Abstract
Altered expression of serum microRNAs (miRNA) has been reported to correlate with carcinogenesis and progression of glioblastoma (GBM). This study assessed the potential diagnostic and prognostic value of serum miR-29b for GBM. Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was used to detect the expression levels of serum miR-29b in 107 patients with GBM patients, 40 patients with anaplastic astrocytoma (AA) and 80 healthy volunteers. The results showed that serum miR-29b levels were much lower in patients with GBM than in those with AA or healthy controls. Receiver operating characteristic (ROC) curve analysis revealed that serum exosomal miR-29b could effectively distinguish GBM patients from AA patients or normal controls. In addition, serum exosomal miR-29b level was significantly increased after treatment. Low serum exosomal miR-29b expression was strongly associated with aggressive clinical findings and shorter survival. Moreover, the Cox regression analysis demonstrated that serum exosomal miR-29b was an independent prognostic indicator. Collectively, serum exosomal miR-29b might be a promising biomarker for predicting prognosis of GBM.
Keywords: Glioblastoma, exosomes, miR-29b, prognosis
Introduction
As the most fatal type of glioma and a frequent primary brain tumor in the central nervous system, glioblastoma (GBM) is characterized by rapid growth, high invasiveness, and treatment resistance [1,2]. Conventional therapies for GBM including surgical resection, radiotherapy, and chemotherapy. Although great improvements have been made for the treatment over the past decades, the prognosis of GBM is still unfavorable [3,4]. Therefore, identification of novel diagnostic and prognostic biomarkers is urgently required to improve the clinical outcome of GBM.
MicroRNA (miRNA) is a group of small noncoding RNAs of 18-25 nucleotides in length that post-transcriptionally regulate the expression of target mRNAs, leading to degradation or translational inhibition of the target mRNAs [5,6]. Evidence has demonstrated that miRNAs are frequently dysregulated in a variety of cancers and associated with various pathologic processes, such as uncontrolled proliferation, metastasis, invasion, and immortality [7,8]. Exosomes are nano-sized vesicles of 30-200 nm and widely present in a variety of biologic fluids, including saliva, urine, blood, and ascites. Exosomes contain a wide range of active molecules, such as miRNAs, mRNAs, and proteins [9-11]. Exosomal miRNAs are highly stable in serum, and can be used as effective and reliable biomarkers for cancer detection and prognosis. For instance, serum exosomal miR-21 was identified as a non-invasive biomarker for hepatocellular carcinoma [12]. Similarly, serum exosomal miR-141 was used as a biomarker fordetection of prostate cancer [13].
Previous studies have shown that miR-29b might play a tumor suppressive role in GBM. For instance, Chung et al. revealed that miR-29b reduced the oncogenic activities of GBM cells by decreasing MMP-2 activity and inhibited aggressiveness by suppressing angiogenesis [14]. Downregulation of miR-29b was observed in GBM tissues. Enforced miR-29b expression resulted in cell apoptosis and S phase arrest in GBM cells, suggesting miR-29b exerted a tumor-suppressive effect in GBM [15]. Likewise, miR-29b expression was significantly downregulated in glioma tissues. Overexpression of miR-29b significantly suppressed glioma cell proliferation and induced apoptosis by directly targeting MYCN [16]. However, the expression pattern of serum exosomal miR-29b in GBM and its association with the clinicopathologic variables of GBM remain poorly known. The primary goal of this study was to explore the potential value of serum exosomal miR-29b as a biomarker for diagnosis and prognosis of GBM.
Materials and methods
Study population
The current study included 107 patients diagnosed with GBM (WHO grade IV), 40 patients with anaplastic astrocytoma (AA, WHO grade III) and 80 healthy controls. Among GBM cases, 56 patients (52.3%) were male. The mean age of all patients was 50.4 years. Among all GBM patients, 79 cases were treated with gross total resection or partial resection. The study protocol was approved by Ethical Committee of Jiangxi Provincial People’s Hospital. Written consents were collected from all the participants prior to the recruitment. The clinical characteristics of the GBM patients are presented in Table 1.
Table 1.
Relationship between clinical factors and serum exosomal miR-29b expression level
| Characteristic | Patients (N=107) | miR-29b high (n=48) | miR-29b low (n=59) | P |
|---|---|---|---|---|
| Sex | 0.2244 | |||
| Man | 56 | 22 | 34 | |
| Woman | 51 | 26 | 25 | |
| Age | 0.7488 | |||
| <50 | 45 | 21 | 24 | |
| ≥50 | 62 | 27 | 35 | |
| Tumor size (cm) | 0.1251 | |||
| <5 | 47 | 25 | 22 | |
| ≥5 | 60 | 23 | 37 | |
| MGMT status | 0.0189 | |||
| Unmethylated | 58 | 20 | 38 | |
| Methylated | 49 | 28 | 21 | |
| IDH1 status | 0.0341 | |||
| Mutated | 36 | 11 | 25 | |
| Wild-type | 71 | 37 | 34 | |
| Preoperative KPS | 0.0404 | |||
| <80 | 38 | 12 | 26 | |
| ≥80 | 69 | 36 | 33 |
MGMT, O-6-methylguanine-DNA methyltransferase; IDH, isocitrate dehydrogenase; KPS, Karnofsky performance scale.
Isolation and purification of exosomes from serum
After harvesting from all the participants, the blood samples were centrifuged at 3,000 rpm for 10 min at 4°C, then the serum samples were stored at -80°C until further analysis. Exosomes were isolated from serum using ExoQuick Exosome Precipitation Solution (System Biosciences, Palo Alto, CA, USA) following the manufacturer’s instructions. The exosome pellet was resuspended in sterile PBS solution, aliquoted in cryogenic vials and stored at -80°C until future use.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA was extracted from the exosomal pelleted suspensions using a miRNeasy Serum/Plasma kit (Qiagen, Hilden, Germany). The RNA concentration was quantified by measuring the A260/A280 absorbance ratios (Nano-Drop Technologies, Wilmington, DE). Quantitative reverse transcription-polymerase chain reaction (qRT-PCR) was performed using the PrimeScript RT Reagent kit and SYBR Premix Ex Taq kit (Takara, Tianjin, China) in the Applied BiosystemsTM 7500 Real-Time PCR Systems (Applied Biosystems, Carlsbad, CA, USA). All reactions were repeated in triplicate. During RNA isolation, 2 μL synthetic Caenorhabditis elegans cel-miR-39 (RiboBio, Guangzhou, China) was added to each sample as a spike-in control. 2-ΔΔCt was used to detect the relative serum exosomal miR-29b levels.
Statistical analysis
The Mann-Whitney test and Kruskal-Wallis test were used to determine differences in serum exosomal miR-29b expression levels between groups. Chi-square test was carried out to evaluate the significance of differences in categorized demographic data. Receiver-operator characteristic (ROC) curve analysis was used to assess the accuracy of discriminating GBM from AA or healthy controls, and the area under the ROC curve (AUC) was calculated. Overall survival (OS) and disease-free survival (DFS) were calculated by the Kaplan-Meier method and log-rank test. OS was defined as the time from treatment to death or last follow-up; DFS was defined as the time from treatment to relapse or death or last follow-up. Univariate and multivariate analyses were performed to identify prognostic indicators using the Cox regression model. All statistical analyses were performed using MedCalc (version 15.6.1) and Graphpad (version 5.0). P<0.05 was considered significant.
Results
Downregulation of serum exosomal miR-29b in GBM
The serum exosomal miR-29b levels were detected in a total of 107 GBM patients, 40 cases with AA, and 80 healthy controls by qRT-PCR. Serum exosomal miR-29b levels were significantly lower in GBM patients than in AA patients (P=0.019) or normal controls (P<0.001, Figure 1A). Serum exosomal miR-29b levels were significantly higher in GBM patients with methylated MGMT status (P=0.016), with wild-type IDH1 status (P=0.012), and better preoperative KPS (P=0.017). To determine whether the serum exosomal miR-29b could be used for monitoring therapeutic response, blood samples were collected from the GBM subjects who received surgical treatment. The serum exosomal miR-29b in paired pre-operative and post-operative blood samples were compared, and we found that serum exosomal miR-29b levels of the postoperative samples were markedly increased compared to the preoperative samples (P=0.009, Figure 1E).
Figure 1.
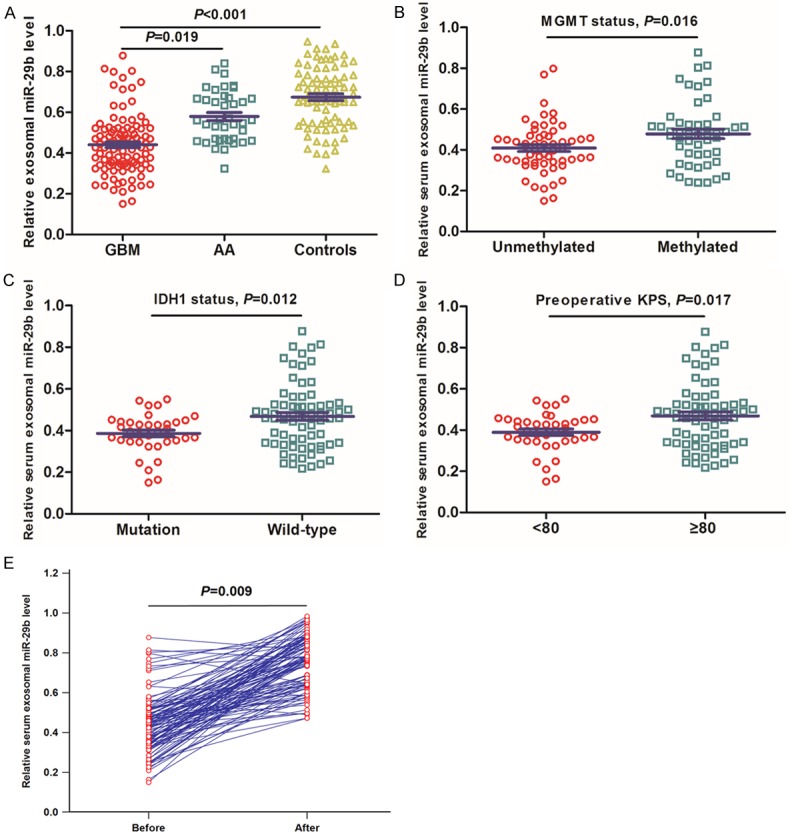
A. Serum exosomal miR-29b expression was significantly lower in GBM patients. B. Serum exosomal miR-29b expression was significantly higher in GBM patients with methylated MGMT status. C. Serum exosomal miR-29b expression was significantly higher in GBM patients with wild-type IDH1 status. D. Serum exosomal miR-29b expression was significantly higher in GBM patients with ≥80 preoperative KPS. E. Comparison of serum exosomal miR-29b levels between pre- and postoperative samples collected from GBM patients.
ROC analysis
ROC curve analysis was carried out to evaluate the diagnostic accuracy of serum exosomal miR-29b for GBM. We found that serum exosomal miR-29b could well identify GBM patients from normal controls, with an AUC of 0.866. The sensitivity and specificity were 83.18% and 81.25%, respectively (Figure 2A). Serum exosomal miR-29b also performed well to discriminate GBM patients from AA patients. Sensitivity was 81.3% and the specificity was 77.5%, with an AUC of 0.825 (Figure 2B).
Figure 2.
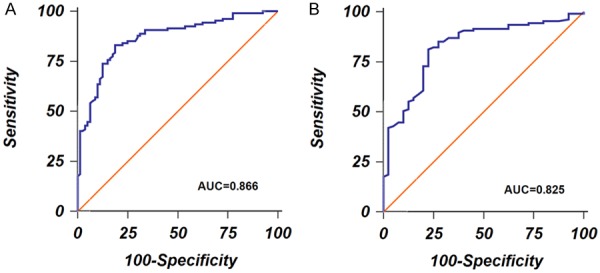
A. ROC curves to discriminate GBM patients and normal controls. B. ROC curves to discriminate GBM patients and anaplastic astrocytomas patients.
Serum exosomal miR-29b expression and clinical variables of GBM patients
All 107 patients with GBM were divided into a low serum exosomal miR-29b expression group (n=59) and high serum exosomal miR-29b expression group (n=48) based on the median value of serum exosomal miR-29b level. As in Table 1, low serum exosomal miR-29b expression was strongly associated with O-6-methylguanine-DNA methyltransferase (MGMT) methylation status (P=0.0189), isocitrate dehydrogenase (IDH) mutation status (P=0.0341), and Karnofsky performance scale (KPS) (P=0.0404). However, no significant relationship was found between serum exosomal miR-29b expression and other factors, including sex (P=0.2244), age (P=0.7488), and tumor size (P=01251).
Serum exosomal miR-29b expression and prognosis of GBM
The Kaplan-Meier method was used to analyze the correlation between serum exosomal miR-29b level and survival rate. Results showed that GBM patients in the low serum exosomal miR-29b group had shorter OS compared to those in the high serum exosomal miR-29b group (P=0.0008, Figure 3A). Likewise, GBM patients in the low serum exosomal miR-29b group had worse DFS than those in the high serum exosomal miR-29b group (P<0.0001, Figure 3B).
Figure 3.
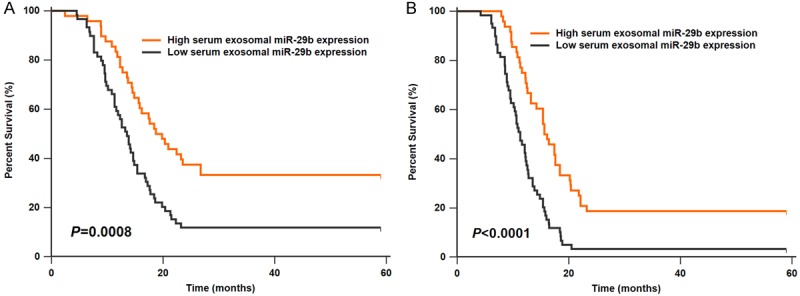
A. GBM patients with high exosomal miR-29b expression had longer overall survival. B. GBM patients with high exosomal miR-29b expression had longer disease-free survival.
Univariate and multivariate analyses for OS in GBM patients (n=107) were performed. In the univariate analysis, MGMT status (HR=3.13, 95% CI=1.65-4.87, P=0.018), IDH1 status (HR=2.89, 95% CI=1.51-4.56, P=0.022), preoperative KPS (HR=2.65, 95% CI=1.41-4.02, P=0.027), and serum exosomal miR-29b (HR=3.46, 95% CI=1.82-5.31, P=0.016) showed significance. By multivariate analysis, MGMT status (HR=2.73, 95% CI=1.47-4.14, P=0.026), IDH1 status (HR=2.50, 95% CI=1.33-3.82, P=0.031), preoperative KPS (HR=2.24, 95% CI=1.16-3.48, P=0.035), and serum exosomal miR-29b (HR=2.98, 95% CI=1.57-4.69, P=0.021) were independent prognostic factors (Table 2).
Table 2.
Univariate and multivariate analysis for overall survival of GBM patients
| Finding | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
|
|
|
|||
| HR (95% CI) | P | HR (95% CI) | P | |
| MGMT status | 3.13 (1.65-4.87) | 0.018 | 2.73 (1.47-4.14) | 0.026 |
| IDH1 status | 2.89 (1.51-4.56) | 0.022 | 2.50 (1.33-3.82) | 0.031 |
| Preoperative KPS | 2.65 (1.41-4.02) | 0.027 | 2.24 (1.16-3.48) | 0.035 |
| Serum exosomal miR-29b | 3.46 (1.82-5.31) | 0.016 | 2.98 (1.57-4.69) | 0.021 |
CI, Confidence Interval; HR, Hazard Ratio.
Discussion
Previous studies have reported the tumor suppressive role of miR-29b in GBM, but this study is the first to evaluate the clinical value of serum exosomal miR-29b in GBM patients. We found that serum exosomal miR-29b levels were significantly lower in GBM subjects than in AA cases and healthy controls. In addition, serum exosomal miR-29b levels in postoperative blood samples were greatly higher than in preoperative blood samples. Moreover, serum exosomal miR-29b could discriminate GBM cases from AA or normal controls with high accuracy. Furthermore, low serum exosomal miR-29b expression was strongly associated with aggressive clinical variables and worse prognosis. Finally, serum exosomal miR-29b was confirmed to be an independent prognostic biomarker. These data suggest that serum exosomal miR-29b is a promising biomarker for the diagnosis and prognosis of GBM.
Downregulation of miR-29b has also been reported in other types of cancer. For instance, Ru et al. revealed that miR-29b expression was decreased in prostate cancer cell lines and tissues, and ectopic expression of miR-29b attenuated the metastatic capability of cancer cells [17]. In pancreatic cancer, miR-29b upregulation or SOX12 inhibition markedly restrained cell proliferation, migration, and invasion, and vice versa [18]. In gastric cancer, miR-29b expression was significantly reduced in cancerous tissues, and in vitro and in vivo analysis showed that upregulation of miR-29b greatly repressed the tumorigenicity by regulating MMP2 [19]. MiR-29b downregulation occurred more frequently in multiple myeloma (MM) plasma and cell lines. MiR-29b overexpression not only dramatically inhibited MM cell proliferation and viability, but also stimulated MM cell cycle arrest and apoptosis. EZH2, Mcl-1 and FOXP1 were its downstream target genes [20-22]. In breast cancer (BC), low miR-29b expression was found to be strongly associated with shorter survival and was an independent prognostic factor for BC [23,24]. Similarly, the expression level of miR-29b was significantly lower in osteosarcoma tissues compared to adjacent normal tissues. miR-29b upregulation significantly inhibited cell proliferation, migration, invasiveness, and induced apoptosis by degrading VEGF or CDK6 expression [25,26]. In colorectal carcinoma (CRC), downregulation of miR-29b was observed both in CRC tissues and cell lines, and closely associated with aggressive clinical data. Elevated miR-29b expression significantly decreased cell proliferation and migration in vitro and inhibited the tumorigenesis in vivo by inversely regulating Tiam1 [27,28]. Wang et al. found that miR-29b expression was dramatically decreased in cell lines and tissues of non-small cell lung cancer (NSCLC). In vitro and in vivo evidence showed that miR-29b inhibition enhanced the oncogenic activities of cancer cells bytargeting MMP2 and PTEN [29]. Furthermore, decreased miR-29b expression was found in esophageal squamous cell carcinoma (ESCC) tissues and cell lines, and overexpression of miR-29b inhibited ESCC cell invasion in vitro and cell growth in vivo by regulating MMP-2 [30]. Li et al. reported that miR-29b expression was dramatically decreased in chronic myelogenous leukemia (CML), and miR-29b overexpression significantly suppressed CML cell growth and colony formation, and promoted apoptosis by negatively regulating ABL1 [31]. These findings indicated miR-29b acted as a tumor suppressor in various tumor types.
Interestingly, miR-29b had been reported to exert an oncogenic function in renal cell carcinoma (RCC) and oral squamous cell carcinoma (OSCC). Xu et al. found that miR-29b expression was markedly increased in cancerous tissues and cell lines of RCC; and high miR-29b expression was strongly correlated with advanced TNM stage and poor survival. In addition, repression of miR-29b could decrease RCC cell proliferation and invasion by negatively regulating KIF1B expression [32]. Yang et al. demonstrated that miR-29b was upregulated in OSCC patients with lymph node metastasis, advanced tumor stage, and recurrence. Moreover, ectopic expression of miR-29b significantly enhanced the tumorigenic properties of OSCC cells through silencing CX3CL1 expression [33]. The opposite role of miR-29b in different cancers suggested this miRNA might function as an oncogene or a tumor suppressor gene, depending on the tumor microenvironment.
In conclusion, we demonstrated that serum exosomal miR-29b expression was significantly reduced in GBM. In addition, its downregulation was associated with unfavorable prognosis of GBM, indicating that serum exosomal miR-29b has potential for GBM detection and prognosis.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 2.Ostrom QT, Gittleman H, Liao P, Vecchione-Koval T, Wolinsky Y, Kruchko C, Barnholtz-Sloan JS. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2010-2014. Neuro Oncol. 2017;19(Suppl 5):v1–v88. doi: 10.1093/neuonc/nox158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van Meir EG, Hadjipanayis CG, Norden AD, Shu HK, Wen PY, Olson JJ. Exciting new advances in neuro-oncology: the avenue to a cure for malignant glioma. CA Cancer J Clin. 2010;60:166–193. doi: 10.3322/caac.20069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Franceschi S, Mazzanti CM, Lessi F, Aretini P, Carbone FG, LA Ferla M, Scatena C, Ortenzi V, Vannozzi R, Fanelli G, Pasqualetti F, Bevilacqua G, Zavaglia K, Naccarato AG. Investigating molecular alterations to profile short- and long-term recurrence-free survival in patients with primary glioblastoma. Oncol Lett. 2015;10:3599–3606. doi: 10.3892/ol.2015.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 6.Calin GA, Croce CM. MicroRNA signatures in human cancers. Nat Rev Cancer. 2006;6:857–866. doi: 10.1038/nrc1997. [DOI] [PubMed] [Google Scholar]
- 7.He L, Hannon GJ. MicroRNAs: small RNAs with a big role in gene regulation. Nat Rev Genet. 2004;5:522–531. doi: 10.1038/nrg1379. [DOI] [PubMed] [Google Scholar]
- 8.Deng D, Liu Z, Du Y. Epigenetic alterations as cancer diagnostic, prognostic, and predictive biomarkers. Adv Genet. 2010;71:125–176. doi: 10.1016/B978-0-12-380864-6.00005-5. [DOI] [PubMed] [Google Scholar]
- 9.Colombo M, Raposo G, Thery C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30:255–289. doi: 10.1146/annurev-cellbio-101512-122326. [DOI] [PubMed] [Google Scholar]
- 10.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proc Natl Acad Sci U S A. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lasser C. Exosomal RNA as biomarkers and the therapeutic potential of exosome vectors. Expert Opin Biol Ther. 2012;12(Suppl 1):S189–S197. doi: 10.1517/14712598.2012.680018. [DOI] [PubMed] [Google Scholar]
- 12.Wang H, Hou L, Li A, Duan Y, Gao H, Song X. Expression of serum exosomal microRNA-21 in human hepatocellular carcinoma. Biomed Res Int. 2014;2014:864894. doi: 10.1155/2014/864894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li Z, Ma YY, Wang J, Zeng XF, Li R, Kang W, Hao XK. Exosomal microRNA-141 is upregulated in the serum of prostate cancer patients. Onco Targets Ther. 2015;9:139–148. doi: 10.2147/OTT.S95565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chung HJ, Choi YE, Kim ES, Han YH, Park MJ, Bae IH. MiR-29b attenuates tumorigenicity and stemness maintenance in human glioblastoma multiforme by directly targeting BCL2L2. Oncotarget. 2015;6:18429–18444. doi: 10.18632/oncotarget.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shin J, Shim HG, Hwang T, Kim H, Kang SH, Dho YS, Park SH, Kim SJ, Park CK. Restoration of miR-29b exerts anti-cancer effects on glioblastoma. Cancer Cell Int. 2017;17:104. doi: 10.1186/s12935-017-0476-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun G, Lu J, Zhang C, You R, Shi L, Jiang N, Nie D, Zhu J, Li M, Guo J. MiR-29b inhibits the growth of glioma via MYCN dependent way. Oncotarget. 2017;8:45224–45233. doi: 10.18632/oncotarget.16780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ru P, Steele R, Newhall P, Phillips NJ, Toth K, Ray RB. MiRNA-29b suppresses prostate cancer metastasis by regulating epithelial-mesenchymal transition signaling. Mol Cancer Ther. 2012;11:1166–1173. doi: 10.1158/1535-7163.MCT-12-0100. [DOI] [PubMed] [Google Scholar]
- 18.Wang L, Wang Z, Huang L, Wu C, Zhang B. MiR-29b suppresses proliferation and mobility by targeting SOX12 and DNMT3b in pancreatic cancer. Anticancer Drugs. 2019;30:281–288. doi: 10.1097/CAD.0000000000000719. [DOI] [PubMed] [Google Scholar]
- 19.Wang T, Hou J, Jian S, Luo Q, Wei J, Li Z, Wang X, Bai P, Duan B, Xing J, Cai J. MiR-29b negatively regulates MMP2 to impact gastric cancer development by suppress gastric cancer cell migration and tumor growth. J Cancer. 2018;9:3776–3786. doi: 10.7150/jca.26263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamato MA, Juli G, Romeo E, Ronchetti D, Arbitrio M, Caracciolo D, Neri A, Tagliaferri P, Tassone P, Amodio N. Inhibition of EZH2 triggers the tumor suppressive miR-29b network in multiple myeloma. Oncotarget. 2017;8:106527–106537. doi: 10.18632/oncotarget.22507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang YK, Wang H, Leng Y, Li ZL, Yang YF, Xiao FJ, Li QF, Chen XQ, Wang LS. Overexpression of microRNA-29b induces apoptosis of multiple myeloma cells through down regulating Mcl-1. Biochem Biophys Res Commun. 2011;414:233–239. doi: 10.1016/j.bbrc.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 22.Wang H, Ding Q, Wang M, Guo M, Zhao Q. MiR-29b inhibits the progression of multiple myeloma through downregulating FOXP1. Hematology. 2019;24:32–38. doi: 10.1080/10245332.2018.1502961. [DOI] [PubMed] [Google Scholar]
- 23.Shinden Y, Iguchi T, Akiyoshi S, Ueo H, Ueda M, Hirata H, Sakimura S, Uchi R, Takano Y, Eguchi H, Sugimachi K, Kijima Y, Natsugoe S, Mimori K. MiR-29b is an indicator of prognosis in breast cancer patients. Mol Clin Oncol. 2015;3:919–923. doi: 10.3892/mco.2015.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu Y, Zhang J, Sun X, Su Q, You C. Down-regulation of miR-29b in carcinoma associated fibroblasts promotes cell growth and metastasis of breast cancer. Oncotarget. 2017;8:39559–39570. doi: 10.18632/oncotarget.17136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhang K, Zhang C, Liu L, Zhou J. A key role of microRNA-29b in suppression of osteosarcoma cell proliferation and migration via modulation of VEGF. Int J Clin Exp Pathol. 2014;7:5701–5708. [PMC free article] [PubMed] [Google Scholar]
- 26.Zhu K, Liu L, Zhang J, Wang Y, Liang H, Fan G, Jiang Z, Zhang CY, Chen X, Zhou G. MiR-29b suppresses the proliferation and migration of osteosarcoma cells by targeting CDK6. Protein Cell. 2016;7:434–444. doi: 10.1007/s13238-016-0277-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Inoue A, Yamamoto H, Uemura M, Nishimura J, Hata T, Takemasa I, Ikenaga M, Ikeda M, Murata K, Mizushima T, Doki Y, Mori M. MicroRNA-29b is a novel prognostic marker in colorectal cancer. Ann Surg Oncol. 2015;22(Suppl 3):S1410–8. doi: 10.1245/s10434-014-4255-8. [DOI] [PubMed] [Google Scholar]
- 28.Wang B, Li W, Liu H, Yang L, Liao Q, Cui S, Wang H, Zhao L. MiR-29b suppresses tumor growth and metastasis in colorectal cancer via downregulating Tiam1 expression and inhibiting epithelial-mesenchymal transition. Cell Death Dis. 2014;5:e1335. doi: 10.1038/cddis.2014.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang H, Guan X, Tu Y, Zheng S, Long J, Li S, Qi C, Xie X, Zhang H, Zhang Y. MicroRNA-29b attenuates non-small cell lung cancer metastasis by targeting matrix metalloproteinase 2 and PTEN. J Exp Clin Cancer Res. 2015;34:59. doi: 10.1186/s13046-015-0169-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Y, Li X, Zhao S. MiR-29b inhibits the progression of esophageal squamous cell carcinoma by targeting MMP-2. Neoplasma. 2015;62:384–390. doi: 10.4149/neo_2015_046. [DOI] [PubMed] [Google Scholar]
- 31.Li Y, Wang H, Tao K, Xiao Q, Huang Z, Zhong L, Cao W, Wen J, Feng W. MiR-29b suppresses CML cell proliferation and induces apoptosis via regulation of BCR/ABL1 protein. Exp Cell Res. 2013;319:1094–1101. doi: 10.1016/j.yexcr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 32.Xu Y, Zhu J, Lei Z, Wan L, Zhu X, Ye F, Tong Y. Expression and functional role of miR-29b in renal cell carcinoma. Int J Clin Exp Pathol. 2015;8:14161–14170. [PMC free article] [PubMed] [Google Scholar]
- 33.Yang CN, Deng YT, Tang JY, Cheng SJ, Chen ST, Li YJ, Wu TS, Yang MH, Lin BR, Kuo MY, Ko JY, Chang CC. MicroRNA-29b regulates migration in oral squamous cell carcinoma and its clinical significance. Oral Oncol. 2015;51:170–177. doi: 10.1016/j.oraloncology.2014.10.017. [DOI] [PubMed] [Google Scholar]


