Abstract
TCP1 ring complex (TRiC) participates in protein folding in cells, regulating the expression of many tumor-related proteins and the cell cycle. Although the clinical significance of its subunits has been widely discussed in various malignancies, limited studies have explored its function in hepatocellular carcinoma (HCC) in the perspective of a complex. This study discusses the clinical significance of the TRiC subunits in HCC patients in terms of expression level, prognostic value, and potential mechanism. We used HCC samples from Nanfang hospital, data from The Cancer Genome Atlas (TCGA) database and information from the Gene Expression Omnibus (GEO) database with statistical methods and Gene Set Enrichment Analysis (GSEA) to analyze the gene expression levels of TRiC subunits along with survival data. We found altered expressions of the TRiC subunits in HCC, including significantly increased TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 expressions as well as decreased CCT6B expression, which predict poor prognosis and are associated with tumor progression. Moreover, the expression levels of these genes were pairwise correlated in HCC, indicating that the function of the entire complex should be explored as a functional macrocosm. Finally, we identified that the overexpressions of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A are involved in the dysregulation of Myc target genes, hypoxia-inducible factor (HIF) target genes and cell cycle especially the G1/S transition. Our study found that all TRiC subunits are aberrantly co-expressed in HCC, and these components have potential as therapeutic targets.
Keywords: Hepatocellular carcinoma, TCP1 ring complex, prognosis, co-expression, liver
Introduction
Protein folding plays an important role in the expression of genes [1]. The TCP1 ring complex (TRiC), also known as cytosolic chaperonin containing t-complex polypeptide 1 or CCT, is a kind of ATP-dependent molecular chaperonins in charge of the highly efficient folding of nascent polypeptides [2,3]. TRiC consists of two symmetry rings with eight paralogous subunits (α, β, γ, δ, ε, ζ-1 or ζ-2, η and θ encoded by TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, or CCT6B, CCT7, and CCT8) composing each ring [4,5]. TRiC mediates the folding of cytoskeletal proteins including tubulins and actins [6,7]. Moreover, as evidenced by its participation in the cell cycle, the upregulation of TRiC is manifested during G1/S transition through the early S phase [8], and the downregulation of subunit TCP1 leads to the inhibition of cell proliferation and cell cycle arrest [9]. Recent studies indicate that the extended list of TRiC clients includes proteins involved in tumorigenesis such as cyclin E [10], the tumor suppressor protein Von Hippel-Lindau (VHL) [11], p21 [12] and p53 [13]. Increased levels of TRiC subunits are manifested in several solid tumors including gastric cancer [14], colorectal cancer [15], breast cancer [16] and non-small cell lung cancer [17], and the knockdown of TRiC subunits inhibits the proliferation and colony formation of gastric cancer and breast cancer cells in vitro [14,16]. But the mutation of CCT6B, resulting in the loss of function in efficient protein folding, was reported in Burkitt lymphoma [18]. In addition to malignancies, the downregulation of TRiC was also reported to be involved in neurodegenerative diseases such as Huntington’s disease, since the subunits of TRiC could prevent disease progression by reducing misfolding proteins [19]. Thus, it is of great significance to elucidate the function and mechanism of TRiC.
Liver cancer ranks sixth in terms of the most commonly diagnosed cancers and is the fourth most common cause of cancer-related deaths worldwide. Hepatocellular carcinoma (HCC), comprising more than three quarters of liver cancer cases, is characterized by high proliferation, heterogeneity, and invasiveness [20]. Due to the cancer’s delayed diagnosis and rapid progression, HCC patients receive limited efficacy from hepatectomy, transcatheter arterial chemoembolization, and other treatments [21]. Limited studies have demonstrated the function and significance of TRiC in HCC. The correlation between the aberrant overexpression of CCT3 and the poor prognosis of HCC patients has been shown, as has the depletion of CCT3 sensitized HCC cells to chemotherapy [22]. Another study defined CCT8 as an oncogene and demonstrated its function of participating in HCC cell proliferation by facilitating S-phase entry [23]. However, all subunits are necessary for the function of TRiC. The loss of any subunits would significantly affect the expression of other subunits at the protein level [24]. Therefore, the components TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7, CCT8 should be a macroscopic structure with abundant functions, which is far from being fully investigated.
Thus, we aimed to demonstrate the clinical significance of TRiC subunit expression and explore the molecular mechanism for their possible implications in diagnosis and treatment.
Material and methods
Patients and tissues
A total of 7 HCC patients enrolled in the study were diagnosed with primary HCC at Nangfang Hospital, Southern Medical University (Guangzhou, China) between January and December of 2015. All patients underwent hepatectomies without pretreatments of radiotherapy, chemotherapy or targeted therapy, and fresh HCC and paired adjacent non-tumor liver specimens were collected. Informed consents were provided by all patients eligible for the collection prior to their participation in the study. The protocol of the present study was approved by the Ethics Committee of Nangfang Hospital, Southern Medical University.
Data source
To compare the RNA expression levels of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 between HCC and the normal controls, data from 351 HCC and 50 liver samples were obtained from The Cancer Genome Atlas (TCGA) database. The HCC dataset of TCGA consists of follow-up information and genome-wide expression profiles. 332 HCC patients with available data on TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 expressions and clinical information were available for the survival analysis.
The GSE89377 dataset, downloaded from the Gene Expression Omnibus (GEO) website (https://www.ncbi.nlm.nih.gov/geo/geo2r/?acc=GSE89377) presented expression profiles from precancerous lesions and early HCC to overt cancer and contains a total of 108 cases, including healthy donors (n=13), with low-graded (n=8) and high-graded chronic hepatitis (n=12), cirrhosis (n=12), low-graded (n=11) and high-graded dysplastic nodules (n=11), early HCC (n=5), HCC during stage I (n=9), stage II (n=12), and stage III (n=14).
The GSE14520 dataset, downloaded from the GEO website (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE14520) includes the gene expression profiles of 233 HCC patients containing the expressions of tumor and paired non-tumor tissues.
The gene set enrichment analysis (GSEA)
Gene sets or pathways related to TRiC subunit expressions in the TCGA dataset were screened by the gene set enrichment analysis (GSEA). Taking TCP1 as an example, the patients were divided into two groups. According to the median expression of each gene, 351 HCC patients were separated into a high expression group and a low expression group. GSEA software was used to generate enrichment data through analysis, annotation and interpretation. The significance thresholds were Normalized Enrichment Score >1, Nominal p-value <0.05 and FDR q-value <0.25.
RNA isolation, cDNA synthesis, and RTqPCR analysis
Total RNA of the HCC samples and adjacent liver tissues was isolated using TRIzol® reagent (Invitrogen; Thermo Fisher Scientific, Waltham, MA, USA), according to the protocol of manufacturer. The RNA concentration was determined using a spectrophotometer. Reverse transcription was performed utilizing PrimeScripTM RT reagent Kit (Takara Biotechnology Co. Ltd. Dalian, China) in a 20 µl reaction volume with 1000 ng RNA, followed by the removal of genomic DNA by DNA Eraser. A qPCR reaction was performed using a SYBR Green PCR kit (Takara Biotechnology Co. Ltd. Dalian, China) and LightCycler® 480 Instrument II (Roche Diagnostics, Basel, Switzerland). The sequences of the primers were as follows: TCP1 sense, 5’-TGGTGCAACCATCCTGAAGT-3’, and anti-sense, 5’-ATAACGCACTGCTTCCTTGC-3’; CCT2 sense, 5’-CTCTTGTCACAGGTGGTGAAATT-3’, and anti-sense, 5’-CTCAGAACAGCCTCCTCCATAA-3’; CCT3 sense, 5’-TGCTGCCAAGATTCAAGTCC-3’, and anti-sense, 5’-TCATCCAATGCCTTGCGGTA-3’; CCT4 sense, 5’-CCTATCAGGACCGCGACAAG-3’, and anti-sense, 5’-AGCTCCACCAGCATTTTATCCA-3’; CCT5 sense, 5’-AAATTGAGCTGATTGCCATCGC-3’, and anti-sense, 5’-TTCGCCTCCTCAATGATCATCTTA-3’; CCT6A sense, 5’-CAGACGGGCCGACTTTTCC-3’, and anti-sense, 5’-AACGAGCATCTTCATGGTGCC-3’; CCT6B sense, 5’-TCCCTCTGAACGGTTAGGCT-3’, and anti-sense, 5’-GTGCAGGCCCATCTCATCG-3’; CCT7 sense, 5’-GATTGGAGGCGAGAGGTACAATT-3’, and anti-sense, 5’-CCACTGAATCATTCTTGATGGCC-3’; CCT8 sense, 5’-GAATGAGGTGGGAGCGTCAG-3’, and anti-sense, 5’-CTACAACGCGCGGCTTCA-3’; GAPDH sense, 5’-CAGGAGGCATTGCTGATGAT-3’, and anti-sense, 5’-GAAGGCTGGGGCTCATTT-3’. The amplification reaction was performed under the following conditions: preliminary denaturation for 2 minutes at 95°C, followed by 40 cycles of denaturation at 95°C for 5 seconds and annealing at 60°C for 20 seconds. A subsequent melting curve analysis was routinely run to ensure the specificity of amplicons. The expression levels of the experimental genes were normalized to the expression of GAPDH, and the relative quantification method (2-ΔΔCt) was performed to calculate the fold changes of the genes.
Statistical analysis
Student’s t-test for independent samples was performed to assess the significant differences between two groups. One-way analysis of variance (ANOVA) and subsequent Dunnett’s multiple comparisons were performed to compare the expression levels among more than two groups. Student’s t-test for paired samples was performed to compare the statistical differences between HCC samples and the paired adjacent liver tissues. A log-rank based survival analysis was applied to assess the statistical differences of overall survival (OS) or disease-free survival (DFS) time between the high and low expression level groups. The Cox proportional hazardous model was used for a univariate analysis in evaluating the prognostic significance of the experimental genes. Linear correlation analysis was performed to evaluate the pairwise correlation among experimental genes. Cox proportional hazardous model and linear correlation analysis were generated by SPSS 22.0 software (IBM, Chicago, USA). Student’s t-test, one-way ANOVA, and log-rank based survival analysis were performed using Graphpad Prism (La Jolla, CA, USA). A heat map was depicted with tools from the Morpheus website (https://software.broadinstitute.org/morpheus/).
Result
TRiC subunits present variable expression in HCC
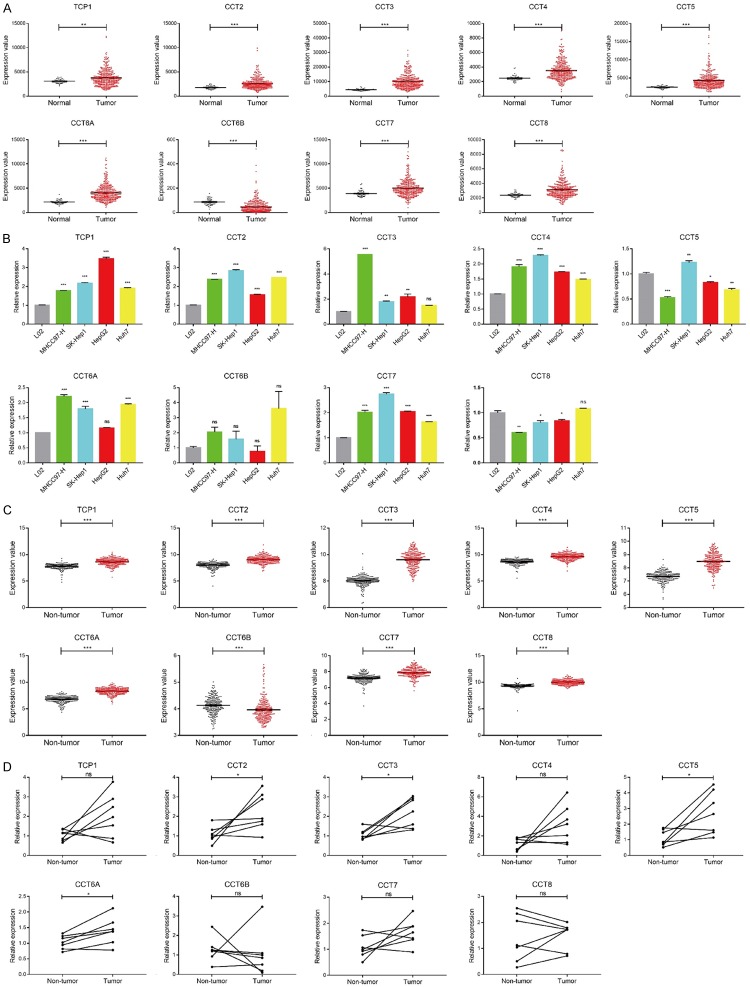
To determine the expression levels of the TRiC subunits in HCC, we compared the RNA expressions of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT7, and CCT8 between the HCC and non-tumor tissues obtained from The Cancer Genome Atlas (TCGA) dataset. In the HCC samples, TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 were overexpressed and CCT6B was downregulated as compared with normal liver tissues (Figure 1A). To verify this result, we measured the mRNA expressions of the TRiC subunits in the HCC cell lines and the normal human hepatic cell line L02 was used as a control. The expression levels of TCP1/CCT2/CCT4/CCT7 in MHC97-H, SK-Hep1, HepG2, and Huh7 were higher than those of the normal controls, and the upregulation of CCT6A was approved in MHC97-H, SK-Hep1 and Huh7, while the expression of CCT3 was elevated in MHC97-H, SK-Hep1, and HepG2 (Figure 1B). However, the expressions of CCT5 and CCT8 were not increased in the HCC cell lines except for that of CCT5 in SK-Hep1, and the CCT6B expression remained unchanged (Figure 1B). We also analyzed a GEO dataset, GSE14520, and found significant overexpression of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 as well as low expression of CCT6B in the tumor lesions compared with the adjacent non-tumor liver tissues (Figure 1C). The significant upregulation of CCT2/CCT3/CCT5/CCT6A was manifested while TCP1/CCT4/CCT6B/CCT7/CCT8 showed little difference between the paired HCC and adjacent non-tumor liver tissue samples collected from the HCC patients at Nanfang Hospital, which may due to the limited size of the samples detected.
Figure 1.
Comparison of TRiC subunit expression between HCC samples and normal or adjacent liver tissues. A. Comparison of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 expression levels between the HCC samples (n=351) and normal liver tissues (n=50) in the TCGA database, assessed by Student’s t-test. B. Expression of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 in the MHC97-H, SK-Hep1, HepG2, and Huh7 cell lines. Human L02, a kind of human hepatic cell line, was normalized as a control in a one-way ANOVA analysis. C. The expression levels of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 between HCC samples and paired adjacent liver tissues (n=223) in the GSE14520 dataset, compared by Student’s t test for paired samples. D. Expressions of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8 (n=7) in HCC and adjacent non-tumor liver specimens, compared by Student’s t test for paired samples. The results above were presented as the means ± standard error of mean (SEM). *P<0.05, **P<0.01, ***P<0.001 and ns indicated non-significant.
Altered expression of TRiC subunits was correlated with the progression and poor prognosis of HCC
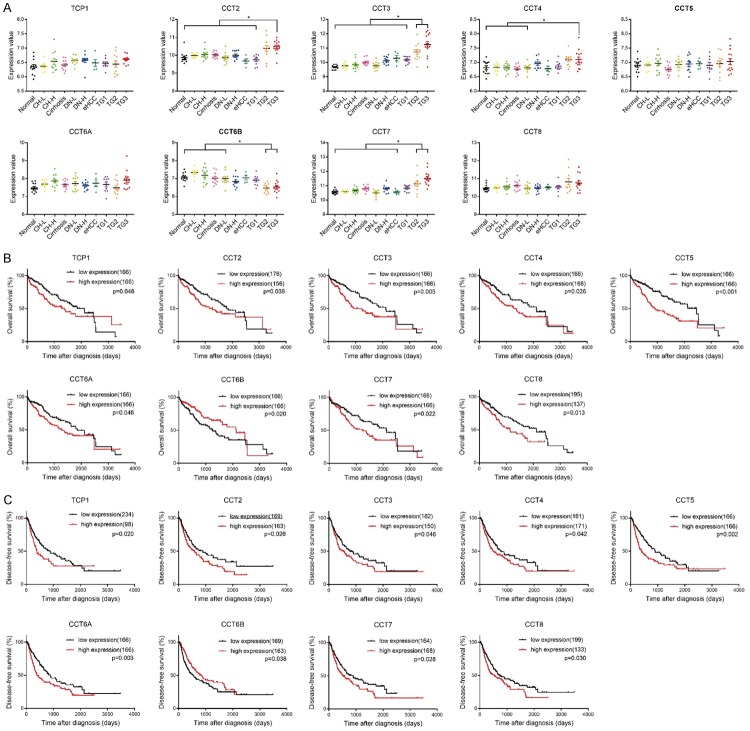
Since the expressions of the TRiC subunits were altered in HCC, we thus investigated the clinical significance of their dysregulation. We first examined the trend of TRiC subunit expressions from precancerous lesions and early HCC to overt cancer. CCT2/CCT3/CCT4/CCT7 were significantly upregulated in the middle and late stages of HCC, especially stages II and III, as compared with chronic hepatitis, cirrhosis, dysplastic nodules and early HCC, but the expression of CCT6B was reduced in HCC during stages II and III (Figure 2A). Then we asked whether their expression affected the prognosis of HCC patients. According to TCGA dataset, overexpressed TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 were respectively correlated with shortened overall survival time and disease-free survival time, yet the results of CCT6B were the opposite (Figure 2B, 2C). A univariate analysis also recognized TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7 as hazardous factors and CCT6B as a protective factor to the survival of HCC patients (Table 1). Hence, these results indicated the association of TRiC subunit expressions with progression and poor prognosis in HCC.
Figure 2.
The association between TRiC subunits expression and progression as well as the prognosis of HCC patients. (A) Expression of TRiC subunits in GSE89377 liver cancer cohort (n=108). Abbreviations were as follows: N, normal; FL, chronic hepatitis with low grade; FH, chronic hepatitis with high grade; CS, cirrhosis; DL, dysplastic nodules with low grade; DH, dysplastic nodules with high grade; eHCC, early HCC; TG1, HCC during stage I; TG2, HCC during stage II; TG3, HCC during stage III. Taking CCT3 as an example, the asterisk * indicated the expression levels of CCT3 were significantly upregulated (P<0.05) in HCC during stages II and III as respectively compared with normal, chronic hepatitis with low grade, chronic hepatitis with high grade, cirrhosis, dysplastic nodules with low grade, dysplastic nodules with high grade, early HCC and HCC during stage I. (B) Overall survival analysis and (C) disease-free survival analysis of TRiC subunits according to the TCGA dataset.
Table 1.
Correlation between expression levels of TRiC subunits and survival time in the TCGA hepatocellular carcinoma cohort
| Variates | Univariate analysis | ||
|---|---|---|---|
|
| |||
| HR | 95% CI | p-value | |
| TCP1 | 1.248 | 1.011-1.541 | 0.039 |
| CCT2 | 1.609 | 1.289-2.010 | 0.000 |
| CCT3 | 1.197 | 1.054-1.359 | 0.006 |
| CCT4 | 1.388 | 1.158-1.665 | 0.000 |
| CCT5 | 1.528 | 1.264-1.848 | 0.000 |
| CCT6A | 1.539 | 1.258-1.883 | 0.000 |
| CCT6B | 0.719 | 0.553-0.935 | 0.014 |
| CCT7 | 1.242 | 1.046-1.474 | 0.013 |
| CCT8 | 1.223 | 0.976-1.533 | 0.080 |
Univariate analysis was presented through the Cox-regression model. Abbreviations are as follows: HR, hazard radio; CI, confidence interval.
TRiC subunits co-expressed at variable levels
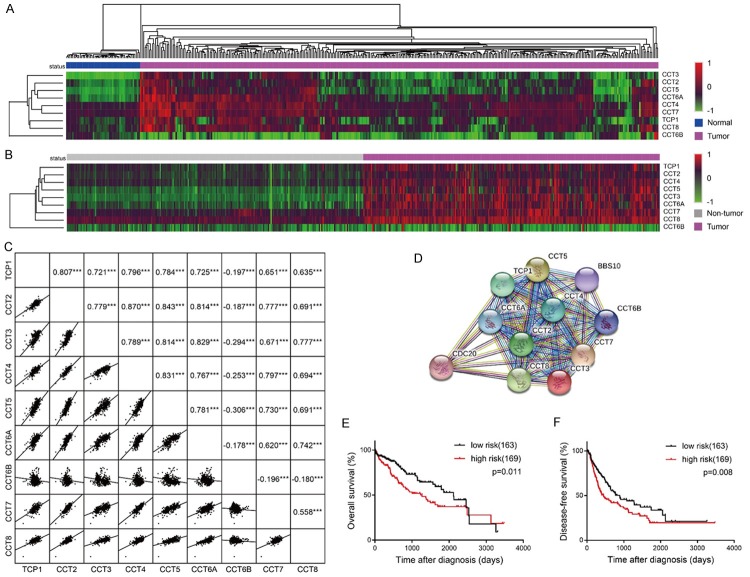
In physiological circumstances, all the subunits are necessary for the chaperonin complex to execute the proper function [25]. We thus hypothesized that the TRiC subunits were co-expressed as a macrocosm. In the HCC cohort of TCGA, the downregulation of CCT6B and the upregulation of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 were simultaneously manifested in about one third of the HCC samples, compared with normal liver tissues (Figure 3A). Besides, the heat map about the information from the GSE89377 dataset indicated that TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 were simultaneously highly expressed, but the CCT6B level was decreased in each HCC sample, compared with its paired non-tumor tissue (Figure 3B). Pairwise correlation according to the GSE14520 dataset also manifested positive correlations among TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8, but CCT6B was negatively correlated with the others (Figure 3C). The protein interactions including all the subunits involved in TRiC were reported by Search Tool for the Retrieval of Interacting Genes/Proteins (STRING), suggesting that the common expression of these subunits plays a role in the chaperonin complex (Figure 3D). The STRING analysis of TRiC also revealed its regulation of the cell cycle-involving CDC20 and interaction with ATP hydrolysis-related BBS10 (Figure 3D). To test the classification ability of the co-expressed genes, we analyzed the correlation between a combination of nine genes and the prognosis of HCC patients. High expression of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 as well as low expression of CCT6B in HCC patients were defined as risk factors. We divided the samples with more than 4 risk factors into a high risk group and the others into a low risk group. The high risk group was correlated with poor prognosis as shown by shorter overall survival times and disease-free survival times (Figure 3E, 3F). The result suggested that a nine-gene signature could notably stratify the poor and favorable survival of the HCC patients. In short, we demonstrated that the role of TRiC in HCC is determined by the variable but synergistic co-expression of its subunits.
Figure 3.
The association among the expressions of the TRiC subunits. (A) Heat map displayed the RNA expression levels of TRiC subunits comparing HCC with normal liver samples in the TCGA dataset. The hierarchical clustering method was performed to cluster genes and disease status respectively. (B) A heat map displayed RNA expression of the TRiC subunits obtained from the GSE14520 dataset when comparing HCC with adjacent non-tumor samples. The hierarchical clustering method was performed according to the general expression levels of genes. (C) A scatterplot matrix analysis plotted the pairwise correlation among TRiC subunits according to the GSE14520 dataset. Correlation coefficients and p-values are presented in the upper right area. (D) Links to TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7, and CCT8 at the protein level were analyzed by STRING. (E) Overall survival analysis and (F) disease-free survival analysis of risk factors. High expressions of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 as well as low expressions of CCT6B were defined as risk factors with the median of each gene as the cutoff, and 332 HCC patients from the TCGA dataset were grouped based on their number of risk factors. Patients with 0-4 risk factors were classified into the low risk group, and those with 5-9 risk factors were classified into the high risk group.
TRiC plays an important role in the upregulation of genes related to cell cycle, HIF and Myc targets
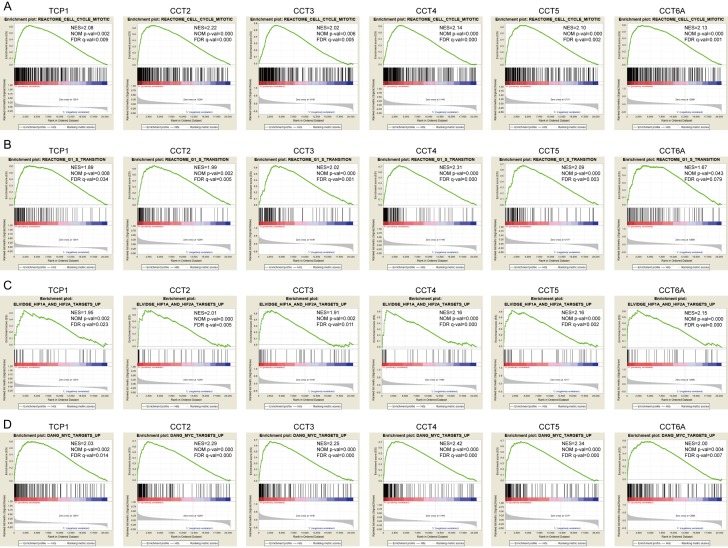
Since the variable expression of TRiC was related to the poor prognosis of HCC patients, we analyzed the TCGA dataset to obtain further insights into the mechanism of TRiC in HCC by performing Gene Set Enrichment Analysis (GSEA). Data from TCGA indicated a strong association between the overexpression of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A and the genes involved in the cell cycle (Figure 4A), especially the G1/S transition (Figure 4B). However, similar results were not shown with the overexpressions of CCT7 or CCT8, or the low expression of CCT6B. Enriched target genes of hypoxia-inducible factor (HIF) were manifested in conditions of overexpressed TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A (Figure 4C), probably associated with the dysregulation of VHL, whose folding is modulated by TRiC. In another aspect, target genes of Myc, an oncogenic transcription factor, were also enriched in these patients (Figure 4D). These results implicated the potential mechanism of the TRiC subunits in HCC.
Figure 4.
Further mechanism exploration to TRiC function using GSEA. A. Gene enrichment in the cell cycle was correlated with the upregulation of TRiC subunits. B. The overexpression of TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A in HCC is associated with enriched genes during G1/S transition of mitosis. C. HIF-related genes were enriched in conditions of overexpressed TRiC subunits. D. Myc target genes were enriched in conditions of elevated mRNA levels of TRiC subunits. The significance thresholds were Normalized Enrichment Score (NES) >1, Nominal p-value (NOM p-val) <0.05 and FDR q-value (FDR q-val) <0.25.
Discussion
The diagnosis and effective treatment of HCC are challenging to clinical physicians and basic researchers and its high incidence makes it imperative to disclose more sensitive and specific biomarkers for early detection and personal treatment strategies [26]. The clinical significance of the altered expression of TRiC has been reported in a variety of cancers. With only a handful of studies, the function and underlying mechanism of TRiC in HCC still requires clear elucidation. The upregulation of CCT3 and CCT8 were respectively reported to promote cell proliferation in HCC, and CCT3 elimination would induce mitotic arrest at the prometaphase and apoptosis [22,23]. In this study, we analyzed the TCGA and GEO datasets and found the aberrant TRiC subunit expression in HCC, compared with either normal controls or adjacent non-tumor liver tissues. The overexpression of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 as well as the low expression of CCT6B were correlated with the poor survival of patients. Interestingly, mutated genes of CCT6B, which could result in the loss of function of TRiC, was disclosed in Burkitt lymphoma, indicating CCT6B might be a potential anti-oncogene. In cell lines and collected HCC specimens, the expression levels of TRiC subunits were not completely the same as those in the TCGA and GEO datasets. This may be due to different characteristics between the HCC samples and the cell lines and the result in collected samples would be more similar to those in the TCGA dataset with enlarged sample sizes. Thus, in our study, we show for the first time, that all TRiC subunits are aberrantly expressed and correlate with the poor prognosis of HCC patients.
To execute the proper function, all the subunits are necessary for the chaperonin complex. The knockdown of any of them may impact the expression of the other components, resulting in reduced activity of the complex [25]. In Hela cells, CCT2 knockdown reduced CCT7 levels and the respective depletion of individual CCT2/CCT5/CCT7 impairs the degradation of the autophagosomes [24]. Thus, all subunits should be investigated as a whole. In our study, we demonstrated a high co-expression of TCP1/CCT2/CCT3/CCT4/CCT5/CCT6A/CCT7/CCT8 and a negative correlation between CCT6B and them in HCC. In addition, a set of nine co-expressed subunits was performed to divide the HCC patients into two groups with notably different survival rates. Our study presented evidence that changes in the expression of a single subunit affect others, and all TRiC subunits should be investigated at the same time.
TRiC is indispensable for the correct folding of many proteins, including tubulins, actins, and cell cycle regulatory proteins [6,27]. Cell cycle disorder is a prominent feature of cancer [28]. Increasing evidence has shown that TRiC is strongly upregulated during the G1/S transition to the early S phase in the cell cycle [8]. CDC20 is required for cell cycle progression, and increased levels of CDC20 have been revealed in the progression of several malignancies [29,30]. It was reported that TRiC was required for the proper folding of CDC20 which subsequently bound to a multi-component E3 ligase to form anaphase promoting complex (APC) [8], and APC is involved in the regulation of key cell cycle regulators, such as cyclin A, cyclin B1, p21 and securin [31]. Aberrant levels of TRiC have been shown to facilitate CDC20 in the cell cycle promotion of human cancers [32]. TRiC is also known to modulate the activity of cyclin E by assisting the folding of nascent cyclin E whose deregulated expression is correlated with aggressive tumor phenotypes [33]. Therefore, TRiC contributes to the folding and activity of cell cycle regulators, and its deregulation is probably associated with cancer progression. Our study confirmed gene enrichment in cell cycle, especially during the G1/S transition, in the presence of overexpressed TRiC subunits. In another aspect, TRiC assembles VHL through the binding site, a 55 amino-acid region corresponding to exon 2 of VHL, whose loss-of-function leads to incorrect folding of VHL and tumorigenesis [34]. With eliminated VHL activity, the decreased degradation of hypoxia-inducible factor (HIF) results in several key hallmarks of cancer, including proliferation, metastasis, and angiogenesis [35,36]. In this study, we discovered enriched HIF target genes respond to the elevated expression of TRiC subunits in HCC cohort of TCGA as shown by GSEA, and we suggest that aberrantly expressed TRiC subunits might lead to the incorrect folding of VHL. In addition, it is also well known that mutations of p53 promote cancer development in several ways. TRiC regulates the protein stability of wild type and mutant p53 by binding to p53 along with co-factors HSP70 and HSP90 [37,38]. The interaction between the binding domain of p53 and TRiC is essential for correct protein folding, and the loss or modulation of the interaction lead to misfolded p53 accumulation which acts in a similar manner as mutant p53 [39]. Also, It is reported that TRiC is able to modulate the folding and function of Signal Transducer and Activator of Transcription 3 (STAT3) [40]. The knockdown of TRiC reduces the phosphorylation of STAT3, while the aberrant activation of STAT3 is highly correlated with many malignant hallmarks in tumor development [41]. We also disclosed the association between enriched Myc target genes and the upregulation of TRiC subunits. In the MCF-7 cell line, elevated expression of the c-Myc protein was reported in conditions of TRiC overexpression [42]. We suggested that the aberrant expression of TRiC might regulate malignant phenotypes in HCC by modulating Myc and its downstream targets. Taken together, the overexpression of TRiC subunits migght be associated with the dysfunction of this chaperone complex, leading to a series of malignant phenotypes in HCC.
In summary, we demonstrated that the RNA expression of TRiC subunits is variable in HCC, which is significantly correlated with the prognosis of HCC patients. Moreover, the nine subunits, TCP1, CCT2, CCT3, CCT4, CCT5, CCT6A, CCT6B, CCT7 and CCT8, were investigated as a whole for the first time in HCC and the interrelation of the expression of these subunits was revealed, suggesting that TRiC should be studied as a functional macrocosm. As the function of TRiC is still not completely understood, our novel discovery may have implications in determining its function in further studies.
Acknowledgements
We would like to thank Jungwoo Eun for sharing information about the GSE89377 dataset and Xinwei Wang for sharing data regarding the GSE14520 dataset online. This paper was carefully edited by Wenwen Li. The research was funded by grants from the National Natural Science Foundation of China (grant nos. 81773008 and 81672756), the Natural Science Foundation of Guangdong Province (grant no. 2017A030311023), and the Guangdong Province Universities and Colleges Pearl River Scholar Funded Scheme (2015).
Disclosure of conflict of interest
None.
References
- 1.Melville MW, McClellan AJ, Meyer AS, Darveau A, Frydman J. The Hsp70 and TRiC/CCT chaperone systems cooperate in vivo to assemble the von Hippel-Lindau tumor suppressor complex. Mol Cell Biol. 2003;23:3141–3151. doi: 10.1128/MCB.23.9.3141-3151.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothman JE. Polypeptide chain binding proteins: catalysts of protein folding and related processes in cells. Cell. 1989;59:591–601. doi: 10.1016/0092-8674(89)90005-6. [DOI] [PubMed] [Google Scholar]
- 3.Broadley SA, Hartl FU. The role of molecular chaperones in human misfolding diseases. FEBS Lett. 2009;583:2647–2653. doi: 10.1016/j.febslet.2009.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Kubota H, Hynes G, Willison K. The chaperonin containing t-complex polypeptide 1 (TCP-1). Multisubunit machinery assisting in protein folding and assembly in the eukaryotic cytosol. Eur J Biochem. 1995;230:3–16. doi: 10.1111/j.1432-1033.1995.tb20527.x. [DOI] [PubMed] [Google Scholar]
- 5.Spiess C, Meyer AS, Reissmann S, Frydman J. Mechanism of the eukaryotic chaperonin: protein folding in the chamber of secrets. Trends Cell Biol. 2004;14:598–604. doi: 10.1016/j.tcb.2004.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yaffe MB, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]
- 7.Sternlicht H, Farr GW, Sternlicht ML, Driscoll JK, Willison K, Yaffe MB. The t-complex polypeptide 1 complex is a chaperonin for tubulin and actin in vivo. Proc Natl Acad Sci U S A. 1993;90:9422–9426. doi: 10.1073/pnas.90.20.9422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yokota S, Yanagi H, Yura T, Kubota H. Cytosolic chaperonin is up-regulated during cell growth. Preferential expression and binding to tubulin at G(1)/S transition through early S phase. J Biol Chem. 1999;274:37070–37078. doi: 10.1074/jbc.274.52.37070. [DOI] [PubMed] [Google Scholar]
- 9.Liu X, Lin CY, Lei M, Yan S, Zhou T, Erikson RL. CCT chaperonin complex is required for the biogenesis of functional Plk1. Mol Cell Biol. 2005;25:4993–5010. doi: 10.1128/MCB.25.12.4993-5010.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Won KA, Schumacher RJ, Farr GW, Horwich AL, Reed SI. Maturation of human cyclin E requires the function of eukaryotic chaperonin CCT. Mol Cell Biol. 1998;18:7584–7589. doi: 10.1128/mcb.18.12.7584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen WJ, Ohh M, Moslehi J, Kondo K, Kaelin WG, Welch WJ. Diverse effects of mutations in exon II of the von Hippel-Lindau (VHL) tumor suppressor gene on the interaction of pVHL with the cytosolic chaperonin and pVHL-dependent ubiquitin ligase activity. Mol Cell Biol. 2002;22:1947–1960. doi: 10.1128/MCB.22.6.1947-1960.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Melki R, Batelier G, Soulié S, Williams RC Jr. Cytoplasmic chaperonin containing TCP-1: structural and functional characterization. Biochemistry. 1997;36:5817–26. doi: 10.1021/bi962830o. [DOI] [PubMed] [Google Scholar]
- 13.Monteith J, McMahon SB. p53: the TRiC is knowing when to fold ‘em. Mol Cell. 2013;50:781–782. doi: 10.1016/j.molcel.2013.06.009. [DOI] [PubMed] [Google Scholar]
- 14.Li LJ, Zhang LS, Han ZJ, He ZY, Chen H, Li YM. Chaperonin containing TCP-1 subunit 3 is critical for gastric cancer growth. Oncotarget. 2017;8:111470–111481. doi: 10.18632/oncotarget.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coghlin C, Carpenter B, Dundas SR, Lawrie LC, Telfer C, Murray GI. Characterization and over-expression of chaperonin t-complex proteins in colorectal cancer. J Pathol. 2006;210:351–357. doi: 10.1002/path.2056. [DOI] [PubMed] [Google Scholar]
- 16.Guest ST, Kratche ZR, Bollig-Fischer A, Haddad R, Ethier SP. Two members of the TRiC chaperonin complex, CCT2 and TCP1 are essential for survival of breast cancer cells and are linked to driving oncogenes. Exp Cell Res. 2015;332:223–235. doi: 10.1016/j.yexcr.2015.02.005. [DOI] [PubMed] [Google Scholar]
- 17.Gao H, Zheng M, Sun S, Wang H, Yue Z, Zhu Y, Han X, Yang J, Zhou Y, Cai Y, Hu W. Chaperonin containing TCP1 subunit 5 is a tumor associated antigen of non-small cell lung cancer. Oncotarget. 2017;8:64170–64179. doi: 10.18632/oncotarget.19369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Love C, Sun Z, Jima D, Li G, Zhang J, Miles R, Richards KL, Dunphy CH, Choi WW, Srivastava G, Lugar PL, Rizzieri DA, Lagoo AS, Bernal-Mizrachi L, Mann KP, Flowers CR, Naresh KN, Evens AM, Chadburn A, Gordon LI, Czader MB, Gill JI, Hsi ED, Greenough A, Moffitt AB, McKinney M, Banerjee A, Grubor V, Levy S, Dunson DB, Dave SS. The genetic landscape of mutations in Burkitt lymphoma. Nat Genet. 2012;44:1321–1325. doi: 10.1038/ng.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao X, Chen XQ, Han E, Hu Y, Paik P, Ding Z, Overman J, Lau AL, Shahmoradian SH, Chiu W, Thompson LM, Wu C, Mobley WC. TRiC subunits enhance BDNF axonal transport and rescue striatal atrophy in Huntington’s disease. Proc Natl Acad Sci U S A. 2016;113:E5655–E5664. doi: 10.1073/pnas.1603020113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 21.Wei WX, Yang ZS, Lu LH, Li J, Lei ZQ, Wang K, Xia Y, Yan ZL, Shen F. Long-term survival after partial hepatectomy for sub-stage patients with intermediate stage hepatocellular carcinoma. Int J Surg. 2018;56:256–263. doi: 10.1016/j.ijsu.2018.06.020. [DOI] [PubMed] [Google Scholar]
- 22.Zhang Y, Wang Y, Wei Y, Wu J, Zhang P, Shen S, Saiyin H, Wumaier R, Yang X, Wang C, Yu L. Molecular chaperone CCT3 supports proper mitotic progression and cell proliferation in hepatocellular carcinoma cells. Cancer Lett. 2016;372:101–109. doi: 10.1016/j.canlet.2015.12.029. [DOI] [PubMed] [Google Scholar]
- 23.Huang X, Wang X, Cheng C, Cai J, He S, Wang H, Liu F, Zhu C, Ding Z, Huang X, Zhang T, Zhang Y. Chaperonin containing TCP1, subunit 8 (CCT8) is upregulated in hepatocellular carcinoma and promotes HCC proliferation. Apmis. 2014;122:1070–1079. doi: 10.1111/apm.12258. [DOI] [PubMed] [Google Scholar]
- 24.Pavel M, Imarisio S, Menzies FM, Jimenez-Sanchez M, Siddiqi FH, Wu X, Renna M, O’Kane CJ, Crowther DC, Rubinsztein DC. CCT complex restricts neuropathogenic protein aggregation via autophagy. Nat Commun. 2016;7:13821. doi: 10.1038/ncomms13821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tam S, Geller R, Spiess C, Frydman J. The chaperonin TRiC controls polyglutamine aggregation and toxicity through subunit-specific interactions. Nat Cell Biol. 2006;8:1155–1162. doi: 10.1038/ncb1477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Behne T, Copur MS. Biomarkers for hepatocellular carcinoma. Int J Hepatol. 2012;2012:859076. doi: 10.1155/2012/859076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Camasses A, Bogdanova A, Shevchenko A, Zachariae W. The CCT chaperonin promotes activation of the anaphase-promoting complex through the generation of functional Cdc20. Mol Cell. 2003;12:87–100. doi: 10.1016/s1097-2765(03)00244-2. [DOI] [PubMed] [Google Scholar]
- 28.Kastan MB, Bartek J. Cell-cycle checkpoints and cancer. Nature. 2004;432:316–323. doi: 10.1038/nature03097. [DOI] [PubMed] [Google Scholar]
- 29.Kimata Y, Baxter JE, Fry AM, Yamano H. A role for the Fizzy/Cdc20 family of proteins in activation of the APC/C distinct from substrate recruitment. Mol Cell. 2008;32:576–583. doi: 10.1016/j.molcel.2008.09.023. [DOI] [PubMed] [Google Scholar]
- 30.Peters JM. The anaphase promoting complex/cyclosome: a machine designed to destroy. Nat Rev Mol Cell Biol. 2006;7:644–656. doi: 10.1038/nrm1988. [DOI] [PubMed] [Google Scholar]
- 31.Harley ME, Allan LA, Sanderson HS, Clarke PR. Phosphorylation of Mcl-1 by CDK1-cyclin B1 initiates its Cdc20-dependent destruction during mitotic arrest. EMBO J. 2010;29:2407–2420. doi: 10.1038/emboj.2010.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Z, Wan L, Zhong J, Inuzuka H, Liu P, Sarkar FH, Wei W. Cdc20: a potential novel therapeutic target for cancer treatment. Curr Pharm Des. 2013;19:3210–3214. doi: 10.2174/1381612811319180005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwang HC, Clurman BE. Cyclin E in normal and neoplastic cell cycles. Oncogene. 2005;24:2776–2786. doi: 10.1038/sj.onc.1208613. [DOI] [PubMed] [Google Scholar]
- 34.Roh SH, Kasembeli M, Bakthavatsalam D, Chiu W, Tweardy DJ. Contribution of the type II chaperonin, TRiC/CCT, to oncogenesis. Int J Mol Sci. 2015;16:26706–26720. doi: 10.3390/ijms161125975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talks KL, Turley H, Gatter KC, Maxwell PH, Pugh CW, Ratcliffe PJ, Harris AL. The expression and distribution of the hypoxia-inducible factors HIF-1alpha and HIF-2alpha in normal human tissues, cancers, and tumor-associated macrophages. Am J Pathol. 2000;157:411–421. doi: 10.1016/s0002-9440(10)64554-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rankin EB, Giaccia AJ. The role of hypoxia-inducible factors in tumorigenesis. Cell Death Differ. 2008;15:678–685. doi: 10.1038/cdd.2008.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Blagosklonny MV, Toretsky J, Bohen S, Neckers L. Mutant conformation of p53 translated in vitro or in vivo requires functional HSP90. Proc Natl Acad Sci U S A. 1996;93:8379–8383. doi: 10.1073/pnas.93.16.8379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walerych D, Olszewski MB, Gutkowska M, Helwak A, Zylicz M, Zylicz A. Hsp70 molecular chaperones are required to support p53 tumor suppressor activity under stress conditions. Oncogene. 2009;28:4284–4294. doi: 10.1038/onc.2009.281. [DOI] [PubMed] [Google Scholar]
- 39.Trinidad AG, Muller PA, Cuellar J, Klejnot M, Nobis M, Valpuesta JM, Vousden KH. Interaction of p53 with the CCT complex promotes protein folding and wild-type p53 activity. Mol Cell. 2013;50:805–817. doi: 10.1016/j.molcel.2013.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kasembeli M, Lau WC, Roh SH, Eckols TK, Frydman J, Chiu W, Tweardy DJ. Modulation of STAT3 folding and function by TRiC/CCT chaperonin. PLoS Biol. 2014;12:e1001844. doi: 10.1371/journal.pbio.1001844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu H, Jove R. The STATs of cancer--new molecular targets come of age. Nat Rev Cancer. 2004;4:97–105. doi: 10.1038/nrc1275. [DOI] [PubMed] [Google Scholar]
- 42.Chen L, Zhang Z, Qiu J, Zhang L, Luo X, Jang J. Chaperonin CCT-mediated AIB1 folding promotes the growth of ERalpha-positive breast cancer cells on hard substrates. PLoS One. 2014;9:e96085. doi: 10.1371/journal.pone.0096085. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]