Abstract
Uterine cervical cancer (UCC) causes more than one quarter of a million deaths per year in many developing countries. Nearly all cases of cervical cancer result from infection with the human papillomavirus. After high-risk HPV infection, most HPV infections are cleared naturally as a result of humoral and cell-mediated immune responses. Only a limited number of patients’ cervical lesions progress through CIN to cervical cancer, from persistent oncogenic human papillomavirus (HPV) infection. This indicated that immunoregulation may play a central role in the HPV-induced carcinogenesis. However, the natural history of clearance of a cervical HPV infection or its progression to a UCC needs clarification. We examined the related immune cells (Th1, Th2, Th17 and Treg cells) and related immune factors (INF-γ, IL-4, IL-10, IL-17, IL-23, TGF-βI) of UCC patients, CIN patients, HPV infected patients, and healthy controls. Compared with healthy controls, patients with UCC or CIN had a lower proportion of Th1 cells, and a higher proportion of Th2, Th17, and Treg cells. IL-4, IL-10, IL-17, IL-23 and TGF-βI concentrations in serum were found to be increased from patients with UCC or CIN, while INF-γ concentration in serum with UCC or CIN decreased. Our findings suggested that there were attractive imbalances of Th1/Th2 and Th17/Treg cells in UCC and CIN patients. HPV persistent infection induced an immunologic dissonance, and the degree of imbalance is aggravated with the progression of the disease.
Keywords: Uterine cervical cancer, cervical intraepithelial neoplasia, T helper 17, T helper 1, T helper 2, Treg
Introduction
Uterine cervical cancer (UCC), is the one of the most common cancers diagnosed in women worldwide, and causes more than one quarter of a million deaths per year as a result of grossly deficient treatments in many developing countries. Nearly all cases of cervical cancer result from infection by the human papillomavirus. In addition, a high proportion of cases goes through a preinvasive phase (cervical intraepithelial neoplasia, CIN) that can be detectable by clinical methods [1,2].
After high-risk HPV infection, most HPV infections are cleared naturally as a result of humoral and cell-mediated immune responses [2]. Only a limited number of patients’ cervical lesions progress through CIN to cervical cancer [3], because of persistent oncogenic human papillomavirus (HPV) infection [4,5]. This indicates that immunoregulation may play a central role in the HPV-induced carcinogenesis. However, the natural history of clearance of a cervical HPV infection or its progression to a UCC needs to be better understood.
Recently, Th17 cells and regulatory T cells (Treg cells) have been defined as two distinct CD4+ T subsets from Th1 and Th2 cells. Th17 is a subset of CD4+RORγt+IL23R+CCR6+CD161+ IL17-producing T cells [6-8]. Th17 cells and their effector cytokines (such as IL-17A, IL-17F, IL-21, IL-22, and IL-26) are increasingly being recognized as key players in the pathogenesis of various autoimmune diseases and in mediating host defensive mechanisms against various infections [9-13]. The autocrine activity of IL-21, activation of STAT3, and induction of the orphan nuclear receptor ROR γt are the key regulators of Th17-cell lineage differentiation [14,15]. However, the nature of Th17 cells is not understood in cancer patients.
Treg cells have a major role in modulating the activity of self-reactive cells. They are characterized by expressing Foxp3 in the nuclei, and are a functionally immunosuppressive subset of T cells. This vitally important function exists alongside the detrimental effects on tumor immunosurveillance and antitumor immunity [16]. Evidences from cancer patients suggest that increased Treg activity may be associated with poor immune responses to tumor antigens and contribute to immune dysfunction [17]. Women with hematologic malignancies after stem cell transplant have low rate of cervical cancer screening for fresh normal T cells [18]. These reports suggested that Treg cells might suppress the antitumor immune response. Whether Treg cells comprise a higher proportion of peripheral blood lymphocytes in patients with HPV infection, UCC, or CIN has not been investigated.
It was reported that the balance between Treg and Th17 cells controlled immune response and was a key factor in regulating helper T cell function relating to the Th1/Th2 shift in autoimmune disease and graft vs. host disease [19]. However, the information on the balance between Th1 and Th2, Treg, and Th17 cells in cancer patients has been minimally reported [20]. In our study, we measured the levels of Th1, Th2 Th17 and Treg cells and the related immune factors in UCC, CIN, and HPV infected patients and healthy controls to detect the possible roles of the imbalance between regulatory and effector cells in the development and progression of uterine cervical cancer.
Materials and methods
Materials and samples
This research was approved by the ethics committee and written informed consent for participation in the study was obtained from each subject. 139 fresh specimens were obtained from the Department of Gynecology, The Affiliated Hospital of Qingdao University. Thirty-eight untreated UCC patients (age range 39-69 years, 46.2±6.9 years), 61 untreated CIN patients (age range 24-56 years, 41.4±5.8 years) and 20 HPV infected patients with normal cervical smear (age range 25-52 years, 40.6±5.5 years) were enrolled in this study. Patients complicated by hypertension, cardiovascular diseases, diabetes, pregnancy, active or chronic infection, connective tissue diseases, endometriosis or with malignant tumor in the past history, were excluded. No initial immunosuppression, radiotherapy, or chemotherapy was performed prior to the surgery. All of the cases were histologically proven and the clinical stage of UCC patients was based on FIGO (International Federation of Gynecology and Obstetrics) 2009. Twenty healthy women (age range 25-68 years, 42.9±7.1 years) were selected as a control group.
Flow cytometric analysis of Th1, Th2, Th17 and Treg cells
Intracellular cytokines were evaluated by flow cytometry to reflex the Th1, Th2 and Th17 cytokine-producing cells. Heparinized peripheral whole blood (200 μl) with an equal volume of Roswell Park Memorial Institute 1640 medium was incubated for 4 h at 37°C, 5% CO2 in the presence of 25 ng/ml of phorbol myristate acetate (PMA), 1 μg/ml of ionomycin, and 1.7 μg/ml Monensin (all from Alexis Biochemicals, San Diego, CA). PMA and ionomycin are T-cell-activating agents that mimic signals generated by the T-cell receptor complex and have the advantage of stimulating T cells of any antigen specificity. Monensin was used to block intracellular transport mechanisms, leading to an accumulation of cytokines in the cells. After incubation, the cells were stained with PE-conjugated anti-γ-IFN, anti-IL-4, anti-IL17 and anti-CD4-FITC, (Caltag Laboratories, Burlingame, CA, USA). Isotype controls were given to enable correct comparisons and confirm antibody specificity. Stained cells were analyzed by flow cytometric analysis using a FACS can cytometer equipped with CellQuest software (BD Bioscience Pharmingen, San Diego, CA).
Circulating CD4+/CD25+/FoxP3+ Tregs were enumerated by flow cytometry. Peripheral blood mononuclear cells (PBMCs) were incubated with anti-CD4-FITC and anti-CD25-PC5 (Beckman Coulter, Immunotech, France) mAb for 30 min at 4°C. Subsequent to washing with PBS, PBMCs were fixed and permeabilized with fixation/permeabilization buffer for 30 min at 4°C, washed twice with permeabilization buffer and stained with anti-human FoxP3-PE mAb, following the manufacturer’s instructions (eBioscience, San Diego, CA, USA). Following a 30-min incubation at 4°C, cells were washed and analyzed by flow cytometry in a Coulter Epics IV Cytometer (Beckman Coulter, Inc, Fullerton, CA, USA) employing Expo32 Software (Beckman Coulter). Cells were gated on viable lymphocytes, following standard forward and sideways scattering parameters. Among cells included in this gate, we evaluated Treg subpopulations as a CD4+/CD25+/FoxP3+ subset. The results are expressed as percentage of triple-positive cells as a proportion of the autofluorescence of CD4+ cells.
Immunohistochemical analysis
The specimens, preserved in Bouin’s solution were washed in 50% and 70% ethanol before embedding in paraffin and subsequently sectioning them at 4 μm. The sections were dewaxed in xylene, rehydrated through a series of ethanol dilutions, and endogenous peroxidase was blocked with 3% H2O2 for 10 min at room temperature. Sections were incubated with the blocking buffer (5% normal goat serum) for 30 min at room temperature. After removing the blocking buffer, the sections were probed with the anti-γ-IFN, anti-IL-4, anti-IL-17 and anti-FoxP3 polyclonal antibodies (Santa Cruz, USA, at a 1:500 dilution) at 4°C for 12 h in a humidified chamber. The sections were washed with PBS three times and incubated with the secondary antibody (at a 1:1,000 dilution) conjugated with peroxidase for 30 min at 37°C followed by PBS wash and incubation with 3, 3-diaminobenzidine (DAB, Sigma, St. Louis, MO) for 5 min. The sections were finally counterstained with hematoxylin to visualize cell nuclei.
Evaluation of immunohistochemical staining
Each specimen was evaluated by two pathologists. The images of specimen were taken digitally with an inverted microscope (Olympus, Japan) and analyzed by Leica Qwin V3 image analysis software. The typical areas were selected under a visual field magnified 200 times. The average grayscale value of the selected area was assessed by the Leica Qwin V3 image analysis software. The value ranged between 0 and 201 and it was inversely proportional to the level of INF-γ, IL-4, IL-17 and Foxp3 proteins.
Enzyme linked immunosorbent assay (ELISA)
The serum concentrations of INF-γ, IL-4, IL-10, IL-17, IL-23, TGF-βI were measured by ELISA, following the manufacturer’s instructions (eBioscience, SanDiego, CA). All samples were measured in duplicate.
Statistical analysis
The results are presented as mean ± S.D. Association between all kinds of parameters among different groups were assessed using either t test, or one-way analysis of variance (ANOVA). P-values smaller than 0.05 were considered significant. Statistical analyses were performed using SPSS 17.0 software (SPSS Inc., Chicago, IL).
Results
Elevated circulating Th1 cells and decreased Th2 cells in patients with UCC, CIN and HPV infection
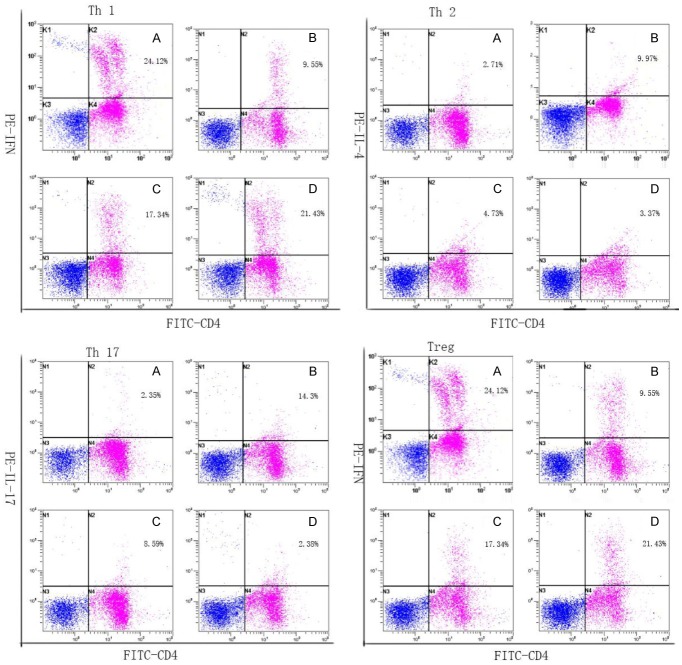
We first analyzed the expression of Th1 and Th2 cells based on cytokine patterns after in vitro activation by PMA/ionomycin in short-term cultures. Compared with healthy controls, patients with UCC or CIN had a lower proportion of Th1 cells (in Table 1 and Figure 1) and a higher proportion of Th2 cells (in Table 1 and Figure 1). Furthermore, the frequencies of Th1 cells decreased and Th2 cells increased in HPV infected patients comparing to the controls. The differences were not significant (P>0.05). Remarkably, an attractive imbalance of Th1/Th2 was observed in UUC and CIN patients.
Table 1.
Frequency of Th1, Th2, Th17, and Treg cells in controls and patients with UCC, CIN, or HPV (%, x̅ ± s)
| Th1 cell | Th2 cell | Th17 cell | Treg cell | |
|---|---|---|---|---|
| Control group (n=20) | 23.12±2.81 | 2.11±0.99 | 1.23±0.41 | 2.00±0.66 |
| HPV group (n=20) | 20.43±3.13 | 3.22±1.47 | 1.44±0.49 | 2.86±0.87a |
| CIN group (n=61) | 16.24±3.76a,b | 4.53±1.55a,b | 7.61±2.49a,b | 4.29±0.8a,b |
| UCC group (n=38) | 9.09±1.56a,b,c | 9.77±2.19a,b,c | 12.41±2.09a,b,c | 5.38±1.14a,b,c |
Compared to the control group;
P<0.05.
Compared to the HPV group;
P<0.05.
Compared to the CIN group;
P<0.05.
Figure 1.
Frequency of Th1, Th2, Th17, and Treg cells in controls and patients with UCC or CIN or HPV. All the cells were stained with PE-conjugated anti-γ-IFN, anti-IL-4, anti-IL17, anti-FoxP3+, and anti-CD4-FITC. Stained cells were analyzed by flow cytometric analysis using a FACS can cytometer equipped with Cell Quest software. The proportions of each cells in representative UCC patients, CIN patients, HPV infective patients and controls were annotated in the figure. Controls (A), UCC patients (B), CIN patients (C) and HPV-infected patients (D).
Elevated circulating Th17 cells and Treg cells in patients with UCC or CIN
We analyzed the expression of Th17 cells based on cytokine patterns after in vitro activation by PMA/ionomycin in short-term cultures. Compared to healthy controls, the percentage of Th17 cells was significantly increased in UCC patients (12.41±2.09%) and CIN patients (7.61±2.49%) as showed (in Table 1 and Figure 1). UCC patients also revealed a significant increase in percentage of Tregs (5.38±1.14%), as did the CIN patients (4.29±0.8%). Although the percentages of Th17 cells and Treg cells in patients with HPV were slightly higher than that in controls, no statistical difference was found (in Table 1 and Figure 1). Furthermore, the frequencies of Th17 cells and Treg cells were positively correlated in uterine cervical cancer patients. Remarkably, an attractive imbalance of Th17/Treg was observed in UUC and CIN patients. In UCC patients with lymph node metastases or vasoinvasion, the ratio of Th17/Treg was significantly higher than that in negative patients respectively (as in our recent research).
INF-γ is downregulated, but IL-4, IL-17 and Foxp3 are upregulated in patients with UCC or CIN
Among the 61 CIN patients, only 21 patients had CIN 3 and endured a cervical conization. Among the 38 UCC patients, only 20 patients needed radical hysterectomy. The control group contains patients who underwent hysterectomy for hysteromyoma. In view of the relationship between CD4+T cells and UCC, we examined the expression of INF-γ, IL-4, IL-17 and Foxp3 proteins by immunohistochemitry. In Table 3, we found the levels of INF-γ protein in UCC and CIN groups were lower than the control group. Furthermore, the levels of INF-γ protein in UCC were even lower than the CIN group (Table 3 and Figure 2). The conditions of IL-4, IL-17, and Foxp3 proteins were similar. They were all higher in UCC and CIN groups than in the control group. In addition, the differences among the UCC group, the CIN group, and the control group were all significant (P<0.05).
Table 3.
Expression of INF-γ, IL-4, IL-17, and Foxp3 protein in UCC, CIN, and controls
| Tissue type | Num | Average grayscale value [mean ± S.D.] | |||
|---|---|---|---|---|---|
|
| |||||
| INF-γ | IL-4 | IL-17 | Foxp3 | ||
| UCC | 20 | 158.40±5.32a,b | 131.33±4.89a,b | 120.34±5.79a,b | 125.43±7.85a,b |
| CIN | 20 | 146.20±7.85a | 154.33±8.55 | 135.66±5.99 | 136.71±5.71 |
| Controls | 20 | 129.40±9.50 | 163.17±7.88 | 143.50±8.83 | 142.29±6.80 |
Compared to the control group;
P<0.05.
Compared to the CIN group;
P<0.05.
Figure 2.
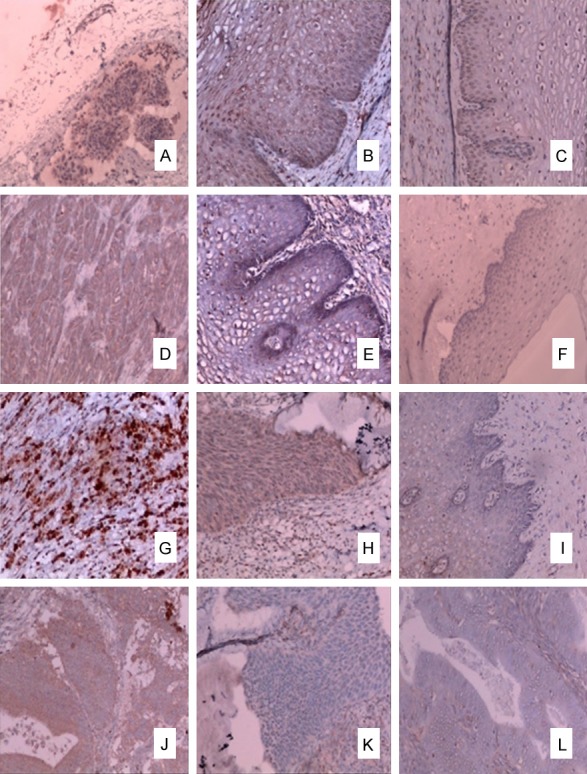
Levels of INF-γ, IL-4, IL-17, and Foxp3 protein in UCC, CIN, and controls. The levels of INF-γ protein in UCC (A) and CIN (B) groups were lower than in the control group (C). Furthermore, the level of INF-γ protein in UCC was even lower than in the CIN group (P<0.05). The levels of IL-4 protein in UCC (D) and CIN (E) groups were higher than in the control group (F). The levels of IL-17 protein in UCC (G) and CIN (H) groups were higher than in the control group (I). The levels of Foxp3 protein in UCC (J) and CIN (K) groups were higher than in the control group (L). The conditions of IL-4, IL-17 Foxp3 and proteins were similar. They were all higher in UCC and CIN groups than in the control group.
IL-4, IL-10, IL-17, IL-23, and TGF-βI concentrations were increased in serum with UCC or CIN, while decreased INF-γ concentration were found in serum with UCC or CIN
Serum INF-γ, IL-4, IL-10, IL-17, IL-23 and TGF-βI were determined in healthy donors and patients with CIN or UCC. As shown in Table 4, the IL-4, IL-10, IL-17, IL-23 and TGF-βI concentrations were significantly higher in UCC and CIN patients than those in control subjects. In addition, INF-γ concentrations were significantly lower in UCC and CIN patients. The results were consistent with the increased prevalence of Th2, Th17, and Treg cells and decreased Th1 cells in PBMCs of patients with UCC or CIN. Significant differences were also found between CIN patients and UCC patients.
Table 4.
Levels of INF-γ, IL-4, IL-10, IL-17, IL-23, and TGF-βI in the serum (pg/ml, x̅ ± s)
| Group | Num | INF-γ | IL-4 | IL-10 | IL-17 | IL-23 | TGF-βI |
|---|---|---|---|---|---|---|---|
| Control | 20 | 136.54±21.79 | 152.25±33.44 | 222.38±63.56 | 20.86±0.65 | 105.45±30.65 | 518.26±157.73 |
| HPV | 20 | 126.80±17.58 | 176.31±23.66 | 216.39±49.80 | 21.11±0.55 | 112.09±26.32 | 539.08±126.36 |
| CIN | 61 | 107.83±13.46a,b | 196.42±28.51a | 226.92±48.28a | 21.52±2.44 | 108.96±28.98 | 553.71±197.29 |
| UCC | 38 | 88.25±12.37a,b,c | 204.38±16.90a | 261.53±31.80a,b,c | 22.78±1.92a,b,c | 148.91±30.97a,b,c | 636.54±108.45a,b |
Compared to the control group;
P<0.05.
Compared to the HPV group;
P<0.05.
Compared to the CIN group;
P<0.05.
Discussion
Cancer development needs to escape immunological surveillance. CD4+T-cell suppression or dysfunction has been reported as a mechanism of causing cancer escape [21,22]. Among the T cells, Treg cells are significant in cancer immune evasion by blocking generation of immunity to tumor antigens in the periphery and by neutralizing tumor-infiltrating effector T cells. It has been demonstrated that the levels of Treg cells increased in cancer patients, and a high number of them related to poor survival [21-23]. The balance between Treg and Th17 cells controls immune response and is a key factor in regulating helper T cell function relating to the Th1/Th2 shift in autoimmune disease and graft vs. host disease [19]. In our study, we systematically determined that the frequencies of Th2, Th17, and Treg cells in PBMCs as well as their related cytokines IL-4, IL-10, IL-17, IL-23, and TGF-βI in serum were prominently increased in patients with UCC and CIN. The Th1 cells in PBMCs as well as serum INF-γ were prominently decreased in patients with UCC and CIN.
In our research, Th1 cell proportions decreased significantly in UCC and CIN patients. However, the Th2 cell proportions markedly increased in the two groups (Table 1). In addition, the difference between the two groups was significant (P<0.05). There was no obvious difference between the HPV group and the control group (P>0.05). The findings verified that a Th1/Th2 shift exists in the patients with uterine cancer and this shift may start from the CIN stage. A similar situation was found, when Th1 and Th2 type cytokines were tested (Table 4).
Consequently, our study showed a Th1/Th2 shift in UCC and CIN patients, which is consistent with other studies in ovarian and mammary cancers [24,25]. Tosolini [26] reported the survival rate was related to the Th1 cells; the lower the number of Th1 cells, the lower the survival rate of colon cancer patients.
Th0 is the precursor cell of Th1 and Th2 cells. It was reported that the related cytokines determined the differentiation direction of Th0 cells [27]. For example, IL-12, IFN-α, and IFN-γ can induce the Th0 cells to Th1 cells, while IL-4 induced the Th0 cells to Th2 cells [28]. Yang P found the Th1/Th2 shift was due to the genic mutation of T-bet [29]. These were consistent with our research. Both INF-γ and Th1 cells were significantly lower in UCC and CIN patients. On the other hand, the IL-4 and Th2 cells were significantly higher in UCC and CIN patients than those in control subjects.
Furthermore, our study also found higher Treg cell and Th17 cell proportions in UCC and CIN patients, compared to the other two groups. In addition, both Th17 and Treg cell proportions were higher in the UCC group than the CIN group and the differences between the two groups were significant (P<0.05). However, there was no obvious difference between the HPV group and the control group (P>0.05). The Th17 cells increased much more strongly than the Treg cells, so the ratio of Th17/Treg increased. Th17/Treg shift was also detected in patients with uterine cervical cancer (Table 2). We next sought to find whether Th17 cell frequency was associated with UCC grade, vasoinvasion or lymph node metastases.
Table 2.
The ratios of Th1/Th2 and Th17/Treg in controls and patients with UCC or CIN or HPV
| Th1/Th2 | Th17/Treg | |
|---|---|---|
| Control group (n=20) | 10.38±7.80 | 0.52±0.13 |
| HPV group (n=20) | 11.17±8.13 | 0.63±0.17 |
| CIN group (n=61) | 3.50±1.51a,b | 1.88±0.80a,b |
| UCC group (n=38) | 1.43±0.71a,b,c | 2.35±0.41a,b,c |
Compared to the control group;
P<0.05.
Compared to the HPV group;
P<0.05.
Compared to the CIN group;
P<0.05.
Previous studies in other cancers [30-32] also demonstrated that Treg cells had obviously higher proportions within PBMCs, and significant differences in the prevalence of Treg cells between early and advanced disease stages. In our study, a statistical difference of Treg cells was found between UCC patients, CIN patients, and the controls. The ratio of Treg cells in UCC patients with early and advanced stages with large population needs study in our next program.
We found the ratio of Th17/Treg in patients with UCC was higher than in the CIN group, and the difference was significant (P<0.05) (Table 3). In addition, the ratio of Th17/Treg in patients with CIN was higher than the HPV and control groups, and the difference was significant (P<0.05). It was verified that Th17/Treg shift existed in the patients with uterine cancer and this shift might start from the CIN stage. A similar situation was found, when Th17 and Treg type cytokines were tested (Table 4).
In Table 4, we see the Th17 secreted cytokines (IL-17 and IL-23) and Treg secreted cytokines (TGF-βI and IL-10) were all significantly higher in the UCC group than the control group. There was no significant difference between the CIN and control groups. However, the Th1 and Th2 representative cytokines INF-γ and IL-4 had already changed by the CIN stage. We tested 20 patients with HPV infection but with normal cervical fluid-based cytology. These patients showed the slight down regulation of Th1 and slight up regulation of Th2, Th17 and Treg. There was no significant difference between the HPV and control groups. Thus, during the HPV infection stage, the Th1/Th2 and Th17/Treg balance has not been broken. Bais found [33] the HPV-infected patients first showed an up regulation of cytokines both secreted by Th1 and Th2, then the Th1 cells decreased and the Th2 cells increased and finally the balance was broken. We speculate the Th17 and Treg cells might experience the same situation, and then the balance is broken. This might explain why a persistent HPV infection can lead to immunologic derangement and finally cause uterine cancer.
Due to clinical constraints, the UCC patients we observed were all stage I-II patients. In the future, we will do some studies on immune status in patients with advanced cervical cancer.
Conclusions
HPV persistent infection induced the immunologic dissonance. Attractive imbalances of Th1/Th2 and Th17/Treg were observed in UCC and CIN patients. These imbalances might involve in the occurrence and development of cervical cancer. Correcting the balance between CD4 cells may be a new target for the therapy of cervical cancer.
Acknowledgements
This work was funded by Shandong Province Medical and Health Technology Problem Items to Fang Yuan (No. 2014WS0178).
Disclosure of conflict of interest
None.
References
- 1.Small W, Bacon MA, Bajaj A, Chuang LT, Fisher BJ, Harkenrider MM, Jhingran A, Kitchener HC, Mileshkin LR, Viswanathan AN, Gaffney DK. Cervical cancer: a global health crisis. Cancer. 2017;123:2404–2412. doi: 10.1002/cncr.30667. [DOI] [PubMed] [Google Scholar]
- 2.Parkin DM, Bray F. Chapter 2: the burden of HPV-related cancers. Vaccine. 2006;24(Suppl 3):S3/11–25. doi: 10.1016/j.vaccine.2006.05.111. [DOI] [PubMed] [Google Scholar]
- 3.Patel S, Chiplunkar S. Host immune responses to cervical cancer. Curr Opin Obstet Gynecol. 2009;21:54–9. doi: 10.1097/GCO.0b013e32831a9890. [DOI] [PubMed] [Google Scholar]
- 4.Hildesheim A, Schiffman MH, Gravitt PE, Glass AG, Greer CE, Zhang T, Scott DR, Rush BB, Lawler P, Sherman ME, et al. Persistence of type-specific human papillomavirus infection among cytologically normal women. J Infect Dis. 1994;169:235–40. doi: 10.1093/infdis/169.2.235. [DOI] [PubMed] [Google Scholar]
- 5.Koshiol J, Lindsay L, Pimenta JM, Poole C, Jenkins D, Smith JS. Persistent human papillomavirus infection and cervical neoplasia: a systematic review and meta-analysis. Am J Epidemiol. 2008;168:123–37. doi: 10.1093/aje/kwn036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Annunziato F, Cosmi L, Santarlasci V, Maggi L, Liotta F, Mazzinghi B, Parente E, Fili L, Ferri S, Frosali F, Giudici F, Romagnani P, Parronchi P, Tonelli F, Maggi E, Romagnani S. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–61. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cosmi L, De Palma R, Santarlasci V, Maggi L, Capone M, Frosali F, Rodolico G, Querci V, Abbate G, Angeli R, Berrino L, Fambrini M, Caproni M, Tonelli F, Lazzeri E, Parronchi P, Liotta F, Maggi E, Romagnani S, Annunziato F. Human interleukin 17-producing cells originate from a CD161+CD4 + T cell precursor. J Exp Med. 2008;205:1903–16. doi: 10.1084/jem.20080397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acosta-Rodriguez EV, Rivino L, Geginat J, Jarrossay D, Gattorno M, Lanzavecchia A, Sallusto F, Napolitani G. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–46. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 9.Korn T, Carrier Y, Gao W, et al. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 10.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 11.Xie JJ, Wang J, Tang TT, Chen J, Gao XL, Yuan J, Zhou ZH, Liao MY, Yao R, Yu X, Wang D, Cheng Y, Liao YH, Cheng X. The Th17/Treg functional imbalance during atherogenesis in ApoE(-/-) mice. Cytokine. 2010;49:185–93. doi: 10.1016/j.cyto.2009.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Zhu X, Ma D, Zhang J, Peng J, Qu X, Ji C, Hou M. Elevated interleukin-21 correlated to Th17 and Th1 cells in patients with immune thrombocytopenia. J Clin Immunol. 2010;30:253–9. doi: 10.1007/s10875-009-9353-1. [DOI] [PubMed] [Google Scholar]
- 13.Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–50. doi: 10.1038/ni0407-345. [DOI] [PubMed] [Google Scholar]
- 14.Ivanov II, McKenzie BS, Zhou L, Tadokoro CE, Lepelley A, Lafaille JJ, Cua DJ, Littman DR. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–33. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Yang XO, Pappu BP, Nurieva R, Akimzhanov A, Kang HS, Chung Y, Ma L, Shah B, Panopoulos AD, Schluns KS, Watowich SS, Tian Q, Jetten AM, Dong C. T helper 17 lineage differentiation is programmed by orphan nuclear receptors ROR alpha and ROR gamma. Immunity. 2008;28:29–39. doi: 10.1016/j.immuni.2007.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mougiakakos D, Choudhury A, Lladser A, Kiessling R, Johansson CC. Regulatory T cells in cancer. Adv Cancer Res. 2010;107:57–117. doi: 10.1016/S0065-230X(10)07003-X. [DOI] [PubMed] [Google Scholar]
- 17.Wang RF. Regulatory T cells and innate immune regulation in tumor immunity. Springer Semin Immunopathol. 2006;28:17–23. doi: 10.1007/s00281-006-0022-7. [DOI] [PubMed] [Google Scholar]
- 18.Hwang JP, Ahmed S, Ariza-Heredia EJ, Duan Z, Zhao H, Schmeler KM, Ramondetta L, Parker SL, Suarez-Almazor ME, Ferrajoli A, Shih YT, Giordano SH, Chiao EY. Low rate of cervical cancer screening among women with hematologic malignancies after stem cell transplant. Biol Blood Marrow Transplant. 2018;24:1094–1098. doi: 10.1016/j.bbmt.2018.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Afzali B, Lombardi G, Lechler RI, Lord GM. The role of T helper 17 (Th17) and regulatory T cells (Treg) in human organ transplantation and autoimmune disease. Clin Exp Immunol. 2007;148:32–46. doi: 10.1111/j.1365-2249.2007.03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kryczek I, Banerjee M, Cheng P, Vatan L, Szeliga W, Wei S, Huang E, Finlayson E, Simeone D, Welling TH, Chang A, Coukos G, Liu R, Zou W. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood. 2009;114:1141–9. doi: 10.1182/blood-2009-03-208249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–9. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 22.Marigo I, Dolcetti L, Serafini P, Zanovello P, Bronte V. Tumor-induced tolerance and immune suppression by myeloid derived suppressor cells. Immunol Rev. 2008;222:162–79. doi: 10.1111/j.1600-065X.2008.00602.x. [DOI] [PubMed] [Google Scholar]
- 23.Nakamura T, Shima T, Saeki A, Hidaka T, Nakashima A, Takikawa O, Saito S. Expression of indoleamine 2, 3-dioxygenase and the recruitment of Foxp3-expressing regulatory T cells in the development and progression of uterine cervical cancer. Cancer Sci. 2007;98:874–81. doi: 10.1111/j.1349-7006.2007.00470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolf D, Wolf AM, Rumpold H, Fiegl H, Zeimet AG, Muller-Holzner E, Deibl M, Gastl G, Gunsilius E, Marth C. The expression of the regulatory T cell-specific forkhead box transcription factor FoxP3 is associated with poor prognosis in ovarian cancer. Clin Cancer Res. 2005;11:8326–31. doi: 10.1158/1078-0432.CCR-05-1244. [DOI] [PubMed] [Google Scholar]
- 25.Krohn M, Listing M, Tjahjono G, Reisshauer A, Peters E, Klapp BF, Rauchfuss M. Depression, mood, stress, and Th1/Th2 immune balance in primary breast cancer patients undergoing classical massage therapy. Supportive Care Cancer. 2011;19:1303–1311. doi: 10.1007/s00520-010-0946-2. [DOI] [PubMed] [Google Scholar]
- 26.Tosolini M, Kirilovsky A, Mlecnik B, Fredriksen T, Mauger S, Bindea G, Berger A, Bruneval P, Fridman WH, Pagès F, Galon J. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
- 27.Ariyasu T, Tanaka T, Fujioka N, Yanai Y, Yamamoto S, Yamauchi H, Ikegami H, Ikeda M, Kurimoto M. Effects of interferon-alpha subtypes on the TH1/TH2 balance in peripheral blood mononuclear cells from patients with hepatitis virus infection-associated liver disorders. In Vitro Cell Dev Biol Anim. 2005;41:50–56. doi: 10.1290/0501008.1. [DOI] [PubMed] [Google Scholar]
- 28.Theodorou GL, Marousi S, Ellul J, Mougiou A, Theodori E, Mouzaki A, Karakantza M. T helper 1 (Th1)/Th2 cytokine expression shift of peripheral blood CD4+ and CD8+ T cells in patients at the post-acute phase of stroke. Clin Exp Immunol. 2008;152:456–463. doi: 10.1111/j.1365-2249.2008.03650.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang P, Qiu G, Wang S, Su Z, Chen J, Wang S, Kong F, Lu L, Ezaki T, Xu H. The mutations of Th1 cell-specific T-box transcription factor may be associated with a predominant Th2 phenotype in gastric cancers. Int J Immunogenet. 2010;37:111–115. doi: 10.1111/j.1744-313X.2010.00899.x. [DOI] [PubMed] [Google Scholar]
- 30.Zhang B, Rong G, Wei H, Zhang M, Bi J, Ma L, Xue X, Wei G, Liu X, Fang G. The prevalence of Th17 cells in patients with gastric cancer. Biochem Biophys Res Commun. 2008;374:533–7. doi: 10.1016/j.bbrc.2008.07.060. [DOI] [PubMed] [Google Scholar]
- 31.Miller AM, Lundberg K, Ozenci V, Banham AH, Hellström M, Egevad L, Pisa P. CD4+CD25 high T cells are enriched in the tumor and peripheral blood of prostate cancer patients. J Immunol. 2006;177:7398–405. doi: 10.4049/jimmunol.177.10.7398. [DOI] [PubMed] [Google Scholar]
- 32.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–3. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 33.Bais AG, Beckmann I, Ewing PC, Eijkemans MJ, Meijer CJ, Snijders PJ, Helmerhorst TJ. Cytokine release in HR-HPV(+) women without and with cervical dysplasia (CIN II and III) or carcinoma, compared with HR-HPV(-) controls. Mediators Inflamm. 2007;2007:24147. doi: 10.1155/2007/24147. [DOI] [PMC free article] [PubMed] [Google Scholar]