Abstract
Activation-induced cytidine deaminase (AID) produces immune-diversity by inducing somatic hypermutations and class-switch recombinations in human immunoglobulin genes. This role of AID in causing genomic mutations, also can potentially cause somatic mutations in various host genes of non-lymphoid tissues, and contribute to tumorigenesis. The goal of the present study was to investigate whether AID expression was involved in the development or progression of colorectal cancer, and the nuclear expression of p53 protein in cancer cells. We examined the pattern of expression of AID and p53 proteins in 71 colorectal adenomas and 122 sporadic colorectal cancers by immunohistochemistry. AID and p53 expression was detected in 57 (46.7%) and 78 (63.9%) out of 122 colorectal cancers, respectively. Statistically, the expression of the AID protein was not associated with the 5-year survival or clinical and pathological parameters, including tumor stage, location, size, and lymph node metastasis (P > 0.05). However, the expression of the AID protein was associated with tumor differentiation (P = 0.004). In addition, a significant association was observed between AID and the nuclear expression of p53 in colorectal cancers (P = 0.0357). Only 3 (4.2%) of the 71 colorectal adenomas showed immunopostivity for AID, resulting in a significant difference between total colorectal cancers and adenomas (P < 0.001). The p53 expression was detected in 7 (9.9%) out of 71 colorectal adenomas. Statistically, AID protein was not associated with the degree of dysplasia and the nuclear expression of p53 in colorectal adenomas (P > 0.05). These results suggest that aberrant expression of the AID protein might play a role in the development of colorectal cancers.
Keywords: Activation-induced cytidine deaminase, immunohistochemistry, tissue microarray, colorectal cancer, p53
Introduction
Scientists have suspected that inflammation and cancer are correlated, with the former somehow involved in the development of the latter. There are clear links between chronic inflammation and cancer. For example, patients harboring Helicobacter pylori (H. pylori), a bacteria that causes gastritis and gastric ulcer, are much more likely to develop gastric cancer than those without this bacteria [1,2]. Individuals with colitis have a lifetime risk of colon cancer that can exceed 40%, and anti-inflammatory medications have been shown to reduce this risk by at least three-quarters [3]. However, the mechanism by which inflammation causes cancer remains unknown. Recently, a study showed that proinflammatory cytokine-mediated aberrant expression of activation-induced cytidine deaminase (AID), in colon epithelial cells, was a genotoxic factor linking inflammation, somatic mutations, and colorectal cancer development [4].
AID, a member of the cytidine deaminase family, is an enzyme that deaminates cytosine to produce uracil in DNA molecules [5]. Expression of AID is restricted to stimulated B cells, especially in germinal centers, and induces class switch recombination that replaces one immunoglobulin heavy chain constant region gene with another and somatic hypermutation [6,7]. Ectopic expression of AID in nonlympoid cells is potentially dangerous to cells, and must be tightly controlled to avoid mutations in genes that could lead to cancer [8]. For example, H. pylori strains induced aberrant expression of AID and upregulation of AID protein resulted in accumulation of nucleotide alterations in the TP53 tumor suppressor gene in gastric cells in vitro [9]. In our previous study, aberrant expression of AID has been observed in H. pylori-positive gastric mucosa and gastric cancer tissues in immunohistochemical analysis [10]. All of these findings suggest that aberrant AID expression can induce mutations in non-immunoglobulin genes including cancer-related genes, and may play a role in the development of human malignancies.
The transcription factor p53 is involved in diverse cellular stresses including DNA damage, apoptosis, and senescence and may serve as a ‘guardian of the genome’ [11]. Once activated, the p53 protein can either induce the expression of p21 (Waf1, Cip-1), which participates in the cellular arrest between G1-S transition, or the expression of bax, Fas, and DR5 [12]. Thus, inactivation of this protein contributes to genomic instability, the accumulation of mutations, and tumorigenesis. Over 6,000 papers have described somatic and germline mutations in p53 in the International Association of Cancer Registries (IARC) database (http://www.iacr.fr/p53). The frequency of p53 mutations in colorectal carcinomas has been consistently reported to be around 50% [13]. The p53 protein has a very short half-life and therefore can be hard to detect in normal tissue. However, longer expression or nuclear accumulation of the p53 protein can result from mutations in the p53 gene, upregulated expression of the wild-type p53 protein to allow repair of damaged DNA, and a defect in the degradation pathway [14]. Since aberrant AID expression in non-B cells can cause mutations of non-Ig genes [15] and the development of human malignancies [16,17]. It is likely that aberrant AID expression may be one of the mutagens in colorectal cancer and induce nuclear accumulation of the p53 protein.
Therefore, to investigate whether aberrant AID expression is involved in the development or progression of colorectal cancers and the nuclear accumulation of p53 protein, in colorectal cancer cells, we analyzed AID and p53 expression in 71 colorectal adenomas and 122 colorectal cancers by immunohistochemistry.
Materials and methods
Tissue samples
Seventy one colorectal adenomas and 122 sporadic colorectal cancers were obtained from the Binzhou Medical University of Shandong. The colorectal cancers stage was classified according to Dukes’ criteria [18]. There were 12, 46, 56, and 8 cases with stages A, B, C, and D disease, respectively. Two pathologists screened the histological sections and selected areas of representative tumor cells. Three and one tissue cores from each cancer sample (0.5 mm in diameter) were obtained and placed in a new recipient paraffin block using a commercially available microarray instrument (Beecher Instruments, Micro-Array Technologies, Silver Spring, MD, USA), according to established methods [19]. One cylinder of normal colon mucosa adjacent to each tumor was also transferred to the recipient block. Colorectal adenomas was taken by biopsy and electrocoagulation.
Immunohistochemistry for AID and p53
For the immunohistochemical analysis, 2 μm sections were cut the day before use and stained according to standard protocols. To maximize the signal on the immunohistochemistry, two strategies were used: antigen retrieval in citrate buffer, and signal amplification with biotinylated tyramide. For the former, heat-induced epitope retrieval was performed by immersing the slides in Coplin jars filled with 10 mmol/L citrate buffer (pH 6.0) and boiling the buffer for 30 min in a pressure cooker (Nordic Ware, Minneapolis, MN, USA) inside of a microwave oven at 700 W; the jars were then cooled for 20 min. For the latter, the Renaissance TSA indirect kit (NEN Life Science, Boston, MA, USA), which included streptavidin-peroxidase and biotinylated tyramide, was used. After rinsing with PBS, the slides were treated with 1% H2O2 in PBS for 15 min at room temperature to eliminate the endogenous peroxidase activity. After washing with TNT buffer (0.1 mol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl and 0.05% Tween 20) for 20 min, the slides were treated with TNB buffer (0.1 mol/L Tris-HCl, pH 7.4, 0.15 mol/L NaCl and 0.5% blocking reagent). The sections were incubated overnight at 4°C with the antibodies (1/100 dilution) to the AID (BD Bioscience, Franklin Lakes, NJ, USA) and p53 proteins (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Detection was carried out using biotinylated goat anti-rabbit antibody (Sigma, St. Louis, MO, USA), followed by incubation with a peroxidase-linked avidin-biotin complex. Diaminobenzidine was used as a chromogen, and the slide was counterstained with Mayer’s hematoxylin. The specificity of the anti-AID antibody was confirmed in our previous study [10]. Staining for the AID antigen was considered positive when > 5% of the cytoplasm stained positively. For p53, the presence of nuclear staining was considered a positive result. The results were reviewed independently by two pathologists. For the negative controls, the slide was treated by replacement of the primary antibody with non-immune serum.
Statistical analysis
The correlation between AID and p53 expression, and various clinicopathologic parameters was assessed using the Chi-square test. Survival curves were constructed using the Kaplan-Meier method and compared using the log rank test. A p value less than 0.05 was considered the limit of statistical significance.
Results
A moderate-to-strong immunopositive response was obtained for AID in the cytoplasm of cancer cells (Figure 1). However, the corresponding normal colon mucosa and surrounding stromal cells, e.g. fibroblasts, were negative or focal weak positive for AID. Aberrant expression of the AID protein was detected in 57 (46.7%) out of 122 colorectal cancers. Based on the differentiation grade of the cancer cells, expression was detected in 100% (6/6), 45.9% (51/111), and 0% (0/5) of well, moderately, and poorly differentiated colorectal cancers. Statistically, AID expression was associated with the differentiation of tumor cells (Chi-square test, P = 0.004) (Table 1). AID expression was observed in 5 (41.6%) out of 12 cases corresponding to stage A, 24 (52.2%) out of 46 stage B, 25 (44.6%) out of 56 stage C, and 3 (37.5%) out of 8 stage D disease. AID expression was found in 52.6% (30/57) of tumors that were ≥ 5 cm among the colorectal cancers. In addition, AID was expressed in 24 (41.4%) out of 58 cases with lymph node metastasis. Statistically, there was no significant relationship between AID expression and the clinical and pathological parameters, including clinical stage, tumor location, tumor size, and lymph node metastasis (Chi-square test, P > 0.05) (Table 1).
Figure 1.

Aberrant AID and p53 expression in colorectal cancer cells. Normal colonic mucosa exhibited focal weak positive staining for AID (A). Colorectal cancers displayed cytoplasmic staining for AID (B, C) and nuclear accumulation of p53 protein (D). Original magnification, ×200.
Table 1.
Correlation of AID and p53 protein expression with clinicopathological parameters in the colorectal cancers
| Parameters | AID protein expression | p value | |
|---|---|---|---|
|
| |||
| + | - | ||
| Adenoma | 3 | 68 | |
| Differentiation | 0.7021 | ||
| Low | 1 | 27 | |
| High | 2 | 41 | |
| P53 expression | 0.6861 | ||
| + | 1 | 6 | |
| - | 2 | 62 | |
| Colorectal cancer | 57 | 65 | |
| Differentiation† | 0.004 | ||
| Well | 6 | 0 | |
| Moderately | 51 | 60 | |
| Poorly | 0 | 5 | |
| L/N metastasis†† | 0.2603 | ||
| + | 24 | 34 | |
| - | 33 | 31 | |
| Stage†† | 0.7908 | ||
| A | 5 | 7 | |
| B | 24 | 22 | |
| C | 25 | 31 | |
| D | 3 | 5 | |
| Side†† | 0.098 | ||
| Right | 8 | 17 | |
| Left | 49 | 48 | |
| Size†† | 0.7603 | ||
| < 5 cm | 27 | 29 | |
| ≥ 5 cm | 30 | 36 | |
| P53 expression† | 0.0357 | ||
| + | 42 | 36 | |
| - | 15 | 29 | |
Chi-Square test, P < 0.05.
Chi-Square test, P > 0.05.
Moderate-to-strong nuclear staining for the p53 protein was found in 78 (63.9%) out of the 122 cancer cases (Table 1). Interestingly, 42 cases were immunopositive for both AID and p53 proteins. Thirty-six cases with p53 nuclear staining were negative for AID, and 29 cases demonstrated negative staining for both proteins. Statistically, the expression of the AID protein was significantly associated with p53 immunostaining (Chi-square test, P = 0.0357).
The 5-years survival rate of patients with AID positive tumors was 29.6%, while that of patients with AID negative tumors was 51.7% (Figure 2). Statistically, no significant association between AID, p53 expression and 5-year survival was found (P > 0.05).
Figure 2.
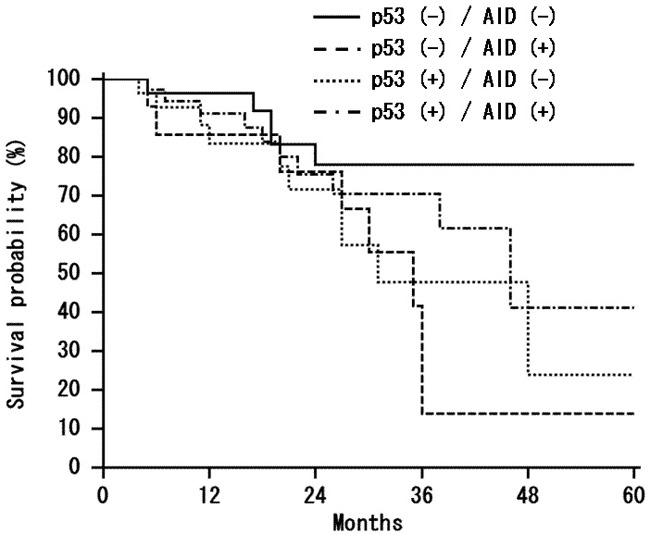
Kaplan-Meier analysis of survival of colorectal cancer patients. Comparative survival analysis of immunohistochemically classified tumors demonstrating negative and positive staining of AID protein showed no significant correlation between AID, p53 overexpression and 5-years survival (P = 0.0899).
In colorectal adenomas, only 3 of the 71 colorectal adenomas showed immunopostivity for AID, resulting in a significant difference between total colorectal cancers and adenomas (Chi-square test, P < 0.001). Nuclear staining for the p53 protein was found in 7 (9.9%) out of the 71 colorectal adenomas (Table 1). Statistically, AID protein was not associated with dysplasia and the nuclear expression of p53 in colorectal adenoma cases (Chi-square test, P > 0.05).
Discussion
Colorectal carcinogenesis has been described as a multistep genetic process, where mutations within several genes, including p53, accumulate during the progression from normal epithelium to adenoma to invasive cancer [20,21]. Chronic inflammatory bowel disease (CIBD) is an important etiologic factor associated with the development of colorectal cancers. Several proinflammatory cytokines, including TNF-α and IL-1β, play an important role in the pathophysiology of CIBD [22,23]. The findings of a recent study suggested that TNF-α induces aberrant AID expression via IκB kinase-dependent nuclear factor (NF)-κB-signaling pathways in human colon epithelial cells. Moreover, aberrant activation of AID in colon cells preferentially induced genetic mutations in the TP53 and c-myc genes [4]. Similar results were found in another study with cag PAI-positive H. pylori induced aberrant expression of the AID protein in human gastric epithelial cells that resulted in multiple mutations in the TP53 tumor suppressor gene and the CTNNB1 oncogene [9]. AID is a key enzyme in the initiation of class switch recombination and somatic hypermutation [6,7]. There is evidence that AID is expressed not only in lymphoid cells but also in non lymphoid cells, including breast cancer cells and hepatocytes [24,25]. In one study, inappropriate expression of AID was induced by proinflammatory cytokine stimulation that was linked to hepatic inflammation and the development of hepatocellular carcinoma [14]. All of these findings suggest that aberrant AID expression may be an important factor involved in the development of colorectal cancer.
It is evident that the p53 tumor suppressor gene acts as a policeman that prevents the propagation of genetically damaged cells. The fact that p53 mutations are common in a variety of human tumors suggests that the p53 protein serves as a critical gatekeeper against the formation of cancer [11]. Since aberrant AID expression can induce genetic mutations, including p53 and c-myc genes, in human colon cells [4], we hypothesized that mutations were caused by aberrant expression of AID stabilize nuclear p53 protein in the cancer tissues. In the present study, we identified nuclear staining for the p53 protein in 63.9% of the colorectal cancers. There was a statistically significant correlation between AID and the nuclear p53 expression. Generally, nuclear accumulation of the p53 protein can be detected in the cancer cells with or without p53 mutations, or decreased p53 protein degradation in response to various cellular stresses, including DNA damage [14]. Although p53 mutations does not account for all the nuclear p53 protein positive cases, our findings suggest that AID-induced DNA damage may lead to mutations in the p53 gene or nuclear accumulation of the p53 protein in the cancer cells with damaged DNA.
In the present study, we examined AID expression in colorectal adenomas and sporadic colorectal cancers. The immunohistochemical studies showed that aberrant AID expression was present 46.7% of the 122 sporadic colorectal cancers. When we evaluated the correlation between aberrant AID expression and colorectal cancer, the expression of the AID protein was not associated with the 5-year survival or the clinical and pathological parameters, including tumor stage, size, location, and lymph node metastasis. The expression of the AID protein was only associated with tumor differentiation. Interestingly, there was a significant difference in AID expression between total colorectal cancers and adenomas. Thus, all of these findings suggest that aberrant expression of the AID protein might play a role in the development of colorectal cancers.
Even though only a small number of cases were evaluated here, our results suggest that aberrant AID expression may play an important role in colorectal cancer carcinogenesis by the accumulation of mutations in tumor-associated genes. Additional studies with a larger patient cohort are needed to verify these initial observations. Functional analysis will certainly broaden our understanding not only of the pathogenesis of colorectal cancer, but also of the mechanisms involved in AID expression in colon epithelial cells.
Acknowledgements
This work was supported by the Natural Science Foundation of Shandong Province (ZR2016HL29, ZR2011HQ025, and ZR2015HL085) and the National Natural Science Foundation of China (No. 81772637).
Disclosure of conflict of interest
None.
References
- 1.Kuper H, Adami HO, Trichopoulos D. Infections as a major preventable cause of human cancer. J Intern Med. 2000;248:171–183. doi: 10.1046/j.1365-2796.2000.00742.x. [DOI] [PubMed] [Google Scholar]
- 2.Farinati F, Cardin R, Cassaro M, Bortolami M, Nitti D, Tieppo C, Zaninotto G, Rugge M. Helicobacter pylori, inflammation, oxidative damage and gastric cancer: a morphological, biological and molecular pathway. Eur J Cancer Prev. 2008;17:195–200. doi: 10.1097/CEJ.0b013e3282f0bff5. [DOI] [PubMed] [Google Scholar]
- 3.Rogler G. Chronic ulcerative colitis and colorectal cancer. Cancer Lett. 2014;345:235–241. doi: 10.1016/j.canlet.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 4.Endo Y, Marusawa H, Kou T, Nakase H, Fujii S, Fujimori T, Kinoshita K, Honjo T, Chiba T. Activation-induced cytidine deaminase links between inflammation and the development of colitis-associated colorectal cancers. Gastroenterology. 2008;135:889–898. doi: 10.1053/j.gastro.2008.06.091. [DOI] [PubMed] [Google Scholar]
- 5.Teng B, Burant GF, Davidson NO. Molecular cloning of an apolipoprotein B messenger RNA editing protein. Science. 1993;260:1816–1819. doi: 10.1126/science.8511591. [DOI] [PubMed] [Google Scholar]
- 6.Sapoznik S, Bahar-Shany K, Brand H, Pinto Y, Gabay O, Glick-Saar E, Dor C, Zadok O, Barshack I, Zundelevich A. Activation-induced cytidine deaminase links ovulation-induced inflammation and serous carcinogenesis. Neoplasia. 2016;18:90–99. doi: 10.1016/j.neo.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Casellas R, Basu U, Yewdell WT, Chaudhuri J, Robbiani DF, Di Noia JM. Mutations, kataegis and translocations in B cells: understanding AID promiscuous activity. Nat Rev Immunol. 2016;16:164–176. doi: 10.1038/nri.2016.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rucci F, Cattaneo L, Marrella V, Sacco MG, Sobacchi C, Lucchini F, Nicola S, Della Bella S, Villa ML, Imberti L, Gentili F, Montagna C, Tiveron C, Tatangelo L, Facchetti F, Vezzoni P, Villa A. Tissue-specific sensitivity to AID expression in transgenic mouse models. Gene. 2006;377:150–158. doi: 10.1016/j.gene.2006.03.024. [DOI] [PubMed] [Google Scholar]
- 9.Matsumoto Y, Marusawa H, Kinoshita K, Endo Y, Kou T, Morisawa T, Azuma T, Okazaki IM, Honjo T, Chiba T. Helicobacter pylori infection triggers aberrant expression of activation-induced cytidine deaminase in gastric epithelium. Nat Med. 2007;13:470–476. doi: 10.1038/nm1566. [DOI] [PubMed] [Google Scholar]
- 10.Kim CJ, Song JH, Cho YG, Cao Z, Kim SY, Nam SW, Lee JY, Park WS. Activation-induced cytidine deaminase expression in gastric cancer. Tumour Biol. 2007;28:333–339. doi: 10.1159/000124239. [DOI] [PubMed] [Google Scholar]
- 11.Levine AJ. p53, the cellular gatekeeper for growth and division. Cell. 1997;88:323–331. doi: 10.1016/s0092-8674(00)81871-1. [DOI] [PubMed] [Google Scholar]
- 12.Mendoza-Rodríguez CA, Cerbón MA. Tumor suppressor gene p53: mechanisms of action in cell proliferation and death. Rev Invest Clin. 2001;53:266–273. [PubMed] [Google Scholar]
- 13.Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003;21:271–276. doi: 10.1002/humu.10175. [DOI] [PubMed] [Google Scholar]
- 14.Petitjean A, Achatz MI, Borresen-Dale AL, Hainaut P, Olivier M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene. 2007;26:2157–2165. doi: 10.1038/sj.onc.1210302. [DOI] [PubMed] [Google Scholar]
- 15.Robbiani DF, Nussenzweig MC. Chromosome translocation, B cell lymphoma, and activation-induced cytidine deaminase. Annu Rev Pathol. 2013;8:79–103. doi: 10.1146/annurev-pathol-020712-164004. [DOI] [PubMed] [Google Scholar]
- 16.Lee Theilen M, Chaudhuri J. Walking the AID tightrope. Nat Immunol. 2010;11:107–109. doi: 10.1038/ni0210-107. [DOI] [PubMed] [Google Scholar]
- 17.Endo Y, Marusawa H, Kinoshita K, Morisawa T, Sakurai T, Okazaki IM, Watashi K, Shimotohno K, Honjo T, Chiba T. Expression of activation-induced cytidine deaminase in human hepatocytes via NF-kappaB signaling. Oncogene. 2007;26:5587–5595. doi: 10.1038/sj.onc.1210344. [DOI] [PubMed] [Google Scholar]
- 18.Astler VB, Coller FA. The prognostic significance of direct extension of carcinoma of the colon and rectum. Ann Surg. 1954;139:846–851. doi: 10.1097/00000658-195406000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kononen J, Bubendorf L, Kallioniemi A, Bärlund M, Schraml P, Leighton S, Torhorst J, Mihatsch MJ, Sauter G, Kallioniemi OP. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat Med. 1998;4:844–847. doi: 10.1038/nm0798-844. [DOI] [PubMed] [Google Scholar]
- 20.Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ. Colorectal carcinoma: a general overview and future perspectives in colorectal cancer. Int J Mol Sci. 2017;18:E197. doi: 10.3390/ijms18010197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen J, Pitmon E, Wang K. Microbiome, inflammation and colorectal cancer. Semin Immunol. 2017;32:43–53. doi: 10.1016/j.smim.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 22.Karki R, Man SM, Kanneganti TD. Inflammasomes and cancer. Cancer Immunol Res. 2017;5:94–99. doi: 10.1158/2326-6066.CIR-16-0269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andoh A, Yagi Y, Shioya M, Nishida A, Tsujikawa T, Fujiyama Y. Mucosal cytokine network in inflammatory bowel disease. World J Gastroenterol. 2008;14:5154–5161. doi: 10.3748/wjg.14.5154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Muñoz DP, Lee EL, Takayama S, Coppé JP, Heo SJ, Boffelli D, Di Noia JM, Martin DI. Activation-induced cytidine deaminase (AID) is necessary for the epithelial-mesenchymal transition in mammary epithelial cells. Proc Natl Acad Sci U S A. 2013;110:E2977–2986. doi: 10.1073/pnas.1301021110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kou T, Marusawa H, Kinoshita K, Endo Y, Okazaki IM, Ueda Y, Kodama Y, Haga H, Ikai I, Chiba T. Expression of activation-induced cytidine deaminase in human hepatocytes during hepatocarcinogenesis. Int J Cancer. 2007;120:469–476. doi: 10.1002/ijc.22292. [DOI] [PubMed] [Google Scholar]


