Abstract
Background: High-mobility graoup box protein 1 (HMGB1) has been shown to mediate a wide range of pathologic responses by interacting with RAGE (receptor for advanced glycation endproducts) and TLRs (Toll-like receptors). Our previous study showed that HMGB1 has been involved in pathogenesis of airway remodeling in an allergen-induced chronic mice asthma model. Increased airway smooth muscle (ASM) mass is a vital feature of airway remodeling. Objective: To evaluate the effect of HMGB1 on proliferation of ASMs and the underlying mechanisms. Methods: Rat airway smooth muscle cells (RASMs) were obtained by primary explant techniques. We investigated the effect of HMGB1 on the proliferation of RASMs. To identify which receptors and signaling pathways be involved in proliferation of RASMs, we performed western blot and CCK-8 assay by specific receptor blockade and inhibition of MAPK (p38, JNK and ERK) and NF-κB signaling pathways. Results: HMGB1 stimulated RASMs proliferation in a dose- and time-dependent manner and also increased proliferating cell nuclear antigen (PCNA) and RAGE expression of RASMs. The inhibitor of RAGE, but not TLR2 and TLR4, reversed HMGB1-induced RASM proliferation and PCNA expression. Incubation of RASMs with HMGB1 caused a rapid increase in P65 and ERK phosphorylation. RASM proliferation and PCNA expression toward HMGB1 were significantly inhibited by the inhibitors of ERK and NF-κB. Conclusion: HMGB1 induces proliferation of RASMs through a RAGE-dependent activation of ERK and NF-κB signaling pathways.
Keywords: HMGB1, RASM cells, cell remodeling, RAGE
Introduction
High mobility group box-1 protein (HMGB1) functions not only as a nuclear factor that stabilizes nucleosome formation, but also as an important mediator to participate in tissue injury, tissue repair, inflammation, and innate and adaptive immunity when present extracellularly [1]. The receptor for advanced glycation endproducts (RAGE) was found to be the first receptor of HMGB1 [2]. HMGB1 can bind to RAGE then to induce activation of mitogen-activated protein kinases (MAPKs) and the NF-кB pathway in rat smooth muscle cells [3]. HMGB1 also can bind to Toll-like receptors (TLRs), and both TLR2 and TLR4 are involved in HMGB1-induced cellular activation and NF-кB activation in macrophages [4].
Increasing evidence demonstrates that the levels of HMGB1 are elevated in many clinical diseases such as infection, rheumatoid arthritis, and cancers [5]. In our previous study [6], elevated sputum and plasma HMGB1 levels were observed in asthmatics and COPD patients and the HMGB1 level showed a negative correlation with the pulmonary function indices such as FEVI, and FEVI/FVC. More importantly, in a recent study we reported that inhibition of HMGB1 activity with anti-HMGB1 antibody decreased the levels of inflammatory mediators and reduced inflammatory cell accumulation, and also reversed airway remodeling in an allergen-induced murine model of chronic asthma [7]. In this study we found that blocking HMGB1 activity obviously decreased the airway smooth muscle thickness in mice.
Allergic asthma is characterized by Th2-typed chronic airway inflammation, and variable airway obstruction, and contributes to airway remodeling [8]. The abnormal proliferation of airway smooth muscle (ASM) is one of the hallmark pathologic features of asthma. Many stimulatory factors including growth factors and proinflammatory cytokines, can induce the excessive proliferation of ASM [9]. The intracellular signaling pathways related to proliferation of ASM mainly include mitogen-activated protein kinases (MAPK) and the NF-kB pathway [9].
Based on these finding above and our previous studies, the present study aims to confirm our hypothesis that in vitro HMGB1 may have a direct effect on the proliferation of ASM, then to elucidate remodeling and the signaling pathway mediating this process.
Materials and methods
Primary rat airway smooth muscle cells (RASMCs) isolation and culture
Primary RASMCs were isolated from trachea and main bronchi of 8-week SD rats which were obtained from the Guangxi Medical University Animal Center. All experimental animal protocols were approved by the Animal Care and Use Committee of the Guangxi Medical University. The trachea and main bronchi were dissected by removing excess connective tissue and were washed in cooled phosphate buffered saline (PBS) solution with antibiotics (100 U/ml penicillin G and streptomycin). Then the epithelium was disrupted by slightly stripping the luminal surface and the trachea and main bronchi were cut into small pieces. They were then incubated in DMEM with 0.1% collagenase solution at 37°C for 4 h. The Cell suspension were placed into a culture flask with complete DMEM/F12, 10% FBS after centrifugation. The flasks were cultured at 37°C in a humidified incubator. Cultured RASMCs were identified by expressed α-smooth muscle actins. Passages four to six were used for all experiments.
Treatment of RASMCs
ASMCs were starved in serum-free DMEM/F12 medium for 24 h before treatment. After reaching confluence, primary RASMCs were plated into 6-well plates before being stimulated with HMGB1 (Sigma, USA) at different concentrations for the indicated time (0 h, 12 h, 24 h and 48 h). Then the primary RASMCs were incubated with HMGB1 in the absence or presence of inhibitors of receptors or signaling pathway (MAPK p38, ERK1/2, JNK, NF-ĸB) inhibitors. The inhibitors of receptors include Cu-CPT22 (SIGMA, USA), a TLR2 inhibitor; CLI-095 (Invivo-Gen, San Diego, USA), a TLR4 inhibitor; and FPSZMI (Merck Millipore, USA), a RAGE inhibitor. The inhibitors of signaling pathways include SB203580, a p38 inhibitor; U0126, an ERK1/2 inhibitor, SP600125, a JNK inhibitor and BAY11-7082, an NF-ĸB inhibitor. All were purchased from Sigma (USA).
RNA extraction and real-time PCR
Single-stranded cDNA was synthesized using the RevertAidTM First Strand cDNA Synthesis Kit (Fermentas, Thermo Scientific). Real-time PCR was performed on the ABI 7900 (USA) using SYBR Premix EX Taq (TaKaRa). The primer sequences were as follows: TLR2: 5’-GGG TCA TCA TCA GCC TCT CC-3’ (sense sequence) and 5’-AGG TCA CTG TTG CTA TGT AGG TG-3’; TLR4: 5’-TGG ATA CGT TTC CTT ATA AG-3’ (sense sequence) and 5’-GAA ATG GAG GCA CCC CTT C-3’; RAGE: 5’-GCC CTC CAG TCT ACT CTC G-3’ (sense sequence) and 5’-TGT GTG GCC ACC CAT TCC AG-3’. GAPDH: 5’-AAG AGA GGC ATC CTC ACC CT-3’ (sense sequence) and 5’-TGGTGAAGACGCCAGTGGA-3’. Relative quantification of target gene expression was normalized to GAPDH expression and was performed by comparing the comparative threshold cycles (2-ΔΔCT).
Detection of cell proliferation
Two methods were performed to evaluate RASMC proliferation: the Cell Counting Kit-8 (CCK-8) assay and the proliferating cell nuclear antigen (PCNA) protein expression detected by western blot. In the CCK-8 assay, RASMCs were plated at 4 × 103 cells/well in 96-well plates and serum-starved for 24 hours. Eight replicates per treatment were used. Then, after treatment described above, the cells were incubated with the CCK-8 solution for 2 hours. Absorbances at 450 nm (A450) of the samples were read using a microplate reader. Trypan blue dye was performed to determine the viability of cells.
Western blotting
The procedure of western blot analysis was performed as previously described [7]. Briefly, RASMCs were lysed in an ice-cold protein extraction buffer (Biyuntian, China) containing the protease inhibitor PMSF (Biyuntian, China). Then the samples were centrifuged for 20 minutes at 12,000 rpm, the supernatants were collected. Protein concentrations of cell lysates were determined by using BCA assay (Biyuntian, China). Whole-cell lysates (50 μg) were separated on a 10% SDS-PAGE and then transferred to nitrocellulose membranes (PALL, USA). After 60 min of incubation at room temperature in 5% non-fat milk in TBST, the membranes were exposed to polyclonal Abs against GAPDH and PCNA (Immunoway, USA), rabbit monoclonal Abs against Erk1/2 and phosphorylated Erk1/2 (Cell Signaling Biotechnology) and rabbit monoclonal Abs against phosphorylated p65 (Cell Signaling Biotechnology). IRDye 800 CW goat anti-rabbit IgG (H+L) (Licor, USA) was used as a secondary antibody. Densitometry was performed using the Odyssey infrared image system (Licor, USA). The levels of proteins of interest were normalized to GAPDH.
Statistical analysis
Statistical analyses were performed using the SPSS 16.0 software. All data were expressed as the means ± SEM. Comparisons between groups were investigated using one-way ANOVA followed by least significant difference (LSD) multiple comparison test. P-values < 0.05 were considered significant.
Results
Effect of HMGB1 on proliferation of RASMCs
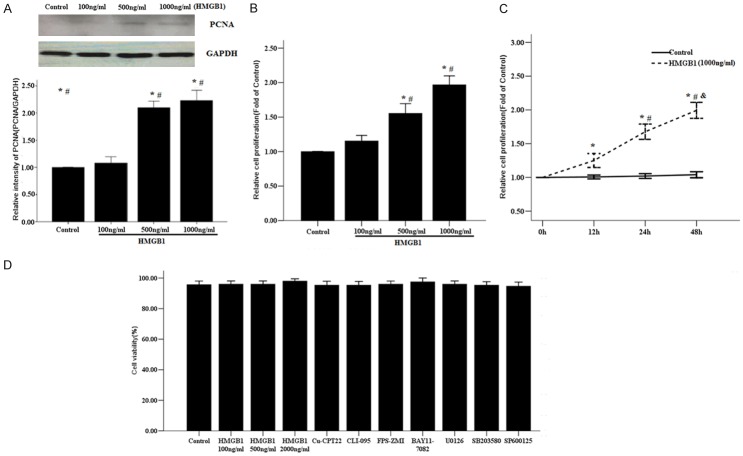
HMGB1 can induce activation of NF-кB, a key regulator of oncogenesis, to promote many types of cell proliferation such as in human mesothelial cells and lung cancer cells [10]. However, the role of HMGB1 on proliferation of RASMCs has not been determined, so we first investigated the impact of HMGB1 on cultured RASMCs. As shown in Figure 1A, 1B, HMGB1 significantly induced proliferation of RASMCs in a dose-dependent manner, compared with control cells. Similarly, HMGB1 also time-dependently significantly stimulated proliferation of RASMCs (Figure 1C).
Figure 1.
Effect of HMGB1 on proliferation of RASMCs. A. Representative western blot analysis for PCNA protein. The primary RASMCs were treated with the indicated concentrations of HMGB1 (100-1000 ng/ml) for 48 h. GAPDH was selected to normalize the PCNA level. *P < 0.05 vs. Control cells; #P < 0.05 vs the RASMCs treated with HMGB1 100 ng/ml. B. Effect of HMGB1 on RASMCs proliferation detected by the CCK-8 assay. The primary RASMCs were treated with HMGB1 for 48 h. *P < 0.05 vs. Control cells; #P < 0.05 vs. the RASMCs treated with HMGB1 100 ng/ml. C. The RASMCs were treated with HMGB1 1000 ng/ml for 0, 12, 24, 48 h. *: P < 0.05 vs. unstimulated cells at 0 h; #: P < 0.05 vs the RASMCs treated with HMGB1 for 12 h; &: P < 0.05 vs the RASMCs treated with HMGB1 for 24 h. D. Cell viability was detected by trypan blue staining. Data are expressed as mean ± SEM from four independent experiments.
HMGB1 had no effect on RASMC viability at concentrations from 100 to 1000 ng/ml. In addition, the inhibitors of receptors and signaling pathways also did not exhibit effects on cell viability (Figure 1D).
HMGB1 predominantly upregulates RAGE in RASMCs
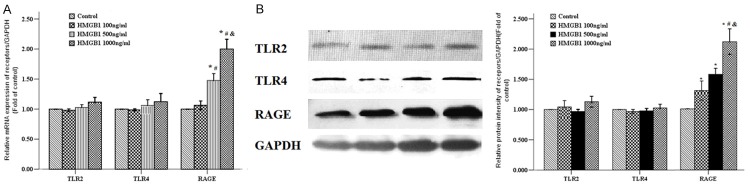
Some studies have demonstrated that TLR2, TLR4, and RAGE are involved in HMGB1-induced immune response of macrophages and in rat smooth muscle cells [3,4]. Therefore we explored the effects of HMGB1 on the expression of RAGE, TLR2, and TLR4 at the mRNA level by RT-PCR and protein levels by western blotting. The RASMCs were stimulated with HMGB1 at concentration of 0, 100, 500 and 1,000 ng/ml for 24 h. Compared with control group and HMGB1 100 ng/ml group, HMGB1 increased the expression of RAGE mRNA of primary RASMCs in 500 and 1000 ng/ml groups (Figure 2A). Similarly, HMGB1 stimulation induced an obvious increase of PCNA protein levels of RASMCs in a dose-dependent fashion (Figure 2B), but no effects of HMGB1 on the expression of TLR2 and TLR4 were observed (Figure 2).
Figure 2.
Effect of HMGB1 on receptors expression of RASMCs. A. The primary RASMCs were treated with the indicated concentrations of HMGB1 (100-1000 ng/ml) for 48 h, then these cells were assessed for TLR2, TLR4 and RAGE mRNA expression by RT-PCR. B. The primary RASMCs were treated with HMGB1 for 48 h, then the cell total proteins were extracted, and the TLR2, TLR4, and RAGE protein expression were determined by western blotting. *: P < 0.05 vs. Control cells; #: P < 0.05 vs. the RASMCs treated with HMGB1 100 ng/ml; &: P < 0.05 vs. the RASMCs treated with HMGB1 500 ng/ml. Data were expressed as mean ± SEM from four independent experiments.
HMGB1-induced proliferation of RASMCs is mediated by RAGE
To determine which receptors are involved in HMGB1-induced proliferation of RASMCs, the cells were incubated with inhibitors of RAGE, TLR2, and TLR4 one hour before HMGB1 (1000 ng/ml) stimulation. FPS-ZMI, a RAGE specific inhibitor significantly suppressed HMGB1-induced proliferation and PCNA protein expression of RASMCs (Figure 3), but there were no obvious changes in levels of proliferation and PCNA protein expression of RASMCs after administration of Cu-CPT22 (a TLR4 inhibitor) and CLI-095 (a TLR2 inhibitor) (Figure 3). These data suggest that HMGB1 signals through RAGE, but not through TLR2 and TLR4, to induce proliferation of RASMCs.
Figure 3.
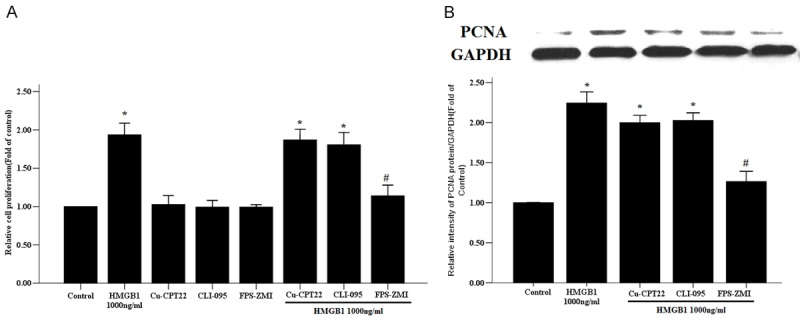
The effects of related receptors on HMGB1-induced proliferation of RASMCs. The primary RASMCs were pretreated with Cu-CPT22 (50 nM), CLI-095 (1 ug/ml) and FPS-ZMI (230 nM) for 1 hour, followed by stimulation with HMGB1 1000 ng/ml for 48 h. A. Cell proliferation was evaluated by a CCK-8 assay. B. The PCNA protein expression was determined by western blot and GAPDH was used as the internal control. *: P < 0.05 vs. unstimulated cells; #: P < 0.05 vs. the RASMCs treated with HMGB1. Data are expressed as mean ± SEM from four independent experiments.
HMGB1 induced RASMC proliferation through ERK1/2 and NF-кB pathways
The previous studies from us and others have demonstrated that HMGB1 can induce activation of NF-ĸB and the MAP kinases in human smooth muscle cells, endothelium, neutrophils, and airway epithelium [3,11-13]. We further assessed whether MAPK and NF-кB signaling pathways contributed to HMGB1-mediated RASMC proliferation. As shown in Figure 4, both the NF-ĸB inhibitor and ERK1/2 inhibitor completely suppressed the proliferation of RASMCs induced by HMGB1. Similarly, NF-кB and ERK1/2 inhibition effectively ameliorated HMGB1-induced PCNA protein expression in RASMCs. However, the proliferation and PCNA protein level of RASMCs were not attenuated by p38 inhibitor SB203580 and JNK inhibitor SP600125 under HMGB1 treatment (Figure 4).
Figure 4.
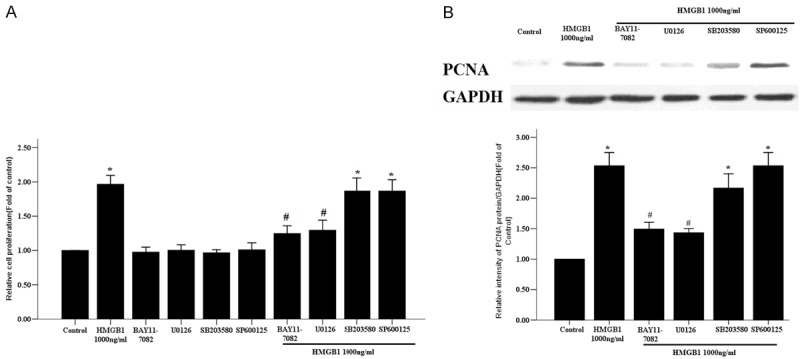
Effects of specific signaling pathway inhibitors on HMGB1-induced proliferation of RASMCs. The primary RASMCs were incubated for 1 h with or without the protein kinase inhibitors BAY11-7082 (10 µM), U0126 (10 µM), SB203580 (10 µM) or SP600125 (50 µM) before stimulation with HMGB1 (1,000 ng/ml) for 48 h. A. The cell proliferation was evaluated by a CCK-8 assay. B. The PCNA protein expression was determined by western blot and GAPDH was used as the internal control. *: P < 0.05 vs. unstimulated cells; #: P < 0.05 vs. the RASMCs treated with HMGB1. Data are expressed as mean ± SEM from four independent experiments.
HMGB1 enhances NF-кB and ERK1/2 activation
To further test whether ERK1/2 and NF-кB signaling pathways are involved in HMGB1-induced RASMC proliferation, we detected the phosphorylation extent of p65 and ERK1/2, which illustrate NF-кB and ERK1/2 signaling pathway phosphorylation activity respectively. As shown in Figure 5A, the levels of phosphorylation of p65 were significantly enhanced in HMGB1 administration groups compared to the control group; moreover, the level of phosphorylation of p65 was increased in the HMGB1 1000 ng/ml group compared to the HMGB1 100 and 500 ng/ml groups. In addition, HMGB1 stimulation induced ERK1/2 hyperphosphorylation in a concentration-dependent manner.
Figure 5.
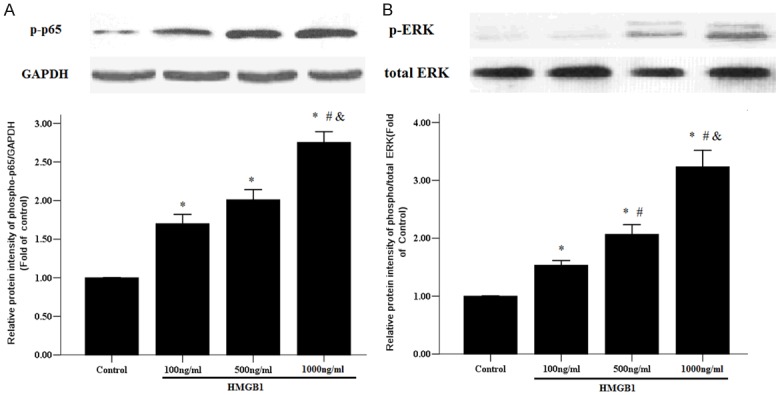
HMGB1 activated NF-ĸB and ERK1/2 signaling pathways. Primary RASMCs were treated with the indicated concentrations of HMGB1 (100-1000 ng/ml) for 12 h, then 50 µg of protein of cell lysates were analyzed for phosphorylation of p65 (A) and extracellular signal-regulated kinase 1/2 (ERK1/2) (B). GAPDH was used as internal control. *: P < 0.05 vs. Control cells; #: P < 0.05 vs. RASMCs treated with HMGB1 100 ng/ml; &: P < 0.05 vs. the RASMCs treated with HMGB1 500 ng/ml. Data are expressed as mean ± SEM from four independent experiments.
RAGE is essential for HMGB1-induced NF-кB and ERK1/2 signaling pathway activity
RAGE activation can stimulate a various number of signaling cascades, including NF-кB, and mitogen activated protein kinase (MAPK), such as ERK-1/2 [14]. In this study HMGB1 had been shown to activate similar signaling through NF-кB and ERK-1/2 (Figure 5), but it was unknown whether HMGB1 binds to RAGE to activate the signaling pathways above. As shown in Figure 6A, RAGE inhibitor FPS-ZMI as well as BAY11-7082 completely abolished HMGB1-induced phosphorylation p65 in RASMCs. Similarly, RAGE inhibitor FPS-ZMI also reversed ERK1/2 hyperphosphorylation by administration of HMGB1 (Figure 6B).
Figure 6.
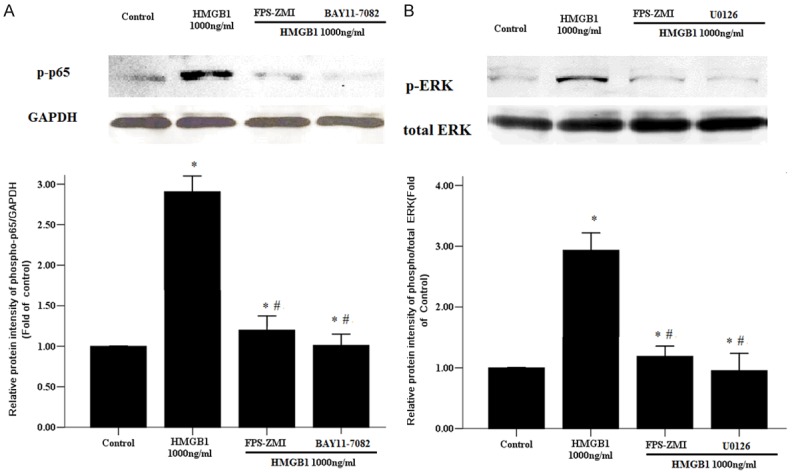
RAGE mediated HMGB1-induced phosphorylation of p65 and ERK1/2. Primary RASMCs were incubated for 1 h with or without FPS-ZMI (230 nM), BAY11-7082 (10 µM), U0126 (10 µM) before stimulation with HMGB1 (1,000 ng/ml) for 12 h. then 50 µg of protein of cell lysates were analyzed for phosphorylation of p65 (A) and extracellular signal-regulated kinase 1/2 (ERK1/2) (B). GAPDH was used as internal control. *: P < 0.05 vs. Control cells; #: P < 0.05 vs. the RASMCs treated with HMGB1 1000 ng/ml. Data are expressed as mean ± SEM from four independent experiments.
Discussion
As far as we know, this is the first report showing that exogenous HMGB1 induces proliferation of airway smooth muscle cells in a dose-dependent manner. Our data also demonstrated that HMGB1-RAGE interaction activates ERK and NF-κB signaling pathways to promote airway smooth muscle cell proliferation. More importantly, our report further confirmed that HMGB1 may play a vital role in airway remodeling by a direct effect on the proliferation of airway smooth muscle.
Increasing evidence shows that extracellular HMGB1 regulates cell proliferation of various kinds of cells including human malignant mesothelioma cells, pancreatic cancer cells, and lung cancer cells [15-17]. HMGB1 has been shown to induce proliferation of human malignant mesothelioma cells by an autocrine circuit [16]. RAGE-HMGB1 coordinately promotes pancreatic cancer cell proliferation and migration through increasing mitochondrial complex I activity, and ATP production [17].
In addition, consistent with our present study, extracellular HMGB1 has been found to promote the proliferation and migration of vessel smooth muscle cell (VSMC) by different mechanisms. The cholesterol loading can stimulate VSMC to actively secrete HMGB1; in turn administration of HMGB1 can promote VSMC proliferation and migration [18]. Glycyrrhizin, a HMGB1 inhibitor, significantly suppressed the proliferation and migration of angiotensin IItreated VSMCs though decreased levels of oxidative stress and inflammation [19]. HMGB1 also enhanced proliferation of pulmonary arterial smooth muscle cells in a c-Jun dependent manner [20].
In this study, we found that HMGB1 promoted RASMC proliferation mainly by binding to RAGE. RAGE is a a transmembrane receptor containing the extracellular domain ligand binding and the amino acid cytoplasmic tail for intracellular signaling activation [15]. RAGE is well-known as a multi-ligand receptor and can interact with various kinds of ligands such as advanced glycation end products (AGEs), S-100 protein, and HMGB1, resulting in the production of reactive oxygen species (ROS), inflammatory pathogenesis, cell proliferation, and autophagy [21]. It is reported that RAGE plays a key role in modulating smooth muscle cell proliferation and migration in a mice model undergoing arterial endothelial denudation injury [22]. Similarly, inhibition of RAGE/ligand interaction decreased S-100 protein-stimulated VSMC proliferation in vitro and proliferating VSMC in both Zucker diabetic and nondiabetic rat models undergoing injury of the carotid artery [23]. Our results are consistent with a recent study, in which S100A8 inhibited PDGF-induced rat ASMCs proliferation in a manner dependent on RAGE [24]. Collectively, these results, together with our research, further emphasized the importance of RAGE in proliferation of smooth muscle cells. TLR2 has been also shown to be involved in PDGF-induced proliferation in rat ASMCs [25]. In addition, the deletion of TLR4 from platelets attenuated promoted pulmonary artery smooth muscle cell proliferation in a serotonin-dependent manner [26]. Although the reason for these different results above is unclear, we speculate that these results may be due to the different stimuli and types of cells used.
The extracellular signal-regulated kinases (ERKs) function in control of cell division, and inhibitors of these enzymes are being explored as anticancer agents. It is well-known that ERK1/2 are widely expressed and are mainly involved in the regulation of mitosis of differentiated cells, resulting in cell proliferation [27]. Many different stimuli, such as growth factors, cytokines, and virus infection, can activate the ERK1/2 pathways. Persistent activation of the ERK1/2 signaling pathway contributes to the increased proliferative rate of tumor cells. More importantly, U-0126, a specific ERK1/2 inhibitor, suppressed mitogen-induced proliferation of airway smooth muscle by inhibition expression of c-Fos and cyclin D1, all of which are downstream from ERK in the signaling cascade [28]. This finding agrees with the observation that U-0126 inhibited HMGB1-induced proliferation of RASMCs in the present study. Previous findings, however, have suggested the PI 3-kinase pathway rather than the ERK pathway resulted in proliferation of airway smooth muscle cells in asthmatics under 10% FBS stimulation, while both PI 3-kinase pathway and ERK pathway controlled growth of airway smooth muscle cells from non-asthmatics stimulated with 10% FBS [29]. In our opinion, the smooth muscle cells obtained from different subjects may explain the above contradictory results.
NF-κB is a family of transcription factors that was originally found in B cells. When cells are under various stimuli, NF-κB is activated and regulates various kinds of corresponding genes which controls biologic functions including immune and inflammatory reactions, angiogenesis, and smooth muscle cell proliferation [30]. Increasing evidence supports the functional importance of NF-κB in promoting airway smooth muscle cell proliferation. Many stimuli, such as PDGF, Th17-associated cytokines, lysophosphatidic acid (LPA), and reactive oxygen species (ROS) angiotensin II promote the proliferation of ASM completely or in part through activation of transcription factor NF-κB [31-34]. Some pharmacologic and proteasome inhibitors also have been shown to suppress ASMC proliferation through inactivation of the NF-κB pathway. Consistent with our study, a recent study suggests that miR-24 overexpression significantly inhibits VSMC proliferation and migration by targeting HMGB1, and that NF-κB nuclear translocation and DNA binding were decreased in association with the down-regulation of HMGB1 [35]. One way for NF-κB to induce smooth muscle cell proliferation is that NF-κB can regulate the cyclin D1 promoter which is the key mediator for the cell cycle [31]. Another important finding shown in the present study was that neither inhibition of the NF-κB pathway nor inhibition of the ERK1/2 pathway could completely abolish proliferation of RASMCs and PCNA protein expression mediated by HMGB1. These results suggest that dual ERK1/2 and NF-κB pathways control proliferation of RASMCs. JNK and p38 Protein Kinases are widely known as critical regulators of transcription to activate the inflammatory response [27], which coincides with our present results in which proliferation and PCNA protein level of RASMCs were not attenuated by p38 inhibitor SB203580 and JNK inhibitor SP600125.
In summary, these results provide evidence that HMGB1-induced RASMCs proliferation is mediated by a RAGE/ERK1/2-NF-κB cascade. These data will provide us with a novel molecular mechanism for HMGB1 to be involved in airway remodeling, and some potential therapeutic strategies targeting the RAGE-dependent ERK1/2-NF-κB pathway for the treatment of asthma.
Acknowledgements
This study was supported by national natural science foundation of China (No: 81600020); the natural science foundation of Guangxi province (No: 2016GXNSFBA380100).
Disclosure of conflict of interest
None.
References
- 1.Lotze MT, Tracey KJ. High-mobility group box 1 protein (HMGB1): nuclear weapon in the immune arsenal. Nat Rev Immunol. 2005;5:331–342. doi: 10.1038/nri1594. [DOI] [PubMed] [Google Scholar]
- 2.Parkkinen J, Raulo E, Merenmies J, Nolo R, Kajander EO, Baumann M, Rauvala H. Amphoterin, the 30-kDa protein in a family of HMG1-type polypeptides. Enhanced expression in transformed cells, leading edge localization, and interactions with plasminogen activation. J Biol Chem. 1993;268:19726–38. [PubMed] [Google Scholar]
- 3.Degryse B, Bonaldi T, Scaffidi P, Müller S, Resnati M, Sanvito F, Arrigoni G, Bianchi ME. The high mobility group (HMG) boxes of the nuclear protein HMG1 induce chemotaxis and cytoskeleton reorganization in rat smooth muscle cells. J Cell Biol. 2001;152:1197–206. doi: 10.1083/jcb.152.6.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Park JS, Svetkauskaite D, He Q, Kim JY, Strassheim D, Ishizaka A, Abraham E. Involvement of toll-like receptors 2 and 4 in cellular activation by high mobility group box 1 protein. J Biol Chem. 2004;279:7370–7. doi: 10.1074/jbc.M306793200. [DOI] [PubMed] [Google Scholar]
- 5.Bianchi ME, Crippa MP, Manfredi AA, Mezzapelle R, Rovere Querini P, Venereau E. High-mobility group box 1 protein orchestrates responses to tissue damage via inflammation, innate and adaptive immunity, and tissue repair. Immunol Rev. 2017;280:74–82. doi: 10.1111/imr.12601. [DOI] [PubMed] [Google Scholar]
- 6.Hou C, Zhao H, Liu L, Li W, Zhou X, Lv Y, Shen X, Liang Z, Cai S, Zou F. High mobility group protein B1 (HMGB1) in asthma: comparison of patients with chronic obstructive pulmonary disease and healthy controls. Mol Med. 2011;17:807–15. doi: 10.2119/molmed.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hou C, Kong J, Liang Y, Huang H, Wen H, Zheng X, Wu L, Chen Y. HMGB1 contributes to allergen-induced airway remodeling in a murine model of chronic asthma by modulating airway inflammation and activating lung fibroblasts. Cell Mol Immunol. 2015;12:409–23. doi: 10.1038/cmi.2014.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saglani S, Lloyd CM. Novel concepts in airway inflammation and remodelling in asthma. Eur Respir J. 2015;46:1796–804. doi: 10.1183/13993003.01196-2014. [DOI] [PubMed] [Google Scholar]
- 9.Pelaia G, Renda T, Gallelli L, Vatrella A, Busceti MT, Agati S, Caputi M, Cazzola M, Maselli R, Marsico SA. Molecular mechanisms underlying airway smooth muscle contraction and proliferation: implications for asthma. Respir Med. 2008;102:1173–81. doi: 10.1016/j.rmed.2008.02.020. [DOI] [PubMed] [Google Scholar]
- 10.Yang H, Rivera Z, Jube S, Nasu M, Bertino P, Goparaju C, Franzoso G, Lotze MT, Krausz T, Pass HI, Bianchi ME, Carbone M. Programmed necrosis induced by asbestos in human mesothelial cells causes high-mobility group box 1 protein release and resultant inflammation. Proc Natl Acad Sci USA. 2010;107:12611–6. doi: 10.1073/pnas.1006542107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Treutiger CJ, Mullins GE, Johansson AS, Rouhiainen A, Rauvala HM, Erlandsson-Harris H, Andersson U, Yang H, Tracey KJ, Andersson J, Palmblad JE. High mobility group 1 B-box mediates activation of human endothelium. J Intern Med. 2003;254:375–85. doi: 10.1046/j.1365-2796.2003.01204.x. [DOI] [PubMed] [Google Scholar]
- 12.Park JS, Arcaroli J, Yum HK, Yang H, Wang H, Yang KY, Choe KH, Strassheim D, Pitts TM, Tracey KJ, Abraham E. Activation of gene expression in human neutrophils by high mobility group box 1 protein. Am J Physiol Cell Physiol. 2003;284:C870–9. doi: 10.1152/ajpcell.00322.2002. [DOI] [PubMed] [Google Scholar]
- 13.Liang Y, Hou C, Kong J, Wen H, Zheng X, Wu L, Huang H, Chen Y. HMGB1 binding to receptor for advanced glycation end products enhances inflammatory responses of human bronchial epithelial cells by activating p38 MAPK and ERK1/2. Mol Cell Biochem. 2015;405:63–71. doi: 10.1007/s11010-015-2396-0. [DOI] [PubMed] [Google Scholar]
- 14.Ott C, Jacobs K, Haucke E, Navarrete Santos A, Grune T, Simm A. Role of advanced glycation end products in cellular signaling. Redox Biol. 2014;9:411–29. doi: 10.1016/j.redox.2013.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang R, Zhang Q, Zeh HJ 3rd, Lotze MT, Tang D. HMGB1 in cancer: good, bad, or both? Clin Cancer Res. 2013;19:4046–57. doi: 10.1158/1078-0432.CCR-13-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jube S, Rivera ZS, Bianchi ME, Powers A, Wang E, Pagano I, Pass HI, Gaudino G, Carbone M, Yang H. Cancer cell secretion of the DAMP protein HMGB1 supports progression in malignant mesothelioma. Cancer Res. 2012;72:3290–3301. doi: 10.1158/0008-5472.CAN-11-3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kang R, Tang D, Schapiro NE, Loux T, Livesey KM, Billiar TR, Wang H, Van Houten B, Lotze MT, Zeh HJ. The HMGB1/RAGE inflammatory pathway promotes pancreatic tumor growth by regulating mitochondrial bioenergetics. Oncogene. 2014;33:567–77. doi: 10.1038/onc.2012.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Porto A, Palumbo R, Pieroni M, Aprigliano G, Chiesa R, Sanvito F, Maseri A, Bianchi ME. Smooth muscle cells in human atherosclerotic plaques secrete and proliferate in response to high mobility group box 1 protein. FASEB J. 2006;20:2565–6. doi: 10.1096/fj.06-5867fje. [DOI] [PubMed] [Google Scholar]
- 19.Chen J, Zhang J, Xu L, Xu C, Chen S, Yang J, Jiang H. Inhibition of neointimal hyperplasia in the rat carotid artery injury model by a HMGB1 inhibitor. Atherosclerosis. 2012;224:332–9. doi: 10.1016/j.atherosclerosis.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Zabini D, Crnkovic S, Xu H, Tscherner M, Ghanim B, Klepetko W, Olschewski A, Kwapiszewska G, Marsh LM. High-mobility group box-1 induces vascular remodelling processes via c-Jun activation. J Cell Mol Med. 2015;19:1151–61. doi: 10.1111/jcmm.12519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bongarzone S, Savickas V, Luzi F, Gee AD. Targeting the receptor for advanced glycation endproducts (RAGE): a medicinal chemistry perspective. J Med Chem. 2017;60:7213–7232. doi: 10.1021/acs.jmedchem.7b00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sakaguchi T, Yan SF, Yan SD, Belov D, Rong LL, Sousa M, Andrassy M, Marso SP, Duda S, Arnold B, Liliensiek B, Nawroth PP, Stern DM, Schmidt AM, Naka Y. Central role of RAGE-dependent neointimal expansion in arterial restenosis. J Clin Invest. 2003;111:959–72. doi: 10.1172/JCI17115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Z, Wang K, Penn MS, Marso SP, Lauer MA, Forudi F, Zhou X, Qu W, Lu Y, Stern DM, Schmidt AM, Lincoff AM, Topol EJ. Receptor for AGE (RAGE) mediates neointimal formation in response to arterial injury. Circulation. 2003;107:2238–43. doi: 10.1161/01.CIR.0000063577.32819.23. [DOI] [PubMed] [Google Scholar]
- 24.Xu YD, Wang Y, Yin LM, Peng LL, Park GH, Yang YQ. S100A8 inhibits PDGF-induced proliferation of airway smooth muscle cells dependent on the receptor for advanced glycation end-products. Biol Res. 2017;50:23. doi: 10.1186/s40659-017-0128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cheng W, Yan K, Chen Y, Zhang W, Ji Z, Dang C. ABCA1 induced proliferation and migration of rat airway smooth muscle cell through blocking TLR2/NF-κB/NFATc1 signaling. J Cell Biochem. 2018;119:7388–96. doi: 10.1002/jcb.27046. [DOI] [PubMed] [Google Scholar]
- 26.Bauer EM, Chanthaphavong RS, Sodhi CP, Hackam DJ, Billiar TR, Bauer PM. Genetic deletion of toll-like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circ Res. 2014;114:1596–600. doi: 10.1161/CIRCRESAHA.114.303662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–2. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 28.Lee JH, Johnson PR, Roth M, Hunt NH, Black JL. ERK activation and mitogenesis in human airway smooth muscle cells. Am J Physiol Lung Cell Mol Physiol. 2001;280:L1019–29. doi: 10.1152/ajplung.2001.280.5.L1019. [DOI] [PubMed] [Google Scholar]
- 29.Burgess JK, Lee JH, Ge Q, Ramsay EE, Poniris MH, Parmentier J, Roth M, Johnson PR, Hunt NH, Black JL, Ammit AJ. Dual ERK and phosphatidylinositol 3-kinase pathways control airway smooth muscle proliferation: differences in asthma. J Cell Physiol. 2008;216:673–9. doi: 10.1002/jcp.21450. [DOI] [PubMed] [Google Scholar]
- 30.De Martin R, Hoeth M, Hofer-Warbinek R, Schmid JA. The transcription factor NF-kappa B and the regulation of vascular cell function. Arterioscler Thromb Vasc Biol. 2000;20:E83–8. doi: 10.1161/01.atv.20.11.e83. [DOI] [PubMed] [Google Scholar]
- 31.Guttridge DC, Albanese C, Reuther JY, Pestell RG, Baldwin AS Jr. NF-kB controls cell growth and differentiation through transcriptional regulation of cyclin D1. Mol Cell Biol. 1999;19:5785–99. doi: 10.1128/mcb.19.8.5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ediger TL, Schulte NA, Murphy TJ, Toews ML. Transcription factor activation and mitogenic synergism in airway smooth muscle cells. Eur Respir J. 2003;21:759–69. doi: 10.1183/09031936.03.00075702. [DOI] [PubMed] [Google Scholar]
- 33.Xie S, Sukkar MB, Issa R, Khorasani NM, Chung KF. Mechanisms of induction of airway smooth muscle hyperplasia by transforming growth factor-beta. Am J Physiol Lung Cell Mol Physiol. 2007;293:L245–53. doi: 10.1152/ajplung.00068.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chang Y, Al-Alwan L, Risse PA, Halayko AJ, Martin JG, Baglole CJ, Eidelman DH, Hamid Q. Th17-associated cytokines promote human airway smooth muscle cell proliferation. FASEB J. 2012;26:5152–60. doi: 10.1096/fj.12-208033. [DOI] [PubMed] [Google Scholar]
- 35.Yang J, Chen L, Ding J, Fan Z, Li S, Wu H, Zhang J, Yang C, Wang H, Zeng P, Yang J. MicroRNA-24 inhibits high glucose-induced vascular smooth muscle cell proliferation and migration by targeting HMGB1. Gene. 2016;586:268–73. doi: 10.1016/j.gene.2016.04.027. [DOI] [PubMed] [Google Scholar]