biosciences).
Luciferase reporter assay
The STAT1 3’-UTR sequences with wild-type or mutant miR-499a binding sites were cloned into the pmirGLO vectors (Promega, Madison, WI, USA). Then the constructed vector was co-transfected with miR-NC or miR-499a mimics using Lipofectamine 2000. After post-transfection for 48 h, the relative luciferase activity was evaluated with the help of a dual-luciferase reporter kit (Promega).
Statistical analysis
All the statistical data were showed in the form of means ± standard debytion (SD) and assessed with the GraphPad Prism (GraphPad Software, San Diego, CA, USA) from at least three independently experiment. Comparisons among different groups were analyzed using the one-way analysis variance (ANOVA) followed by Bonferroni’s comparison test or Student’s t-test and P < 0.05 was considered significant.
Results
MiR-499a levels are down-regulated while STAT1 levels are up-regulated in response to LPS stimulation
To determine the roles of miR-499a and STAT1 in HUVECs response to LPS stimulation, we firstly identified the rational dosing and the time course of LPS action. HUVECs were treated with a varying concentration of LPS (0 ng/mL, 50 ng/mL, 100 ng/mL, and 150 ng/mL) at different time points (0 h, 12 h, 24 h, and 36 h). Then the expression of miR-499a was detected and we found miR-499a expression was down-regulated after LPS treatment in a dose- and time-dependent manner in HUVECs (Figure 1A, 1B). Actually, when HUVECs were subjected to 100 ng/mL LPS for 24 h, miR-499a level decreased by approximately 50%. Afterwards, we treated HUVECs with 0 ng/mL or 100 ng/mL LPS for 24 h, and the mRNA and protein expression of STAT1 in HUVECs was significantly up-regulated in LPS treated group (Figure 1C, 1D). Thus, we illustrated that the expression of miR-499a was decreased while STAT1 was increased in LPS-induced HUVECs.
Figure 1.
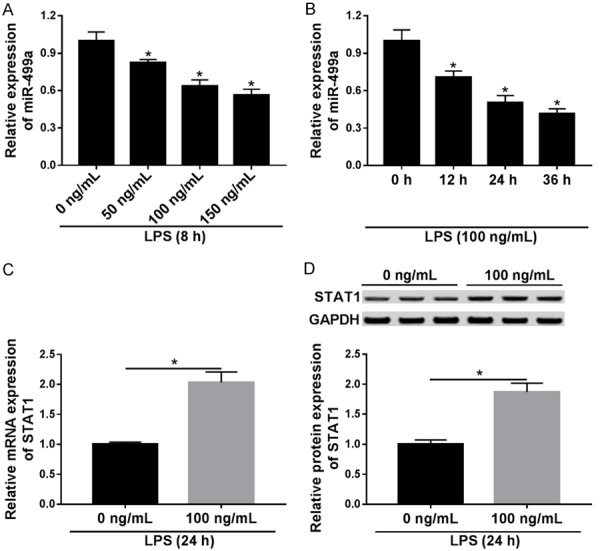
MiR-499a level is down-regulated while STAT1 level is up-regulated in response to LPS stimulation. A, B. MiR-499a expression was measured using qRT-PCR in HUVECs incubated with different concentrations of LPS at different time points. C, D. The mRNA and protein levels of STAT1 were detected with pRT-PCR or western blot in HUVECs treated with 100 ng/mL LPS for 24 h. *P < 0.05.
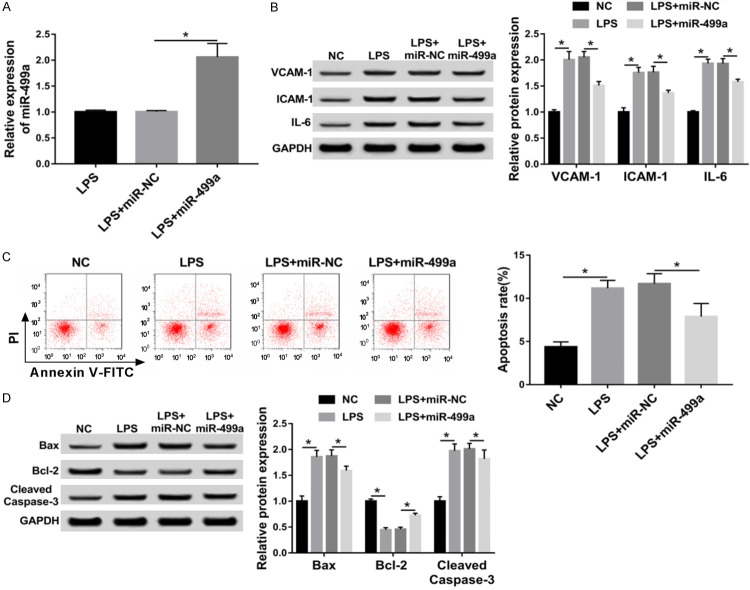
MiR-499a protects HUVECs from LPS-induced inflammatory injury and apoptosis
To explore the biologic function of miR-499a in LPS-induced inflammatory injury and apoptosis in HUVECs, HUVECs were transfected with miR-499a or miR-NC in the presence of 100 ng/mL LPS for 24 h. Then qRT-PCR analysis showed that the expression of miR-499a was markedly up-regulated after transfection with miR-499a mimics compared with miR-NC in HUVECs (Figure 2A). Subsequently, the levels of inflammatory cytokines (IL-6) and adhesion molecules (VCAM-1 and ICAM-1) were measured using western blot and the results showed LPS treatment increased the level of VCAM-1, ICAM-1 and IL-6, which could be reversed by overexpressed miR-499a in HUVECs (Figure 2B). In the meanwhile, we also found miR-499a mimics transfection inhibited LPS-induced apoptosis in HUVECs, reflected by the decreased apoptosis rate, and cleaved caspase-3 and Bax expression, as well as the increased anti-apoptosis protein Bcl-2 level (Figure 2C, 2D). In all, overexpressed miR-499a could protect HUVECs from LPS-induced inflammatory injury and apoptosis.
Figure 2.
MiR-499a protects HUVECs from LPS-induced inflammatory injury and apoptosis. HUVECs were transfected with miR-499a mimics or miR-NC after treatment with 100 ng/mL LPS for 24 h. A. The expression of miR-499a was detected with qRT-PCR. B. Western blot was applied to examine the levels of VCAM-1, ICAM-1 and IL-6. C. The percentage of apoptotic cells was analyzed by flow cytometry. D. Apoptosis-related factors, including cleaved caspase-3, Bax and Bcl-2 were measured by western blot. *P < 0.05.
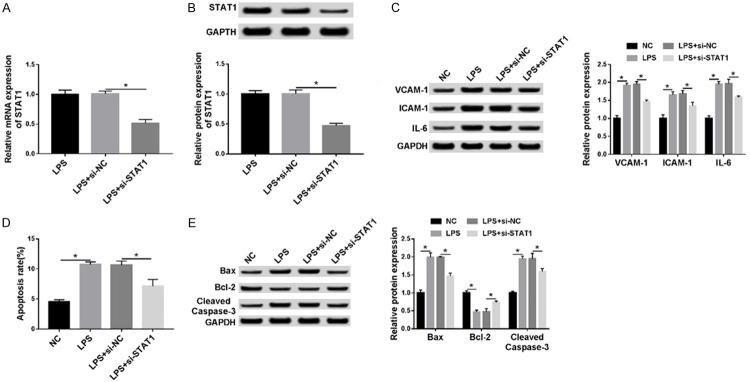
STAT1 promotes LPS-induced inflammatory injury and apoptosis in HUVECs
We further investigated the biological function of STAT1 in LPS-induced injury in HUVECs, and HUVECs were transfected with si-STAT1 or si-NC in the presence of 100 ng/mL LPS for 24 h. Immediately, the expression of STAT1 was detected using qRT-PCR or western blot assay and results showed that the STAT1 expression, whether mRNA or protein, was significantly decreased after transfection with si-STAT1 compared with the control in HUVECs (Figure 3A, 3B). After that, a functional experiment was performed and we demonstrated that low expression STAT1 could attenuate LPS-induced inflammatory injury and apoptosis in HUVECs (Figure 3C-E). These results suggested that STAT1 might promote LPS-induced inflammatory injury and apoptosis in HUVECs.
Figure 3.
STAT1 promotes LPS-induced inflammatory injury and apoptosis in HUVECs. HUVECs were transfected with si-STAT1 or si-NC in the presence of 100 ng/mL LPS for 24 h. A. The mRNA level of STAT1 was examined by qRT-PCR. B, C. The protein expression of STAT1, VCAM-1, ICAM-1 and IL-6 was measured with western blot. D. The percentage of apoptotic cells was analyzed using flow cytometry. E. Apoptosis-related proteins, cleaved caspase-3, Bax and Bcl-2 were detected by western blot. *P < 0.05.
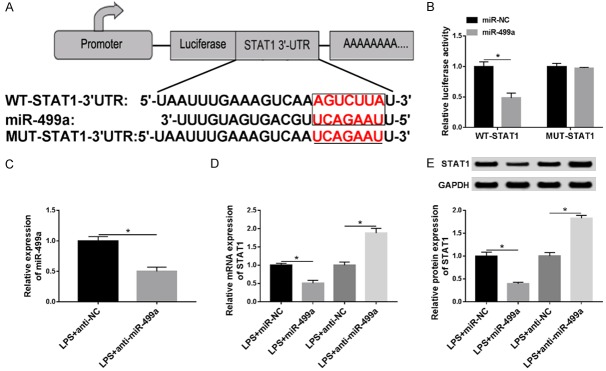
MiR-499a directly targets STAT1 and negatively regulates STAT1 expression in HUVECs
Based on the effects of miR-499a and STAT1 on LPS-induced inflammatory injury and apoptosis in HUVECs, we hypothesized that STAT1 might involve in the miR-499a-mediated repression on LPS-induced HUVECs injury. To verify this hypothesis, Targetscan prediction tool was applied and STAT1 was predicted that might be a target of miR-499a with a putative binding site (Figure 4A). Subsequently, luciferase reporter assay was performed and we discovered overexpressed miR-499a reduced the luciferase activities of the STAT1-WT reporter vector but not STAT1-MUT reporter vector in HUVECs (Figure 4B). Furthermore, to investigate the regulatory mechanism between miR-499a and STAT1, HUVECs was further transfected with anti-miR-499a or anti-NC in the presence of 100 ng/mL LPS for 24 h and decreased miR-499a expression in the group transfection with miR-499a inhibitor was identified (Figure 4C). miR-499a mimic transfection inhibited STAT1 expression, while miR-499a inhibitor transfection promoted STAT1 expression, at both miRNA and protein levels in HUVECs treated with LPS (Figure 4D, 4E). Thus, these data indicated that miR-499a suppressed STAT1 expression in HUVECs.
Figure 4.
MiR-499a directly targets STAT1 and negatively regulates STAT1 expression in HUVECs. A. The potential binding sites between miR-499a and STAT1 were predicted. B. Luciferase activity was analyzed in HUVECs co-transfected with STAT1-WT or STAT1-MUT and miR-NC, miR-499a. C. The expression of miR-499a was detected in HUVECs after transfection with miR-499a inhibitor in the presence of 100 ng/mL LPS for 24 h. D, E. The expression of STAT1 was determined using qRT-PCR or western blot respectively in HUVECs transfected with miR-NC or miR-499a in the presence of 100 ng/mL LPS for 24 h. *P < 0.05.
MiR-499a protects HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression
To further explore whether STAT1 participating in the miR-499a-mediated protection on LPS-induced HUVECs inflammatory injury and apoptosis, HUVECs were transfected with anti-NC, anti-miR-499a, anti-miR-499a + si-NC, anti-miR-499a + si-ATAT1 after treatment with 100 ng/mL LPS for 24 h. Then western bolt analysis showed miR-499a inhibitor transfection promoted the LPS-induced increase of VCAM-1, ICAM-1 and IL-6 level, which could be alleviated by down-regulated STAT1 in HUVECs (Figure 5A). Afterwards, we also demonstrated miR-499a inhibition stimulated LPS-induced HUVECs apoptosis, reflected by the increased apoptosis rate and cleaved caspase-3, Bax level, as well as the decreased expression of anti-apoptosis protein Bcl-2, while decreased STAT1 could counteract these effects (Figure 5B, 5C). Altogether, these results manifested miR-499a could protect HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression.
Figure 5.
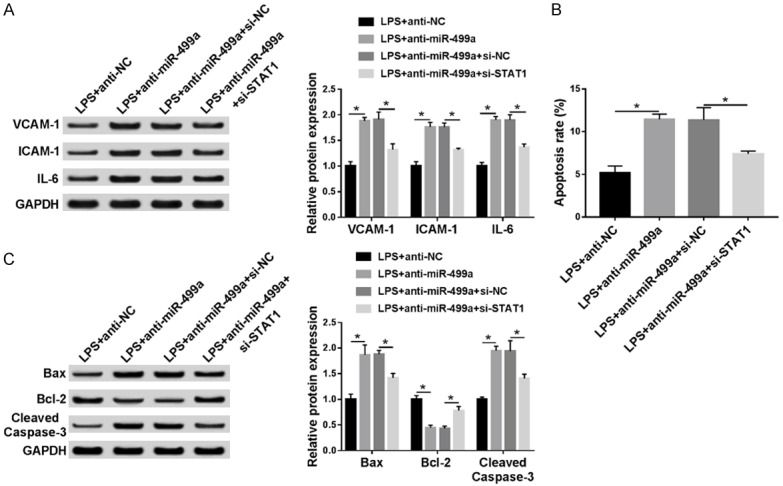
MiR-499a protects HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression. HUVECs were transfected with anti-NC, anti-miR-499a, anti-miR-499a + si-NC, anti-miR-499a + si-ATAT1 after treatment with 100 ng/mL LPS for 24 h. A. The levels of VCAM-1, ICAM-1 and IL-6 were examined using western blot in HUVECs. B. The apoptosis rate was analyzed using flow cytometry in HUVECs. C. Apoptosis-related proteins, Bcl-2 and cleaved caspase-3, Bax were determined by western blot in HUVECs. *P < 0.05.
Discussion
The vascular endothelium, formed by ECs, is a multifunctional organ and is closely implicated in the regulation of vascular structure and tone [21]. ECs are metabolically active with important endocrine, paracrine as well as autocrine functions, participate in the command of blood vessel development, growth and differentiation; command of vascular tone; command of leukocyte trafficking; command of vascular barrier; command of platelet function, coagulation, as well as fibrinolysis, essential for the maintenance of vascular homeostasis under physiological conditions [22-24]. Thus, abnormal dysfunction and activation in ECs may contribute to the development of many vascular inflammatory diseases [25,26]. Besides that, the activated vascular endothelium can express diverse adhesion molecules (VCAM-1, ICAM-1), which can recruit leukocytes, and modulate leukocyte attachment on the vascular ECs at the early stage during inflammation [26].
In recent years, it has been reported that miRNAs participate in the post-transcriptional-mediated modulation of gene expression programs of ECs. For example, miR-16 or miR-424 overexpression represses proliferation, migration, and cord formation of ECs in vitro, and overexpressed miR-16 also reduces the ability of ECs to form blood vessels in vivo, which regulating cell-intrinsic angiogenic activity of ECs [27]; MiRNA-204 enhances vascular endoplasmic reticulum stress and endothelial dysfunction by targeting Sirtuin1 [28]; MiR-4505 aggravates LPS-induced vascular endothelial migration capacity by targeting heat shock protein A12B [29]. All the researches indicated that miRNAs might be prime candidates for miRNA-based therapeutic applications toward vascular disease prevention.
In this research, we focused on the role of LPS in miR-499a expression and explored the biological function of miR-499a in LPS-induced inflammatory injury and apoptosis in HUVECs. The results demonstrated that LPS stimulated ECs inflammatory injury, reflected by the increased expression of inflammatory cytokines (IL-6) and adhesion molecules (ICAM-1 and VCAM-1), and LPS also induced the apoptosis of ECs by promoting the expression of Bax and cleaved caspase 3 expression. Subsequently, we found miR-499a expression was remarkable down-regulated after LPS treatment in a dose- and time-dependent manner in HUVECs, suggesting the regulatory role of miR-499a in the LPS-induced HUVECs. After that, functional experiment was performed and the results exhibited up-regulated miR-499a could decreased the level of IL-6, ICAM-1 and VCAM-1, thereby protecting HUVECs from LPS-induced inflammatory injury, besides that, overexpressed miR-499a also inhibited LPS-induced HUVECs apoptosis by suppressing the expression of cleaved caspase-3 and Bax. Therefore, we illustrated that miR-499a could protect ECs from LPS-induced inflammatory injury and apoptosis.
MiRNAs can involve in the post-transcriptional control of the target mRNAs by predominantly binding to the 3’untranslated regions (UTR) of the target gene [30]. Thus, to further investigate the underlying mechanism of miR-499a on the protection of ECs, the potential targets of miR-499a in HUVECs were predicted and confirmed using the Targetscan prediction tools and luciferase reporter assay, and we confirmed that STAT1 was a target of miR-499a because of the reduction of the luciferase activities of the STAT1-WT reporter vector in HUVECs induced by overexpressed miR-499a. STAT1 has been investigated to be a momentous go-between in initiating and sustaining the inflammatory response in atherosclerosis by the coordination of crosstalk between IFN γ and TLR4 in ECs [31]. The pro-inflammatory genes expression regulation has been implied to depend on signal integration between TLR4 and IFNs by combinatorial actions of the STAT1 complexes ISGF3 and γ-activated factor (GAF), and Nuclear Factor-κB (NF-κB) to active vascular and immune cells and injury the vascular endothelium [31]. In the current examination, we discovered the mRNA and protein expression of STAT1 in HUVECs was significantly up-regulated after LPS treatment, suggesting STAT1 might involve in the LPS-induced ECs injury and then functional experiment was performed and results showed STAT1 promoted LPS-induced inflammatory injury by enhancing the expression of IL-6, ICAM-1 and VCAM-1 and apoptosis by inhibiting cleaved caspase-3 and Bax expression in HUVECs. In the meanwhile, we also conducted rescue assay and we demonstrated that miR-499a could protect HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression.
In conclusion, our researches suggested that miR-499a protected HUVECs from LPS-induced inflammatory injury and apoptosis by regulating STAT1 expression. These findings may help us to better understand the pathogenesis of ECs injury, and provide a novel insight to assist researchers and clinicians in developing potential therapeutic strategies for sepsis.
Disclosure of conflict of interest
None.
References
- 1.Liu J, Li G, Chen C, Chen D, Zhou Q. MiR-6835 promoted LPS-induced infl ammation of HUVECs associated with the interaction between TLR-4 and AdipoR1 in lipid rafts. PLoS One. 2017;12:e0188604. doi: 10.1371/journal.pone.0188604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sturtzel C. Endothelial cells. Adv Exp Med Biol. 2017;1003:71–91. doi: 10.1007/978-3-319-57613-8_4. [DOI] [PubMed] [Google Scholar]
- 3.Bourque C, Zhang Y, Fu M, Racine M, Greasley A, Pei Y, Wu L, Wang R, Yang G. H2S protects lipopolysaccharide-induced infl ammation by blocking NFκB transactivation in endothelial cells. Toxicol Appl Pharmacol. 2018;338:20–29. doi: 10.1016/j.taap.2017.11.004. [DOI] [PubMed] [Google Scholar]
- 4.Otsuka F, Finn AV, Yazdani SK, Nakano M, Kolodgie FD, Virmani R. The importance of the endothelium in atherothrombosis and coronary stenting. Nat Rev Cardiol. 2012;9:439–453. doi: 10.1038/nrcardio.2012.64. [DOI] [PubMed] [Google Scholar]
- 5.Gimbrone MA Jr. Endothelial dysfunction, hemodynamic forces, and atherosclerosis. Thromb Haemostasis. 1999;82:722–726. [PubMed] [Google Scholar]
- 6.Zhang CH. The role of infl ammatory cytokines in endothelial dysfunction. Basic Res Cardiol. 2008;103:398–406. doi: 10.1007/s00395-008-0733-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cui J, Zhou B, Ross SA, Zempleni J. Nutrition, microRNAs, and human health. Adv Nutr. 2017;8:105–112. doi: 10.3945/an.116.013839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 9.Kingsley SMK, Bhat BV. Role of microRNAs in sepsis. Infl amm Res. 2017;66:553–569. doi: 10.1007/s00011-017-1031-9. [DOI] [PubMed] [Google Scholar]
- 10.Fernandez-Hernando C, Suarez Y. MicroRNAs in endothelial cell homeostasis and vascular disease. Curr Opin Hematol. 2018;25:227–236. doi: 10.1097/MOH.0000000000000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scott E, Loya K, Mountford J, Milligan G, Baker AH. MicroRNA regulation of endothelial homeostasis and commitment-implications for vascular regeneration strategies using stem cell therapies. Free Radic Biol Med. 2013;64:52–60. doi: 10.1016/j.freeradbiomed.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 12.Hou YY, Lee JH, Chen HC, Yang CM, Huang SJ, Liou HH, Chi CC, Tsai KW, Ger LP. The association between miR-499a polymorphism and oral squamous cell carcinoma progression. Oral Dis. 2015;21:195–206. doi: 10.1111/odi.12241. [DOI] [PubMed] [Google Scholar]
- 13.Shan YF, Huang YH, Chen ZK, Huang KT, Zhou MT, Shi HQ, Song QT, Yu ZP, Deng AM, Zhang QY. miR-499A>G rs3746444 and miR-146aG>C expression and hepatocellular carcinoma risk in the Chinese population. Genet Mol Res. 2013;12:5365–5371. doi: 10.4238/2013.November.7.11. [DOI] [PubMed] [Google Scholar]
- 14.Toraih EA, Ismail NM, Toraih AA, Hussein MH, Fawzy MS. Precursor mir-499a variant but not mir-196a2 is associated with rheumatoid arthritis susceptibility in an egyptian population. Mol Diagn Ther. 2016;20:279–295. doi: 10.1007/s40291-016-0194-3. [DOI] [PubMed] [Google Scholar]
- 15.Ji Q, Jiang Q, Yan W, Li X, Zhang Y, Meng P, Shao M, Chen L, Zhu H, Tian N. Expression of circulating microRNAs in patients with ST segment elevation acute myocardial infarction. Minerva Cardioangiologica. 2015;63:397–402. [PubMed] [Google Scholar]
- 16.Wu Y, Yang L, Mei X, Yu Y. Selective inhibition of STAT1 reduces spinal cord injury in mice. Neurosci Lett. 2014;580:7–11. doi: 10.1016/j.neulet.2013.11.055. [DOI] [PubMed] [Google Scholar]
- 17.Chmielewski S, Piaszyk-Borychowska A, Wesoly J, Bluyssen HA. STAT1 and IRF8 in vascular infl ammation and cardiovascular disease: diagnostic and therapeutic potential. Int Rev Immunol. 2016;35:434–454. doi: 10.3109/08830185.2015.1087519. [DOI] [PubMed] [Google Scholar]
- 18.Sikorski K, Czerwoniec A, Bujnicki JM, Wesoly J, Bluyssen HA. STAT1 as a novel therapeutical target in pro-atherogenic signal integration of IFNγ, TLR4 and IL-6 in vascular disease. Cytokine Growth Factor Rev. 2011;22:211–219. doi: 10.1016/j.cytogfr.2011.06.003. [DOI] [PubMed] [Google Scholar]
- 19.Hansson GK, Hermansson A. The immune system in atherosclerosis. Nat Immunol. 2011;12:204–212. doi: 10.1038/ni.2001. [DOI] [PubMed] [Google Scholar]
- 20.Fu Y, Hu X, Cao Y, Zhang Z, Zhang N. Saikosaponin an inhibits lipopolysaccharide-oxidative stress and infl ammation in Human umbilical vein endothelial cells by preventing TLR4 translocation into lipid rafts. Free Radic Biol Med. 2015;89:777–785. doi: 10.1016/j.freeradbiomed.2015.10.407. [DOI] [PubMed] [Google Scholar]
- 21.Fishman AP. Endothelium: a distributed organ of diverse capabilities. Ann N Y Acad Sci. 1982;401:1–8. doi: 10.1111/j.1749-6632.1982.tb25702.x. [DOI] [PubMed] [Google Scholar]
- 22.Busse R, Fleming I. Vascular endothelium and blood flow. Handb Exp Pharmacol. 2006:43–78. doi: 10.1007/3-540-36028-x_2. [DOI] [PubMed] [Google Scholar]
- 23.Pober JS, Sessa WC. Evolving functions of endothelial cells in infl ammation. Nat Rev Immunol. 2007;7:803–815. doi: 10.1038/nri2171. [DOI] [PubMed] [Google Scholar]
- 24.Minshall RD, Malik AB. Transport across the endothelium: regulation of endothelial permeability. Handb Exp Pharmacol. 2006;176:107–144. doi: 10.1007/3-540-32967-6_4. [DOI] [PubMed] [Google Scholar]
- 25.Zhou M, Ding Y, Cai L, Wang Y, Lin C, Shi Z. Low molecular weight fucoidan attenuates experimental abdominal aortic aneurysm through interfering the leukocyte-endothelial cells interaction. Mol Med Rep. 2018;17:7089–7096. doi: 10.3892/mmr.2018.8765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tettey CO, Yang I, Shin HM. Smilax china leaf extracts suppress pro-infl ammatory adhesion response in human umbilical vein endothelial cells and proliferation of HeLa cells. Arch Physiol Biochem. 2018;30:1–5. doi: 10.1080/13813455.2018.1520262. [DOI] [PubMed] [Google Scholar]
- 27.Chamorro-Jorganes A, Araldi E, Penalva LO, Sandhu D, Fernandez-Hernando C, Suarez Y. MicroRNA-16 and microRNA-424 regulate cell-autonomous angiogenic functions in endothelial cells by targeting vascular endothelial growth factor receptor-2 and fibroblast growth factor receptor-1. Arterioscler Thromb Vasc Biol. 2011;31:2595–2606. doi: 10.1161/ATVBAHA.111.236521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nemecz M, Alexandru N, Tanko G, Georgescu A. Role of microRNA in endothelial dysfunction and hypertension. Curr Hypertens Rep. 2016;18:87. doi: 10.1007/s11906-016-0696-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang X, Chen Y, Wang L, Kang Q, Yu G, Wan X, Wang J, Zhu K. MiR-4505 aggravates lipopolysaccharide-induced vascular endothelial injury by targeting heat shock protein A12B. Mol Med Rep. 2018;17:1389–1395. doi: 10.3892/mmr.2017.7936. [DOI] [PubMed] [Google Scholar]
- 30.Shukla GC, Singh J, Barik S. MicroRNAs: processing, maturation, target recognition and regulatory functions. Mol Cell Pharmacol. 2011;3:83–92. [PMC free article] [PubMed] [Google Scholar]
- 31.Chmielewski S, Olejnik A, Sikorski K, Pelisek J, Blaszczyk K, Aoqui C, Nowicka H, Zernecke A, Heemann U, Wesoly J, Baumann M, Bluyssen HA. STAT1-dependent signal integration between IFNgamma and TLR4 in vascular cells refl ect pro-atherogenic responses in human atherosclerosis. PLoS One. 2014;9:e113318. doi: 10.1371/journal.pone.0113318. [DOI] [PMC free article] [PubMed] [Google Scholar]