Abstract
Background: Studies have reported that the unfolded protein response of ER (UPRER) declines in the several organs of aging mice. However, changes of UPRER during the aging process in the intestine are rarely reported. Our previous studies have demonstrated that Saponins from Panax japonicus (SPJ) have anti-aging effects in different murine models and can modulate ER stress. In the present study, we focused on age-dependent expressions of UPRER in the intestine of 6- to 24-month-old rats by an immunohistochemical (IHC) method and determined whether SPJ could regulate the three different UPRER branches of the colon in aging rats. Methods: Aging rats had been treated with different doses (10 and 30 mg/kg) of SPJ for 6 months, which were mixed with feed, since they were 18 months old. Then the expressions of GRP78 and three different UPRER branches were determined by immunohistochemistry (IHC). Results: Total expressions of GRP78 and p-JNK increased, and other UPRER proteins decreased in the colon of aging rats, while SPJ treatment relieved the corresponding changes in aging rats. Here we also found different patterns of GRP78 and the three UPRER branches in the different layers of colon in rats. Conclusion: The study demonstrated that UPRER declined and GRP78 increased in the colon of aging rats. SPJ could reverse most URPER changes in the colon of aging rats. This study also showed different expression patterns of the three branches of UPRER in different layers of the colon in rats.
Keywords: Aging, endoplasmic reticulum, unfolded protein response, intestine, saponins from Panax japonicus
Introduction
The endoplasmic reticulum (ER) is a major position for the synthesis and folding of about one-third of the proteome in a whole cell. ER homeostasis can be interrupted by many endogenous and exogenous environmental conditions, finally leading to ER stress (ERS) and triggering the unfolded protein response (UPRER) [1]. UPRER is activated to restore ER homeostasis and prevent the further damage of cells. Under conditions of ER homeostasis, binding of ER chaperone GRP78 inhibits the activation of three stress sensors (IRE1α, PERK, and ATF6). When ERS happens, GRP78 dissociates and activates the above three stress sensors, resulting in the decrease of protein translation and increase of the folding capacity of the ER to relieve ERS.
ERS plays an important role in the aging process and is proposed as one of the hallmarks of aging [2]. Decline of UPRER is observed in several organs of aging rodents, including brain, heart, kidney, and liver [3-5]. However, the changes of UPRER during the aging process in the intestine are rarely reported. Recent studies of old flies have shown an age-associated increase of PERK activity in ISCs, while inhibition of PERK activity in ISCs extends lifespan and promotes the homeostasis of intestinal epithelium [6]. However, it remains unclear whether ERS in the specific cellular types of mammalian intestine is changed during the aging process. In the present study, we focused on age-dependent expressions of UPRER in the intestine of 6- to 24-month-old rats by an immunohistochemical (IHC) method.
Saponins from Panax japonicus (SPJ) are the main active components of Panax japonicus, which is a substitute for ginseng roots in the minority ethnic group of China. Our previous studies have demonstrated that SPJ can reduce D-galactose-induced brain aging in rats, mitigate neuroinflammation in the brain of naturally aging rat, and reverse the decreased expressions of the tight junction proteins in the colon of naturally aging rats [7-9]. SPJ could also regulate ERS, alleviating the expression of GRP78 in fatty liver fibrosis of mice [10]. Therefore, we hypothesized that SPJ might regulate the ERS of the intestine in aging rats.
Materials and methods
Materials
Panax japonicus (PJ) was received from Chunmuying Medicinal Materials Planting Base (Enshi, Hubei) and the voucher specimen was conserved in the Institute of Chinese Herb Medicine, Medical College of China Three Gorges University (Yichang, China). SPJ were extracted from Panax japonicus and analyzed by High Performance Liquid Chromatography (HPLC) in accordance with our previous methods [7].
Animals
Male Sprague-Dawley rats were provided by the Animal Center of China Three Gorges University (Yichang, China). All experiments were approved by the Ethics Committee of China Three Gorges University, and performed according to the guidelines of the National Institutes of Health on the care and use of animals. The rats were fed with chow and water ad libitum and housed in an environmentally controlled room (12 h light/12 h dark cycle; 23 ± 3°C). Thirty rats were randomly divided into an aging control group (n = 10) and SPJ-treated groups (n = 20) when they were 18 months old. SPJ were mixed with a normal diet and final dosages of rats in SPJ-treated groups were 10 mg/kg and 30 mg/kg respectively on the basis of Chinese Pharmacopoeia and our previous experiment [9]. Rats had been administered SPJ consecutively for 6 months until they were 24 months old. 6-month old rats were used as the adult control group (n = 10).
Immunohistochemistry (IHC) and immunofluorescence staining
After the rats were sacrificed, the colon samples had been fixed in 4% formaldehyde for at least 24 h, and then they were embedded in paraffin. Slices (4 μm-thick) were deparaffinized, rehydrated, and boiled with citrate buffer for 5 min by high pressure method. After cooling to room temperature, the slices were incubated with 5% bovine serum albumin or non-fat milk for 30 min at room temperature. Then the slices were stained with specific primary antibodies and incubated in a humidified chamber at 4°C overnight. Antibodies to GRP78, p-PERK, and ATF6α were purchased from Santa Cruz (CA, USA). Antibodies of IRE1, p-IRE1, XBP1, ATF4, eIF2α, and p-eIF2α were purchased from Abcam (Cambridge, UK). Antibody of p-JNK was obtained from Cell Signaling Technology (Boston, MA, USA). Next morning, Slices were incubated with secondary antibodies for 30 min at room temperature. Finally, slices were rinsed with phosphate buffer saline and visualized with diaminobenzidine for 5-10 min. Photos were acquired by a light microscope (Olympus).
For the co-staining of GRP78 with CD68, antibody to GRP78 was purchased from Santa Cruz (CA, USA) and antibody to CD68 was obtained from Abcam (Cambridge, UK). Staining was visualized by secondary antibodies, which were Alexa Fluor conjugates, and then processed with DAPI for 10 min. Photos were captured by a confocal laser scanning microscope (NIKON, Japan).
Quantification and statistical analysis of the immunostaining
The IHC positive result was brown yellow. All slides were reviewed blindly by two pathologists. Photos of three rat slices from each group were observed and captured, and then the Image J software (Rawak Software, Inc. Germany) was applied to analyze the average optical density (OD) value of positive expression in each power (×200) view. Positive cells were counted on 5 well-stained, randomly selected, microscopic fields at ×200 magnification for each region of interest. Data were expressed as mean ± SD and analyzed by one-way analysis of variance with post hoc analysis. SPSS software (version 13.0; IBM, Armonk, NY, USA) was used for statistical analysis. P < 0.05 was considered significant.
Results
Effects of aging on GRP78 expression in the different layers of colon in rats and the regulatory roles of SPJ
To determine whether ERS changed in the colon of aging rats, we first examined the expression of GRP78 in the colon. As shown in Figure 1A, GRP78 was obviously expressed in the cytoplasm of the surface epithelium, upper crypt, and some cells in the lamina propria of aging rats. GRP78 was also found in the cytoplasm and some nuclei of smooth muscle cells in the muscularis. A significant increase of GRP78 in each layer was found in aging rats compared with that in adult rats. The results suggested that ERS was enhanced in the intestine of aging rats. Treatment with SPJ in aging rats significantly down-regulated the GRP78 expression in each layer of the colon, indicating that SPJ can modulate intestinal ERS in aging rats.
Figure 1.
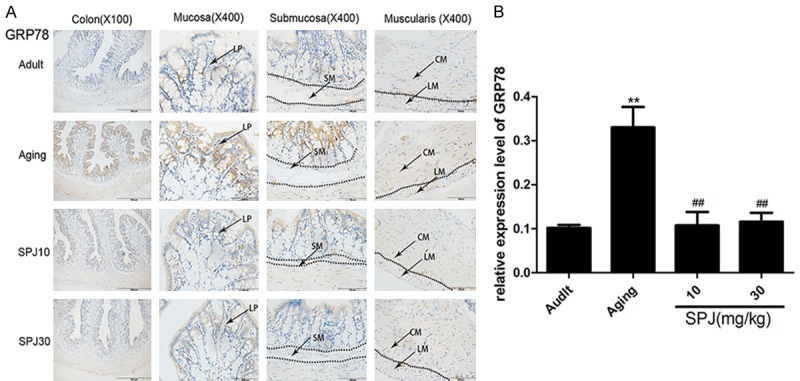
Effects of aging on GRP78 expression in the colon of rats and the regulatory roles of SPJ. A. Representative pictures of GRP78 expression pattern in the different layers of colon in rats by IHC. B. Quantitative analysis of global GRP78 protein immunostaining. Data are expressed as mean ± SD (n = 6). *P < 0.05 vs. adult group rats; **P < 0.01 vs. adult group rats; #P < 0.05 vs. aging group rats; ##P < 0.01 vs. aging group rats. LP, lamina propria; SM, submucosa; CM, circular muscle; LM, longitudinal muscle.
Effects of aging on IRE1/XBP1 branch signaling pathway in the different layers of colon in rats and the regulatory roles of SPJ
Among the three distinct UPR signaling pathways, IRE1 is the most evolutionarily conserved. Once ERS happens, IRE1 is activated to cause the activation of XBP1 and subsequent conversion into spliced XBP1 (XBP1s), which can translate and regulate a series of transcriptional targets to keep ER proteostasis [11]. Studies have also shown that XBP1 is linked to the activation of JNK [12]. We next determined the expression of IRE1, p-IRE1, XBP1, and p-JNK in the colon of rats. Results indicated that IRE1 was expressed widely in the surface epithelium, crypt, lamina propria, submucosa, and muscularis (Figure 2A). While p-IRE1 was expressed mainly in the surface epithelium, and muscularis, only a little was found in some cell types in the lamina propria of mucosa and submucosa (Figure 2C). The density of IRE1 expression was much higher than that of p-IRE1. Furthermore, IRE1 was found in the nucleus, and p-IRE1 was only expressed in the cytoplasm. Especially, there was more obvious expression of IRE1 in some cells with large size nucleus in the lamina propria. Total expressions of IRE1 and p-IRE1 were decreased in the colon of aging rats. Although the expression of IRE1 was decreased in the inner circular muscle, it was increased in the outer longitudinal muscle during the aging process. SPJ increased the total expressions of both proteins, but down-regulated the expression of IRE1 in the muscularis. IRE1 was expressed in the whole crypt of adult and aging rats, but only expressed in the upper crypt of SPJ-treated rats. Expression of p-IRE1 was decreased in the surface epithelium, but increased in the muscularis during the aging process. SPJ increased the expression of p-IRE1 in the surface epithelium and muscularis. XBP1 could be found mostly in the cytoplasm of surface epithelium, and nuclei of cells in the submucosa and muscularis (Figure 2E). The expression pattern of XBP1 in the lower part and upper part of crypt was different. XBP1 was expressed in the nuclei of the upper crypt and the cytoplasm of the lower crypt. Expression of XBP1 was not changed in the colon of aging rats. P-JNK was widely expressed in the nucleus of cells of surface epithelium, crypt, lamina propria, submucosa, and muscularis in the colon (Figure 2G). Expression of p-JNK was increased in the colon of aging rats. Treatment with SPJ in aging rats significantly down-regulated the p-JNK expression in each layer of colon.
Figure 2.
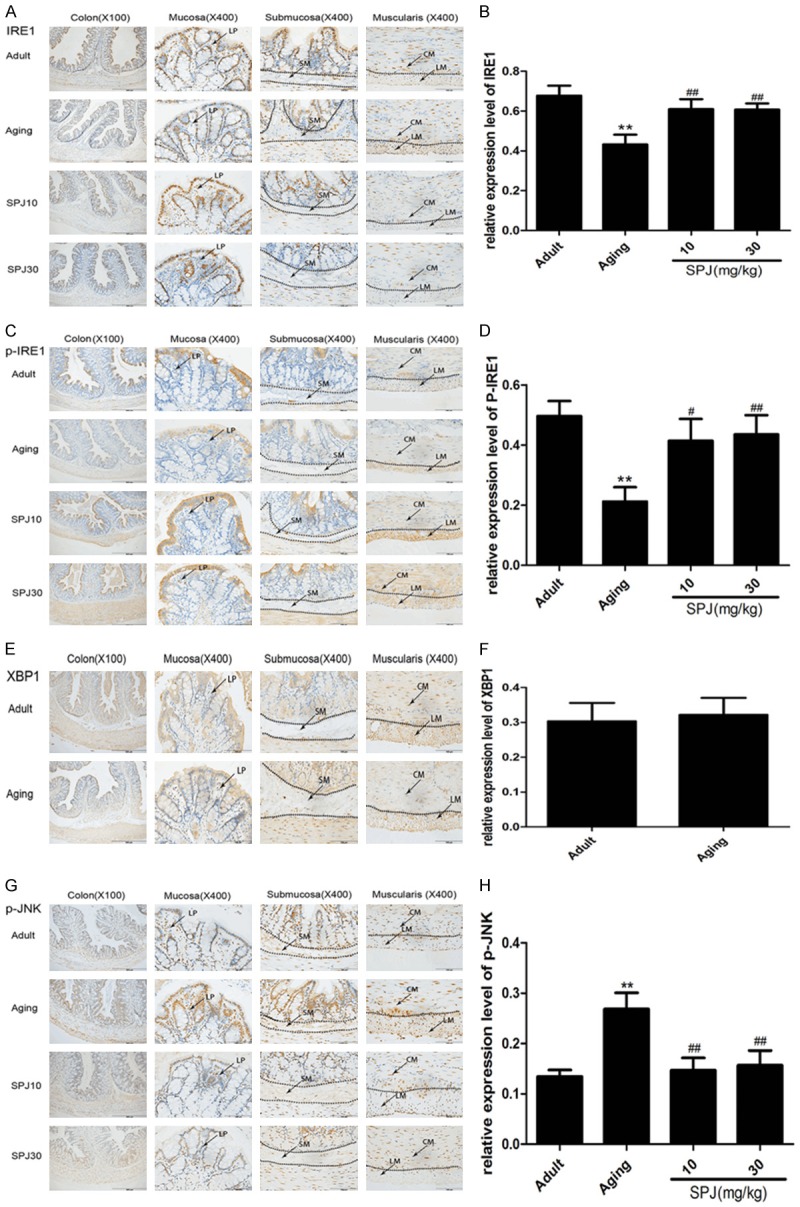
Effects of aging on IRE1/XBP1 branch signaling pathway in the colon of rats and the regulatory roles of SPJ. A. Representative pictures of IRE1 expression pattern in the different layers of colon in rats by IHC. B. Quantitative analysis of global IRE1 protein immunostaining. C. Representative pictures of p-IRE1 expression pattern in the different layers of colon in rats by IHC. D. Quantitative analysis of global p-IRE1 protein immunostaining. E. Representative pictures of XBP1 expression pattern in the different layers of colon in rats by IHC. F. Quantitative analysis of global XBP1 protein immunostaining. G. Representative pictures of p-JNK expression pattern in the different layers of colon in rats by IHC. H. Quantitative analysis of global p-JNK protein immunostaining. Data are expressed as mean ± SD (n = 6). *P < 0.05 vs. adult group rats; **P < 0.01 vs. adult group rats; #P < 0.05 vs. aging group rats; ##P < 0.01 vs. aging group rats. LP, lamina propria; SM, submucosa; CM, circular muscle; LM, longitudinal muscle.
Effects of aging on PERK/eIF2α signaling pathway in the different layers of the colon in rats and the regulatory roles of SPJ
Dissociation of GRP78 from PERK promotes PERK homodimerization and autophosphorylation to activate the kinase domain of itself and phosphorylate eIF2α, finally reducing the translational initiation and enhancing the expressions of downstream transcriptional factors such as ATF4 and CHOP [11,13]. Here, we found that p-PERK was mainly expressed in the nuclei of upper crypt of aging rats, and the nuclei of muscularis in adult and aging groups (Figure 3A). eIF2α was mainly expressed in the cytoplasm of surface epithelium, nuclei of some cells in the upper crypt and lamina propria of mucosa, submucosa and muscularis (Figure 3C). The expressions of p-PERK and eIF2α were low in the colon, and not changed during the aging process. The expression patterns of p-eIF2α and ATF4 were similar, and they were both widely expressed in the nuclei of surface epithelium, whole crypt, lamina propria of mucosa, and muscularis (Figure 3E and 3G). Expressions of p-eIF2α and ATF4 both decreased in the colon of aging rats. Treatment with SPJ in aging rats significantly up-regulated the p-eIF2α and ATF4 expressions in each layer of colon. However, we did not detect any expression of CHOP in the colon of each group (data not shown).
Figure 3.
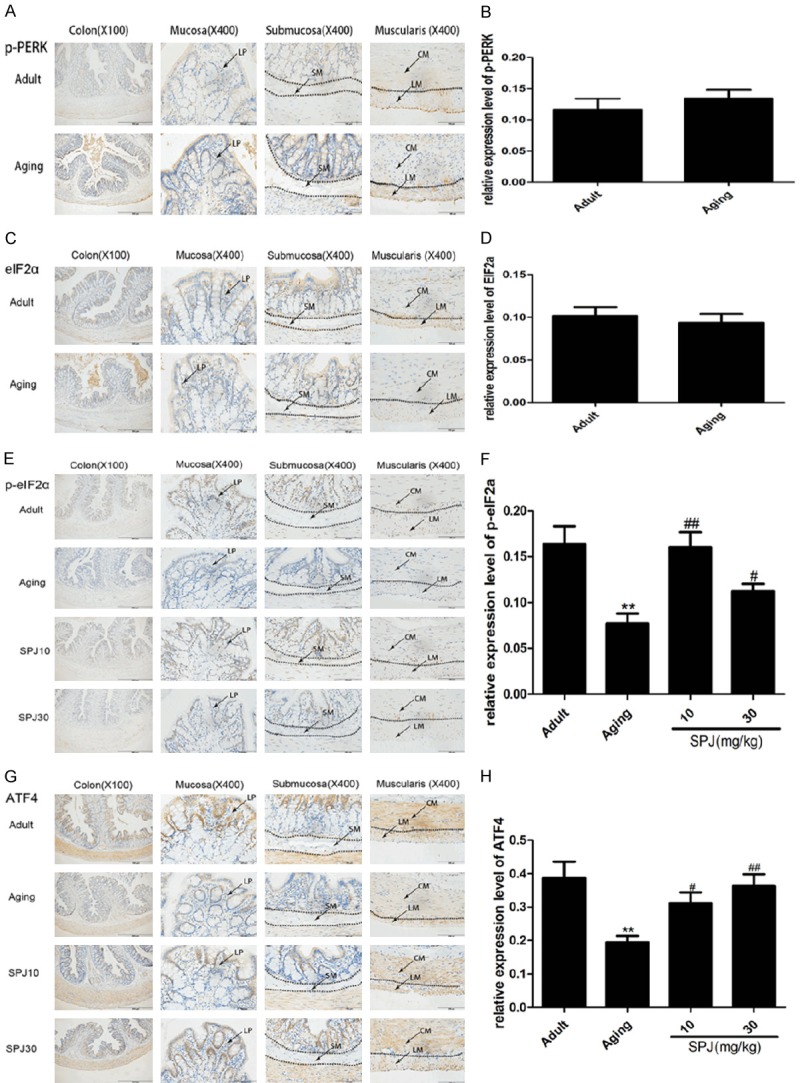
Effects of aging on PERK/eIF2α signaling pathway in the colon of rats and the regulatory roles of SPJ. A. Representative pictures of p-PERK expression pattern in the different layers of colon in rats by IHC. B. Quantitative analysis of global p-PERK protein immunostaining. C. Representative pictures of eIF2α expression pattern in the different layers of colon in rats by IHC. D. Quantitative analysis of global eIF2α protein immunostaining. E. Representative pictures of p-eIF2α expression pattern in the different layers of colon in rats by IHC. F. Quantitative analysis of global p-eIF2α protein immunostaining. G. Representative pictures of ATF4 expression pattern in the different layers of colon in rats by IHC. H. Quantitative analysis of global ATF4 protein immunostaining. Data are expressed as mean ± SD (n = 6). *P < 0.05 vs. adult group rats; **P < 0.01 vs. adult group rats; #P < 0.05 vs. aging group rats; ##P < 0.01 vs. aging group rats. LP, lamina propria; SM, submucosa; CM, circular muscle; LM, longitudinal muscle.
Effects of aging on ATF6α expression in the different layer of colon in rats and the regulatory roles of SPJ
Studies have demonstrated that there are two different types of ATF6 in the mammalian genome (ATF6α and ATF6β), but only ATF6α is required to activate UPR downstream gene expression [11]. Here we discovered that ATF6α was mostly expressed in the cytoplasm of surface epithelium, and nuclei of some cells in the crypt (Figure 4A). Expression of ATF6α was decreased in the colon of aging rats. However, treatment with SPJ in aging rats could not change the expression level of ATF6α.
Figure 4.
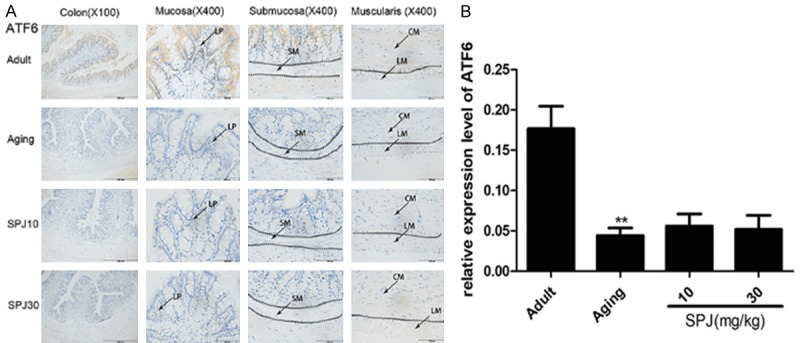
Effects of aging on ATF6α expression in the different layer of colon in rats and the regulatory roles of SPJ. A. Representative pictures of ATF6α expression pattern in the different layers of colon in rats by IHC. B. Quantitative analysis of global ATF6α protein immunostaining. Data are expressed as mean ± SD (n = 6). *P < 0.05 vs. adult group rats; **P < 0.01 vs. adult group rats; #P < 0.05 vs. aging group rats; ##P < 0.01 vs. aging group rats. LP, lamina propria; SM, submucosa; CM, circular muscle; LM, longitudinal muscle.
Discussion
Aging is an inevitable consequence of processes and characterized by age-dependent physiological and functional decline [14,15]. UPRER can be used to detect the homeostasis of ER and activate downstream mechanisms that allow cells to resolve or adapt to ER stress (ERS). Studies in model organisms have shown that the ability of UPRER regulators to activate downstream targets declines with age. Age-associated decline of the UPRER may play an important role in the aging process [2]. Studies have also demonstrated that intestinal inflammation is often found in elderly people and at the same time ERS is closely associated with intestinal inflammation [16,17]. However, at present there are only a few reports about the changes of ERS in the intestine during the aging process [6]. Here we found that the expressions of GRP78 and p-JNK increased, while three different UPRER branches decreased with age in the colon of rats, suggesting the decreased ability of UPRER to restore the protein folding led to the enhanced ERS. SPJ could reverse many changes of UPRER in aging rats, showing that SPJ could restore the protein folding ability of UPRER to alleviate the ERS.
The three sensors of the UPR are IRE1, PERK and ATF6. GRP78 disassociates from the above three sensors upon ER stress, thus triggering the UPR and enabling the activities of GRP78 to reduce the accumulation of misfolded and unfolded proteins. There are two enzymatic activities of IRE1α, including an endoribonuclease (RNase) domain and a serine/threonine kinase domain. On release from GRP78, dimerization of IRE1α elicits RNase activity, leading to the splicing XBP1 into XBP1s, which finally induces the transcription of a series of molecular chaperones and protein-folding enzymes, including p-JNK [12]. Here we just detected the expression of XBP1, but couldn’t determine the expression of XBP1s by IHC because of current technology limitation. Although XBP did not change in the colon of aging rats, this does not imply that XBP1s cannot change with age. We found that IRE1 and p-IRE1 decreased with age, while p-JNK increased with age. Because JNK is involved in not only ERS, but also some other signaling pathways, for example, MAPK signaling pathway. Our previous studies have discovered that MAPK signaling pathway enhances in the colon of aging rats by western blot [9], which is consistent with the results here by IHC.
Breakdown of intestinal epithelium homeostasis occurs during the aging process [18-20], and studies have demonstrated that long-term homeostasis of the intestinal epithelium is significantly impacted by ER stress [21-23]. Deletion of IRE1α in intestinal epithelial cells results in failure of intestinal epithelial barrier function [22]. Here we found that almost every UPRER protein was expressed in the surface epithelium and the expression changes in the surface epithelium were consistent with those in the total colon. In accordance with our results, some UPRER genetic deficiency mice show increased expression of GRP78, for example in IRE1β-deficient mice, Xbp1ΔIEC mice, Atf6α-/- mice [12,24,25]. Perhaps the decreased UPRER induced the increase of GRP78 in the surface epithelium of aging rats. Almost every UPRER protein determined here could be found in the surface epithelium of colon, suggesting that ERS might play a potential role in the homeostasis of surface epithelium during the aging process.
ER stress is a common feature of intestinal secretory cells such as goblet cells and Paneth cells [12,26]. Goblet cells are mostly in the crypt of colon and they produce anti-microbial molecules and the mucus barrier. Here we found that IRE1, p-EIF2α and p-JNK were expressed in the whole crypt, while GRP78, ATF4, and ATF6α were mainly found in the upper crypt. Expressions of GRP78 and p-JNK increased, while other proteins decreased in the crypt of aging rats, meaning that enhanced ERS by damaged UPRER might lead to the damage of secretory function in goblet cells during the aging process.
This experiment also determined that ERS existed in the lamina propria of colonic mucosa. The mucosal lamina propria contains some cell types of the innate and adaptive immune systems, including macrophages and large numbers of lymphocytes [27,28]. Intestinal immune systems play an essential role in mucosal homeostasis, infection and inflammation. The age-related decline in intestinal function is also a consequence of complex inflammatory conditions that are associated with increased protein misfolding [26,29]. Previous studies about the relationship between the ER stress and intestinal inflammation were mainly focused on the intestinal epithelium. Now more attention is being given to the roles of ER stress on the immune cells in the intestinal inflammation and aging [28,30]. Highly secretory immune cells are susceptible to environmental conditions that impose ER stress. Thus, inflammation is an important factor in the induction of ER stress, and changes of UPRER may be a sensitive hallmark of inflammation. Here it seemed that the expression position of GRP78 was in the cytoplasm of macrophages and the expression position of p-JNK was in the lymphocytes. We also found that IRE1 was expressed in the submucosa of colon, which also looked like in the macrophages. Especially the expression of GRP78 in macrophages increased and expression of IRE1 in macrophages decreased with aging, implying that ERS of macrophages could play an important role in the aging process. We further conducted a double immunofluorescence of GRP78 with CD68 (one marker of macrophages). The results showed that co-localization of GRP78 and CD68 was found majorly in the lamina propria of aging group and low dose of the SPJ group, while treatment of 30 mg/kg SPJ can significantly alleviate the co-localization in aging rats (Figure 5). Consistent with our results, Song et al have reported that aged macrophages exhibit diminished IRE1 activation and increased susceptibility to ER stress-dependent apoptosis [31]. SPJ inhibited the expression of GRP78 and enhanced the expression of IRE1 in macrophages, indicating that SPJ can modulate the ERS of macrophages. Our previous studies have discovered that Chikusetsusaponin V, a major constituent of SPJ, can mitigate the inflammation of LPS-induced macrophages [32]. All the above results implied that macrophages could be a major target of SPJ during the aging process.
Figure 5.
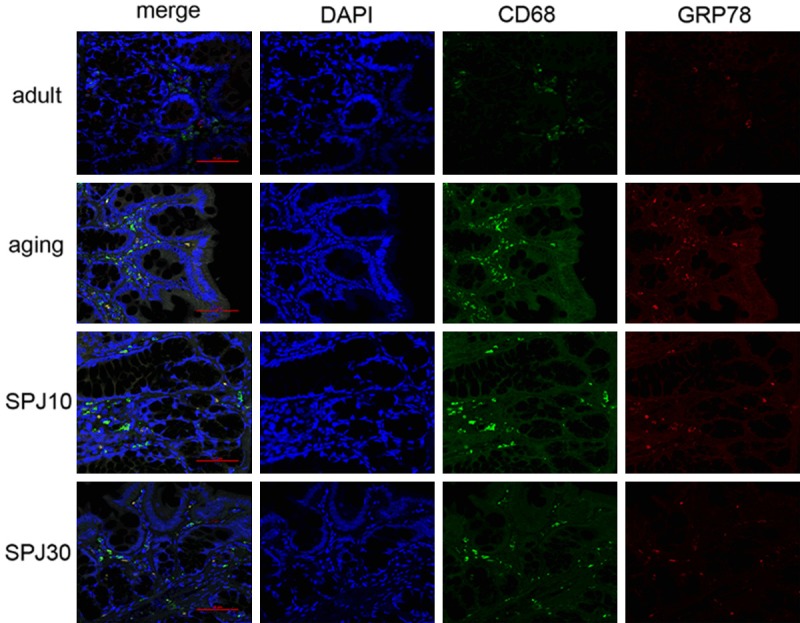
Co-staining of GRP78 with CD68 in the lamina propria of colon in different groups (×400). Red-GRP78, Green-CD68, Blue-DAPI.
Our studies also showed that changes in UPRER proteins in the muscularis during the aging process were similar to those in the mucosa. Expressions of GRP78 and p-JNK increased, while three different UPRER branches decreased with age in the muscularis of colon, except for p-IRE1, which increased during the aging process in rats. Studies have shown that force generation reduces in the bioengineered rings from aged colonic smooth muscle cells [33] and ERS have been involved in some disease models of smooth muscle tissues [34,35], suggesting that ERS might be associated with decrease of contraction tone of smooth muscle cells during the aging process. Although SPJ reversed the expressions of many ERS-related proteins in the muscularis during the aging process, it continued to increase the expression of IRE1 and p-IRE1. This might be a side effect of SPJ if it could be a potential drug for the treatment of some intestinal diseases.
Conclusions
Our study showed that the UPRER decreased in the colon of aging rats, while GRP78 and p-JNK increased during the aging process. Our results also illustrated distinct expression patterns of GRP78 and three different UPRER branches within the different layers of the colon (Figure 6 and Table 1), suggesting that these proteins might have different roles in the colon of aging rats. SPJ could reverse most URPER changes in the colon of aging rats, indicating that SPJ might have a role in delaying the decline of some intestinal functions during the aging process by modulating the URPER.
Figure 6.
Different layer patterns in the colon.
Table 1.
Expression patterns of aging effects on the UPRER signaling pathway in the different layer of colon in rats and the regulatory role of saponins from Panax japonicus
| Aging (vs. adult group) | SPJ (vs. aging group) | |
|---|---|---|
| Surface epithlium | 1. GRP78↑ | 1. GRP78↓ |
| 2. IRE1↓, p-IRE1↓, XBP1- , p-JNK↑ | 2. IRE1↑, p-IRE1↑, p-JNK↓ | |
| 3. p-eIF2a↓, ATF4↓ | 3. p-eIF2a↑, ATF4↑ | |
| 4. ATF6↓ | 4. ATF6- | |
| Lamina propria | 1. GRP78↑ | 1. GRP78↓ |
| 2. IRE1↓, XBP1-, p-JNK↑ | 2. IRE1↑, p-JNK↓ | |
| 3. p-eIF2a↓ | 3. p-eIF2a↑ | |
| Upper crypt | 1. GRP78↑ | 1. GRP78↓ |
| 2. IRE1↓, XBP1-, p-JNK↑ | 2. IRE1↑, p-JNK↓ | |
| 3. p-eIF2a↓, ATF4↓ | 3. p-eIF2a↑, ATF4↑ | |
| 4. ATF6↓ | 4. ATF6- | |
| Lower crypt | 1. IRE1↓, XBP1-, p-JNK↑ | 1. IRE1↑, p-JNK↓ |
| 2. p-eIF2a↓ | 2. p-eIF2a↑ | |
| Muscularis | 1. GRP78↑ | 1. GRP78↓ |
| 2. IRE1↓, p-IRE1↓, XBP1-, p-JNK↑ | 2. IRE1↓, p-IRE1↑, p-JNK↓ | |
| 3. p-eIF2a↓, ATF4↓ | 3. p-eIF2a↑, ATF4↑ | |
| 4. ATF6↓ | 4. ATF6- |
“↑” means increase, “↓” means decrease, “-” means no change.
Acknowledgements
This study was supported by grants from the Chinese National Natural Science Foundation (No. 81873349, No. 81503423 and No. 81403307).
Disclosure of conflict of interest
None.
References
- 1.Walter P, Ron D. The unfolded protein response: from stress pathway to homeostatic regulation. Science. 2011;334:1081–1086. doi: 10.1126/science.1209038. [DOI] [PubMed] [Google Scholar]
- 2.Sands WA, Page MM, Selman C. Proteostasis and ageing: insights from long-lived mutant mice. J Physiol. 2017;595:6383–6390. doi: 10.1113/JP274334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hussain SG, Ramaiah KV. Reduced eIF2alpha phosphorylation and increased proapoptotic proteins in aging. Biochem Biophys Res Commun. 2007;355:365–370. doi: 10.1016/j.bbrc.2007.01.156. [DOI] [PubMed] [Google Scholar]
- 4.Naidoo N, Ferber M, Master M, Zhu Y, Pack AI. Aging impairs the unfolded protein response to sleep deprivation and leads to proapoptotic signaling. J Neurosci. 2008;28:6539–6548. doi: 10.1523/JNEUROSCI.5685-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Paz Gavilán M, Vela J, Castaño A, Ramos B, del Río JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol Aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 6.Wang LF, Ryoo HD, Qi Y, Jasper H. PERK limits drosophila lifespan by promoting intestinal stem cell proliferation in response to ER stress. PLoS Genet. 2015;11:e1005220. doi: 10.1371/journal.pgen.1005220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang T, Di G, Yang L, Dun Y, Sun Z, Wan J, Peng B, Liu C, Xiong G, Zhang C, Yuan D. Saponins from Panax japonicus attenuate D-galactose-induced cognitive impairment through its anti-oxidative and anti-apoptotic effects in rats. J Pharm Pharmacol. 2015;67:1284–1296. doi: 10.1111/jphp.12413. [DOI] [PubMed] [Google Scholar]
- 8.Deng LL, Yuan D, Zhou ZY, Wan JZ, Zhang CC, Liu CQ, Dun YY, Zhao HX, Zhao B, Yang YJ, Wang T. Saponins from Panax japonicus attenuate age-related neuroinflammation via regulation of the mitogen-activated protein kinase and nuclear factor kappa B signaling pathways. Neural Regen Res. 2017;12:1877–1884. doi: 10.4103/1673-5374.219047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dun YY, Liu M, Chen J, Peng DL, Zhao HX, Zhou ZY, Wang T, Liu CQ, Guo YH, Zhang CC, Yuan D. Regulatory effects of saponins from Panax japonicus on colonic epithelial tight junctions in aging rats. J Ginseng Res. 2018;42:50–56. doi: 10.1016/j.jgr.2016.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yuan D, Xiang TT, Huo YX, Liu CQ, Wang T, Zhou ZY, Dun YY, Zhao HX, Zhang CC. Preventive effects of total saponins of panax japonicus on fatty liver fibrosis in mice. Arch Med Sci. 2018;14:396–406. doi: 10.5114/aoms.2016.63260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hetz C, Chevet E, Oakes SA. Proteostasis control by the unfolded protein response. Nat Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kaser A, Lee AH, Franke A, Glickman JN, Zeissig S, Tilg H, Nieuwenhuis EE, Higgins DE, Schreiber S, Glimcher LH, Blumberg RS. XBP1 links ER stress to intestinal inflammation and confers genetic risk for human inflammatory bowel disease. Cell. 2008;134:743–756. doi: 10.1016/j.cell.2008.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Palam LR, Baird TD, Wek RC. Phosphorylation of eIF2 facilitates ribosomal bypass of an inhibitory upstream ORF to enhance CHOP translation. J Biol Chem. 2011;286:10939–10949. doi: 10.1074/jbc.M110.216093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martinez G, Duran-Aniotz C, Cabral-Miranda F, Vivar JP, Hetz C. Endoplasmic reticulum proteostasis impairment in aging. Aging Cell. 2017;16:615–623. doi: 10.1111/acel.12599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taylor RC. Aging and the UPR(ER) Brain Res. 2016;1648:588–593. doi: 10.1016/j.brainres.2016.04.017. [DOI] [PubMed] [Google Scholar]
- 16.Guigoz Y, Dore J, Schiffrina EJ. The inflammatory status of old age can be nurtured from the intestinal environment. Curr Opin Clin Nutr. 2008;11:13–20. doi: 10.1097/MCO.0b013e3282f2bfdf. [DOI] [PubMed] [Google Scholar]
- 17.Kaser A, Blumberg RS. Endoplasmic reticulum stress and intestinal inflammation. Mucosal Immunol. 2010;3:11–16. doi: 10.1038/mi.2009.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ayyaz A, Jasper H. Intestinal inflammation and stem cell homeostasis in aging drosophila melanogaster. Front Cell Infect Mi. 2013;3:98. doi: 10.3389/fcimb.2013.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tremblay S, Cote NML, Grenier G, Duclos-Lasnier G, Fortier LC, Ilangumaran S, Menendez A. Ileal antimicrobial peptide expression is dysregulated in old age. Immun Ageing. 2017;14:19. doi: 10.1186/s12979-017-0101-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Moorefield EC, Andres SF, Blue RE, Van Landeghem L, Mah AT, Santoro MA, Ding S. Aging effects on intestinal homeostasis associated with expansion and dysfunction of intestinal epithelial stem cells. Aging (Albany NY) 2017;9:1898–1915. doi: 10.18632/aging.101279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cao SS, Wang M, Harrington JC, Chuang BM, Eckmann L, Kaufman RJ. Phosphorylation of eIF2α is dispensable for differentiation but required at a posttranscriptional level for paneth cell function and intestinal homeostasis in mice. Inflamm Bowel Dis. 2014;20:712–722. doi: 10.1097/MIB.0000000000000010. [DOI] [PubMed] [Google Scholar]
- 22.Zhang HS, Chen Y, Fan L, Xi QL, Wu GH, Li XX, Yuan TL, He SQ, Yu Y, Shao ML, Liu Y, Bai CG, Ling ZQ, Li M, Liu Y, Fang J. The endoplasmic reticulum stress sensor IRE1α in intestinal epithelial cells is essential for protecting against colitis. J Biol Chem. 2015;290:15327–15336. doi: 10.1074/jbc.M114.633560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hosomi S, Grootjans J, Tschurtschenthaler M, Krupka N, Matute JD, Flak MB, Martinez-Naves E, Gomez Del Moral M, Glickman JN, Ohira M, Lanier LL, Kaser A, Blumberg R. Intestinal epithelial cell endoplasmic reticulum stress promotes MULT1 up-regulation and NKG2D-mediated inflammation. J Exp Med. 2017;214:2985–2997. doi: 10.1084/jem.20162041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cao SS, Zimmermann EM, Chuang BM, Song B, Nwokoye A, Wilkinson JE, Eaton KA, Kaufman RJ. The unfolded protein response and chemical chaperones reduce protein misfolding and colitis in mice. Gastroenterology. 2013;144:989–1000. doi: 10.1053/j.gastro.2013.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bertolotti A, Wang X, Novoa I, Jungreis R, Schlessinger K, Cho JH, West AB, Ron D. Increased sensitivity to dextran sodium sulfate colitis in IRE1beta-deficient mice. J Clin Invest. 2001;107:585–593. doi: 10.1172/JCI11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McGuckin MA, Eri RD, Das I, Lourie R, Florin TH. ER stress and the unfolded protein response in intestinal inflammation. Am J Physiol-Gastr L. 2010;298:G820–32. doi: 10.1152/ajpgi.00063.2010. [DOI] [PubMed] [Google Scholar]
- 27.Joeris T, Muller-Luda K, Agace WW, Mowat AM. Diversity and functions of intestinal mononuclear phagocytes. Mucosal Immunol. 2017;10:845–864. doi: 10.1038/mi.2017.22. [DOI] [PubMed] [Google Scholar]
- 28.Grootjans J, Kaser A, Kaufman RJ, Blumberg RS. The unfolded protein response in immunity and inflammation. Nat Rev Immunol. 2016;16:469–484. doi: 10.1038/nri.2016.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mabbott NA, Kobayashi A, Sehgal A, Bradford BM, Pattison M, Donaldson DS. Aging and the mucosal immune system in the intestine. Biogerontology. 2015;16:133–145. doi: 10.1007/s10522-014-9498-z. [DOI] [PubMed] [Google Scholar]
- 30.Hasnain SZ, Lourie R, Das I, Chen AC, McGuckin MA. The interplay between endoplasmic reticulum stress and inflammation. Immunol Cell Biol. 2012;90:260–270. doi: 10.1038/icb.2011.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Song Y, Shen H, Du W, Goldstein DR. Inhibition of x-box binding protein 1 reduces tunicamycin-induced apoptosis in aged murine macrophages. Aging Cell. 2013;12:794–801. doi: 10.1111/acel.12105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang T, Dai YW, Dun YY, Zhang CC, Wan JZ, Deng LL, Zhou ZY, Liu CQ, Yuan D. Chikusetsusaponin V inhibits inflammatory responses via NF-κB and MAPK signaling pathways in LPS-induced RAW 264.7 macrophages. Immunopharmacol Immunotoxicol. 2014;36:404–411. doi: 10.3109/08923973.2014.960088. [DOI] [PubMed] [Google Scholar]
- 33.Somara S, Bashllari D, Gilmont RR, Bitar KN. Real-time dynamic movement of caveolin-1 during smooth muscle contraction of human colon and aged rat colon transfected with caveolin-1 cDNA. Am J Physiol Gastrointest Liver Physiol. 2011;300:G1022–1032. doi: 10.1152/ajpgi.00301.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Duan XH, Chang JR, Zhang J, Zhang BH, Li YL, Teng X, Zhu Y, Du J, Tang CS, Qi YF. Activating transcription factor 4 is involved in endoplasmic reticulum stress-mediated apoptosis contributing to vascular calcification. Apoptosis. 2013;18:1132–1144. doi: 10.1007/s10495-013-0861-3. [DOI] [PubMed] [Google Scholar]
- 35.Fang X, Zhang M, Guo J, Jin Z. Effects of insulin-like growth factor-1 on endoplasmic reticulum stress and autophagy in rat gastric smooth muscle cells cultured at different glucose concentrations in vitro. Mol Cell Biochem. 2019;451:11–20. doi: 10.1007/s11010-018-3388-7. [DOI] [PubMed] [Google Scholar]


