Abstract
Objectives: Diabetic kidney disease (DKD) is the primary microvascular complication of diabetes. The incidence rate of DKD has increased worldwide, and DKD has become one of the most important causes of end-stage renal disease. In this study, we aimed to investigate the effects of hawthorn leaf flavonoids (HLF) on oxidative stress injury of renal tissue in DKD rats, and elucidate their mechanism(s) of action. Methods: A total of 35 male Sprague Dawley rats were randomly divided into the control group (CON group) and model group. Rats in the model group were fed a diet containing high sugar and fat and were injected with streptozotocin (STZ) into the abdominal cavity to induce diabetes. Diabetic rats that showed >50% increase in 24 h urine volume and >30 mg of 24 h urine protein excretion were selected as DKD model rats. After DKD models were successfully established, model rats were randomly divided into the diabetic kidney disease group (DKD group), irbesartan group (IRB group), and hawthorn leaf flavonoids group (HLF group). All rats were sacrificed at 12 weeks (w) after DKD models were established. Body weight and 24 h urinary protein levels were measured at 4 w, 8 w, and 12 w, respectively. Blood was collected to measure the levels of urea nitrogen, creatinine, triglyceride, nitric oxide, malondialdehyde, and superoxide dismutase. Pathologic changes in renal tissue were examined by hematoxylin and eosin (H&E) and Masson staining. Protein expression of p38MAPK and p-p38MAPK was determined by immunohistochemistry. Results: Our data showed that HLFs improved the general condition and body weight, and reduced the levels of urinary protein in model rats. Rats in the DKD group had more serious pathological damage in the kidney when compared to rats in the HLFs group. In addition, rats in the HLF group had significantly lower levels of urea nitrogen, creatinine, triglyceride, and malondialdehyde, and significantly higher levels of nitric oxide and superoxide dismutase than rats in the DKD group. Furthermore, p38MAPK and p-p38MAPK protein levels were significantly higher in rats in the DKD group compared to rats in the HLF group. Conclusions: HLFs have a protective effect against DKD in rats. The underlying mechanism may involve the reduction of oxidative stress by inactivation of the p38MAPK signaling pathway in renal tissues.
Keywords: Hawthorn leaves flavonoids, diabetic kidney disease, p38MAPK
Introduction
Currently, diabetes mellitus (DM) is one of the ten leading causes of death and one of the costliest chronic diseases worldwide [1]. Diabetic kidney disease (DKD), one of the most common and serious vascular complications of DM, has become a major contributor of end-stage renal disease (ESRD) [2]. Because traditional Chinese medicine (TCM) has a unique advantage in treating DKD, innovative development and mechanistic studies of TCM have become a current hotspot [3,4]. Hawthorn (Crataeguspinnatifida), a genus of the Rosaceae family, is commonly distributed in Asia, Europe, North and Central America. It is commonly used as a delicious daily food source, and its leaves are used for making tea in China. The hawthorn leaf has been shown to treat various diseases, such as hyperlipidemia, atherosclerosis, and dyspepsia [5-7]. To date, various chemical constituents have been identified in the hawthorn leaf, and these include flavonoids, triterpenoids, steroids, lignans, organic acids, and nitrogen-containing compounds. Among these compounds, flavonoids are the most abundant chemical components [8]. In recent years, hawthorn leaf flavonoids (HLF), a natural flavonoid, have received extensive attention both domestically and overseas. In previous studies, it has been reported that HLF possesses a broad spectrum of biologic properties, including anti-inflammatory [9] and antioxidant activities [10], and amelioration of hepatic steatosis [11] and anticancer [12]. Moreover, HLFs play a protective role in streptozotocin (STZ)-induced renal dysfunction DKD rats [13,14]. In recent years, significant progress has been made in understanding the lipid-regulating, hypoglycemic, and anti-cerebral ischemia properties of HLF. However, the effects and underlying molecular mechanism of HLF in DKD are rarely reported. In this study, we treated type 2 DKD rats with HLF and determined the characteristic changes in renal function, p38 mitogen activated protein kinase (p38MAPK) signaling, and oxidative stress injury. We examined the underlying mechanism of action of HLF in the improvement of oxidative stress injury of renal tissues in DKD rats to provide evidence supporting therapy.
Materials and methods
Experimental animals and main pharmacologic reagents
Clean grade male Sprague Dawley (SD) rats (160-200 g) (purchased from the experimental animal center of Southwest Medical University (Luzhou, China), quality certification number: SYXK2013-065). STZ (Streptozotocin) were purchased from Sigma-Aldrich Chemical Company (St. Louis, MO, USA); ABC kit, DAB kit and biotinylated goat anti-rabbit IgG were purchased from Bioss (Beijing, China). Rabbit anti-rat p38MAPK and p-p38MAPK polyclonal antibodies were purchased from Boster (Beijing, China). Irbesartan was obtained from Hangzhou Sanofi-Aventis Minsheng Co., Ltd. (Hangzhou, China). HLF (≥ 93.5% pure total flavonoids extracted from hawthorn leaves) were obtained from LinyiAikang Pharmaceutical Co., Ltd. (Linyi, China). The contents of HLF were determined using HPLC and UV-spectrophotometry, and the compounds were recognized as quercetin, vitexin-4”-O-glucoside, vitexin-2”-O-rhamnoside, and hyperoside.
Experimental design
Thirty-five male SD rats were acclimatized for 1 week and randomly divided into a normal control group (CON; n = 8) and model group (n = 27). Rats in the model group were fed a high-sugar and high-fat diet for 4 weeks, and were then injected with 40 mg/kg of STZ intraperitoneally (i.p.). Rats in the CON group were fed a basic diet and received an i.p. injection of citrate buffer solution. Rats with blood glucose level of ≥ 16.7 mmol/L post-injection were selected as diabetes model rats. Diabetic rats that showed >50% increase in 24 h urine volume and >30 mg of 24 h urine protein excretion were selected as DKD model rats. Twenty-four successfully constructed DKD rats were randomly divided into a DKD group, HLF group, and IRB group (n = 8 per group). Rats in the HLF and IRB groups were intragastrically gavaged with 200 mg/kg HLFs and 17.5 mg/kg IRB, respectively. In addition, rats in the CON and DKD groups were gavaged with an equal volume of distilled water. After 12 weeks of treatment, rats were euthanized and kidneys and blood were collected. Kidney tissues were fixed in 4% neutral paraformaldehyde solution, dehydrated, and embedded in paraffin. Remaining tissues were stored at -80°C for subsequent use.
Observation parameters
General condition
The mental state, water consumption, fur, body mass, urine, stool, and activity of the rats were observed. Body mass was recorded at 4, 8, and 12 weeks after the establishment of DKD models.
Quantitation of 24 h urine protein
24 h urine was collected from rats at 4, 8, and 12 weeks after DKD models were established, and urine protein concentration was determined using the Coomassie Blue G-250 assay.
Serum biomarkers
Levels of blood urea nitrogen (BUN), serum creatinine (Scr), triglyceride (TG), nitrogen monoxide (NO), malonaldehyde (MDA), and superoxide dismutase (SOD) were measured using an automated biochemical analyzer (Beijing Pulang Medical Equipment Co., Ltd., China).
Kidney tissue pathomorphology
Kidney tissues were dehydrated, embedded in paraffin, and cut into 4 µm-thick sections for routine H&E and Masson staining.
Activity of p38MAPK signaling pathway
The activity of the p38MAPK signaling pathway was determined by the expression levels of p38MAPK and p-p38MAPK in kidney tissues and immunohistochemistry was performed to compare the p38MAPK and p-p38MAPK expression. The Avidin-Biotin-Complex method was performed according to the kit instructions. Briefly, kidney tissue paraffin sections were dewaxed and rehydrated for heat-induced antigen retrieval. Subsequently, tissue sections were blocked with goat serum (to prevent non-specific antigen binding), incubated with diluted anti-p38MAPK (1:150) and anti-p-p38MAPK (1:100) antibodies at 4°C overnight, incubated with horseradish peroxidase-labeled goat anti-rabbit IgG, and restained with the avidin-biotin complex and DAB. Next, tissue sections were dehydrated, cleared, and sealed with glycerin gelatin. Brownish-yellow granules were considered a positive signal. The percentages of positive staining cells in the kidney tissues were determined semi-quantitatively using an Image-Pro Plus analysis system.
Statistical analysis
Measured data are ex-pressed as mean ± SD and analyzed by one-way ANOVA. P<0.05 was considered significant. SPSS 19.0 software was used in this study.
Results
Effect of HLF on the general condition and body mass of rats
DKD rats showed increased food and water consumption and urine volume, decreased activity, decreased energy, reduced body weight and darker fur. However, these findings were improved in rats in the HLF group. At week 4, body mass was significantly lower in DKD rats than in CON rats (P<0.01), but significantly higher in HLF and IRB rats than in DKD rats (both P<0.05) (Table 1).
Table 1.
Effect of hawthorn leaf flavonoids on body mass of rats (x̅ ± s, g)
| Group | n | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| CON | 8 | 345.75 ± 16.23 | 420.60 ± 15.42 | 489.90 ± 15.51 |
| DKD | 8 | 298.27 ± 12.57* | 275.38 ± 11.37* | 266.97 ± 13.14* |
| IRB | 8 | 324.74 ± 13.29# | 344.65 ± 9.17# | 368.86 ± 18.27# |
| HLF | 8 | 321.72 ± 11.94# | 348.86 ± 7.78# | 360.25 ± 8.36# |
P<0.01 compared with CON group at each time point;
P<0.05 compared with DKD group at each time point.
Effect of HLF on 24 h urine protein concentration of rats
Starting at week 4, the 24 h urine protein concentration was significantly higher in rats in the DKD group compared to rats in the CON group (P<0.01) and gradually increased over time. However, the 24 h urine protein concentration was significantly lower in rats in the HLF and IRB groups than in rats in the DKD group (both P<0.05) (Table 2).
Table 2.
Effect of hawthorn leaf flavonoids on urine protein concentration in each group (x̅ ± s, mg/24 h)
| Group | n | Week 4 | Week 8 | Week 12 |
|---|---|---|---|---|
| CON | 8 | 11.58 ± 0.96 | 13.89 ± 0.82 | 16.31 ± 1.82 |
| DKD | 8 | 62.25 ± 11.93* | 82.49 ± 14.37* | 94.53 ± 13.97* |
| IRB | 8 | 39.15 ± 7.86# | 43.85 ± 8.52# | 47.63 ± 9.89# |
| HLF | 8 | 41.09 ± 8.37# | 44.18 ± 5.17# | 47.12 ± 6.45# |
P<0.01 compared with CON group at each time point;
P<0.05 compared with DKD group at each time point.
Effect of HLF on serum BUN, Scr, TG, NO, MDA and SOD levels in rats
Rats in the DKD group had significantly elevated serum levels of BUN, Scr, TG, and MDA and significantly reduced levels of NO and SOD compared to rats in the CON group (all P<0.01). In contrast, rats in the HLF and IRB groups had significantly decreased levels of BUN, Scr, TG, and MDA and significantly increased levels of NO and SOD compared to rats in the DKD group (all P<0.05) (Table 3).
Table 3.
Effect of hawthorn leaf flavonoids on BUN, Scr, TG, NO, MDA and SOD levels in rats (n = 8, x̅ ± s)
| Group | BUN (mmol/L) | Scr (μmol/L) | TG (mmol/L) | NO (μmol/L) | MDA (nmol/mL) | SOD (μg/mL) |
|---|---|---|---|---|---|---|
| CON | 6.08UN | 48.78UN, S | 0.668UN | 33.67UN, S | 8.757UN | 78.57UN, Sc |
| DKD | 11.85UN, S* | 78.17UN, S* | 2.697UN* | 19.82UN, S* | 14.68UN* | 40.18UN, S* |
| IRB | 8.548UN# | 56.08UN, Sc# | 1.478UN# | 27.08UN, S# | 10.78UN# | 70.23UN, S# |
| HLF | 10.35UN, S# | 62.51UN, S# | 1.311UN# | 25.23UN, S# | 11.13UN# | 67.92UN, S# |
P<0.01 compared with CON group at each time point;
P<0.05 compared with DKD group at each time point.
Effect of HLF on kidney tissue pathomorphology
Kidney tissue sections were examined following H&E and Masson staining. Whereas the kidney tissues of rats in the CON group showed no significant abnormalities, those of DKD rats revealed glomerular mesangial cell hyperplasia, mesangial expansion, basement membrane thickening, vacuolar degeneration of renal tubular epithelial cells, and renal interstitial inflammatory cell infiltration. Pathologic injury of the kidney tissues was significantly improved in rats in the HLF groups compared to rats in the DKD group (Figures 1 and 2).
Figure 1.
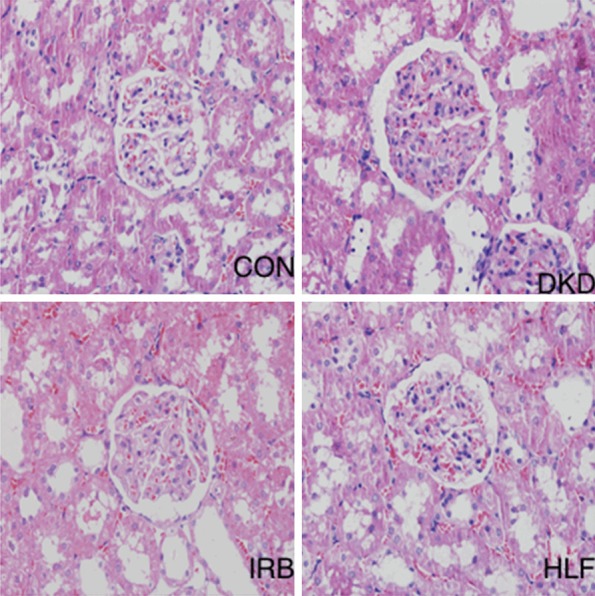
Hematoxylin/eosin staining of pathomorphologic changes in kidney tissues of experimental rats (200×).
Figure 2.
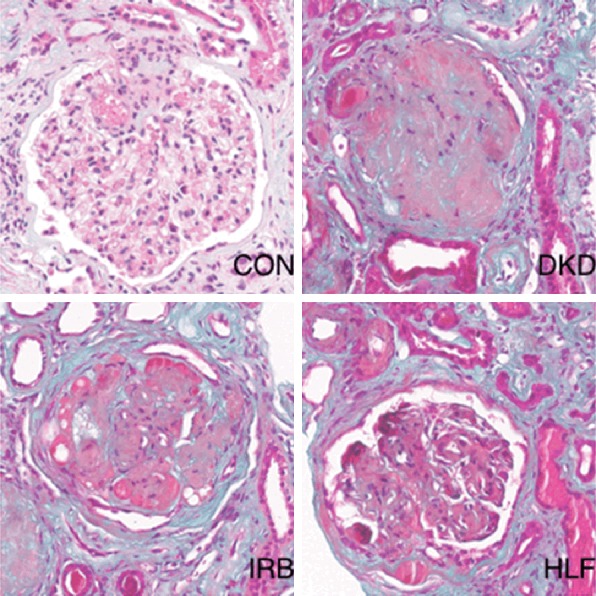
Masson staining of pathomorphologic changes in kidney tissues of different groups (400×).
Effect of HLF on p38MAPK and p-p38MAPK protein expression in rat kidney tissues
Immunohistochemical staining showed that p38MPAK and p-p38MAPK protein expression was significantly upregulated in kidney sections of rats in the DKD group compared to those of rats in the CON group (both P<0.05). In contrast, p38MPAK and p-p38MAPK protein expression was significantly downregulated in kidney sections of rats in the HLF and IRB groups compared to those of rats in the DKD group (both P<0.05) (Figure 3).
Figure 3.
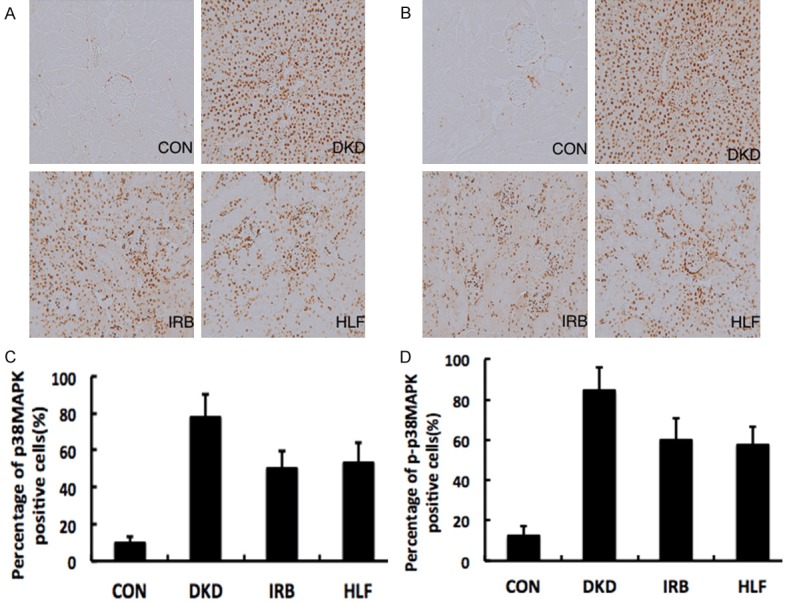
Immunochemical analysis of the (A) p38MAPK (B) and p-p38MAPK expression in the kidney of different groups (200×). Quantitative analysis of (C) p38MAPK and (D) p-p38MAPK expression in the kidney of different groups. The expression of p38MPAK and p-p38MAPK was significantly higher in the DKD group than in the HLF and IRB groups.
Discussion
As described in various pharmacopoeias, hawthorn is one of the oldest pharmaceutical plants, and both its fruits and leaves have medicinal value with favorable therapeutic effects for digestive diseases, lipid metabolic disorders, cardiovascular diseases, and other disorders [15-17]. In China, hawthorn has been used in the clinic for over 2000 years, but a limited number of studies have examined its pharmacological mechanisms. Hawthorn leaves have been reported to contain flavonoids, flavone-C-glycosides, catechins, amines, triterpene saponins, and oligomeric procyanidins, among which, flavonoids are the main bioactive compounds [18]. The antioxidant properties of HLF have recently received increasing attention. By examining the effect of HLF on the lipid peroxidation level in mice with alcoholic liver injury, Li et al. [19] found that HLF have potent free radical scavenging and antioxidant activities and HLF can increase endogenous antioxidant level, clear free radicals, inhibit free radical-mediated lipid peroxidation-induced liver injury, and protect hepatocyte structure and functions. Su et al. [20] showed that HLF can reduce oxidative stress injury, inhibit renal cell apoptosis, lower inflammatory mediator levels, and protect the kidney tissues of type 2 diabetes rats. In our study, we evaluated changes in renal function, oxidative stress, and p38MAPK signaling in DKD rats treated with HLF, and found that HLF significantly decreased BUN, Scr, TG and MDA levels, increased SOD activity, and attenuated pathologic injury of kidney tissues, thereby indicating that HLFs can inhibit oxidative stress injury of kidney tissue and protect renal function in type 2 DKD rats.
The pathogenesis of DKD is complex and involves multiple factors, including genetic factors, glucose metabolism disorder, hemodynamic changes, inflammation, and cytokines [21]. Oxidative stress participates in and promotes DKD development and progression [22]. In a hyperglycemic environment, various factors from endothelial cells lead to the overproduction of reactive oxygen species (ROS), which exceeds the clearance capacity of antioxidants in the body. Accumulation of ROS induces oxidative stress in renal cells and activates p38MAPK and other associated signaling pathways. Activated p38MAPK can participate in cellular stress, inflammation, and apoptosis through multiple pathways and further promote DKD development and progression [23,24]. Inhibition of the p38MAPK signaling pathway can significantly lower the risk of proteinuria in DKD, decrease the infiltration of macrophages and T cells, reduce the secretion of inflammatory mediators, and ultimately delay DKD progression [25,26]. A previous study demonstrated that hyperglycemia and metabolic disorder during DKD induces oxidative stress in the body, and oxidative stress-induced p38MAPK activation in turn leads to cysteine aspartic protease-3 activation and cell apoptosis [27]. In this study, we showed that p-p38MAPK protein expression was upregulated in kidney tissue of DKD rats but was downregulated upon HLF intervention. Together, these findings demonstrate that HLF can regulate p38MAPK signaling in kidney tissue of DKD rats.
In summary, HLF can reduce renal damage and improve renal function in DKD rats. The remarkable renal protective and disease-delaying effects of HLF may be mediated through the regulation of p38MAPK signaling and attenuation of oxidative stress injury.
Disclosure of conflict of interest
None.
References
- 1.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33:62–69. [Google Scholar]
- 2.Guo C, Han F, Zhang C, Xiao W, Yang Z. Protective effects of oxymatrine on experimental diabetic nephropathy. Planta Med. 2014;80:269–276. doi: 10.1055/s-0033-1360369. [DOI] [PubMed] [Google Scholar]
- 3.Huang K, Liu W, Lan T, Xie X, Peng J, Huang J, Wang S, Shen X, Liu P, Huang H. Berberine reduces fibronectin expression by suppressing the S1P-S1P2 receptor pathway in experimental diabetic nephropathy models. PLoS One. 2012;7:43874. doi: 10.1371/journal.pone.0043874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen C, Huang K, Hao J, Huang J, Yang Z, Xiong F, Liu P, Huang H. Polydatin attenuates AGEs-induced upregulation of fibronectin and ICAM-1 in rat glomerular mesangial cells and db/db diabetic mice kidneys by inhibiting the activation of the SphK1-S1P signaling pathway. Mol Cell Endocrinol. 2016;427:45–56. doi: 10.1016/j.mce.2016.03.003. [DOI] [PubMed] [Google Scholar]
- 5.Fan C, Yan J, Qian Y, Wo X, Gao L. Regulation of lipoprotein lipase expression by effect of hawthorn flavonoids on peroxisome prolifera- tor response element pathway. J Pharmacol Sci. 2006;100:51–58. doi: 10.1254/jphs.fp0050748. [DOI] [PubMed] [Google Scholar]
- 6.Herbst M, Roberts JM, Rosier PT, Gowing DJ. Seasonal and interannual variability of canopy transpiration of a hedgerow in southern England. Tree Physiol. 2007;27:321–333. doi: 10.1093/treephys/27.3.321. [DOI] [PubMed] [Google Scholar]
- 7.Wang T, An Y, Zhao C, Han L, Boakye-Yiadom M, Wang W, Zhang Y. Regulation effects of Crataegus pinnatifida leaf on glucose and lipids metabolism. J Agric Food Chem. 2011;59:4987–4994. doi: 10.1021/jf1049062. [DOI] [PubMed] [Google Scholar]
- 8.Wu J, Peng W, Qin R, Zhou H. Crataegus pinnatifida: chemical constituents, pharmacology, and potential applications. Molecules. 2014;19:1685–1712. doi: 10.3390/molecules19021685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fu JH, Zheng YQ, Li P, Li XZ, Shang XH, Liu JX. Hawthorn leaves flavonoids decreases inflammation related to acute myocardial ischemia/reperfusion in anesthetized dogs. Chin J Integr Med. 2013;19:582–8. doi: 10.1007/s11655-012-1250-4. [DOI] [PubMed] [Google Scholar]
- 10.Miftode AM, Stefanache A, Spac AF, Miftode RF, Miron A, Dorneanu V. In vitro measurement of total antioxidant capacity of crataegus macracantha loddleaves. Rev Med Chir Soc Med Nat Iasi. 2016;120:452–6. [PubMed] [Google Scholar]
- 11.Li Z, Xu J, Zheng P, Xing L, Shen H, Yang L, Zhang L, Ji G. Hawthorn leaf flavonoids alleviate nonalcoholic fatty liver disease by enhancing the adiponectin/AMPK pathway. Int J Clin Exp Med. 2015;8:17295–307. [PMC free article] [PubMed] [Google Scholar]
- 12.Mustapha N, Pinon A, Limami Y, Simon A, Ghedira K, Hennebelle T, Chekir-Ghedira L. Crataegus azarolus leaves induce antiproliferative activity, cell cycle arrest, and apoptosis in human HT-29 and HCT-116 colorectal cancer cells. J Cell Biochem. 2016;117:1262–72. doi: 10.1002/jcb.25416. [DOI] [PubMed] [Google Scholar]
- 13.Zhang P, Zhang PX. Protection of hawthorn leaves flavonoids for the diabetic rats. Pharmacology and Clinics of Chinese Materia Medica. 2015;31:114–117. [Google Scholar]
- 14.Zhao XL, Qi SF, Zhang B, et al. Effect of hawthorn leaves flavonoids on the expression of IGF-1 and IGFBP-3 in kidney of type 2 diabetic rats. Chinese Traditional Patent Medicine. 2014;36:2187–2190. [Google Scholar]
- 15.Ahn KS, Hahm MS, Park EJ, Lee HK, Kim IH. Corosolic acid isolated from the fruit of Crataegus pinnatifida var. psilosa is a protein kinase C inhibitor as well as a cytotoxic agent. Planta Med. 1998;64:468–470. doi: 10.1055/s-2006-957487. [DOI] [PubMed] [Google Scholar]
- 16.Niu C, Chen C, Chen L, Cheng K, Yeh C, Cheng J. Decrease of blood lipids induced by Shan-Zha (fruit of Crataegus pinnatifida) is mainly related to an increase of PPARalpha in liver of mice fed high-fat diet. Horm Metab Res. 2011;43:625–630. doi: 10.1055/s-0031-1283147. [DOI] [PubMed] [Google Scholar]
- 17.Zhang PC, Xu SX. Flavonoid ketohexosefuranosides from the leaves of Crataegus pinnatifida Bge. var. major N.E.Br. Phytochemistry. 2001;57:1249–53. doi: 10.1016/s0031-9422(01)00170-4. [DOI] [PubMed] [Google Scholar]
- 18.Liu RH, Yu BY. [Study on the chemical constituents of the leaves from Crataegus pinnatifida Bge. var. major N. E. Br] . Zhong Yao Cai. 2006;29:1169–73. [PubMed] [Google Scholar]
- 19.Li ST, Zhang ZJ, Du C, et al. Effects of hawthorn leaves flavonoids on lipid peroxidation in mice with alcoholic liver injury. Chinese Journal of Gerontology. 2014;34:1012–1014. [Google Scholar]
- 20.Su J, Zhou SY, Kan MC, et al. Protective effect of hawthorn leaves flavonoids on the kidneys of type 2 diabetic rats. Information on Traditional Chinese Medicine. 2017;34:22–27. [Google Scholar]
- 21.Dronavalli S, Duka I, Bakris GL. The pathogenesis of diabetic nephropathy. Nat Clin Pract Endocrinol Metab. 2008:444–452. doi: 10.1038/ncpendmet0894. [DOI] [PubMed] [Google Scholar]
- 22.Singh DK, Winocour P, Farrington K. Oxidative stress in early di abetic nephropathy: fueling the fire. Nat Rev Endocrinol. 2011;7:176. doi: 10.1038/nrendo.2010.212. [DOI] [PubMed] [Google Scholar]
- 23.Ahad A, Ahsan H, Mujeeb M, Siddiqui WA. Gallic acid ameliorates renal functions by inhibiting the activation of p38 MAPK in experimentally induced type 2 diabetic rats and cultured rat proximal tubular epithelial cells. Chem Biol Interact. 2015;240:292–303. doi: 10.1016/j.cbi.2015.08.026. [DOI] [PubMed] [Google Scholar]
- 24.Zuo L, Du Y, Lu M, Gao J, Hu R, Zhang S, Wang Y, Zhu H, Zhou Q, Wei W, Wang Y. Atorvastatin inhibits hyperglycemia- induced expression of osteopontin in the diabetic rat kidney via the p38 MAPK pathway. Mol Biol Rep. 2014;41:2551–2558. doi: 10.1007/s11033-014-3113-x. [DOI] [PubMed] [Google Scholar]
- 25.Lim AK, Tesch GH. Inflammation in diabetic nephropathy. Mediators Inflamm. 2012;2012:146154. doi: 10.1155/2012/146154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzeng TF, Liou SS, Chang CJ, Liu IM. The ethanol extract of Lonicera japonica (Japanese honeysuckle) attenuates diabetic nephropathy by inhibiting p-38 MAPK activity in streptozotocin-induced diabetic rats. Planta Med. 2014;80:121–129. doi: 10.1055/s-0033-1360196. [DOI] [PubMed] [Google Scholar]
- 27.Wu Gedunqiqige, Zhao ZJ, Jiang YF, et al. Protectory effects of Tang Shen Ping Capsule on kidneys in STZ-induced diabetic nephropathy rats and TGF-β1/p38MAPK signal pathway. China Journal of Traditional Chinese Medicine and Pharmacy. 2012;27:1092–1097. [Google Scholar]


