Abstract
BORIS is a transcription factor aberrantly expressed in human cancers that can regulate the expression of estrogen receptors in endometrial cancer and breast cancer. We evaluated the expression of BORIS and the estrogen receptors alpha (ER-α) and beta (ER-β) in ten cell lines derived from cervical cancer using RT-PCR and Western-blot. We also evaluated 54 cervical tissues: normal epithelia, low-grade intraepithelial lesions (LSIL), high-grade intraepithelial lesions (HSIL), and invasive squamous carcinomas (SC) using immunohistochemistry. In the cell lines, BORIS mRNA and protein expressions are associated with ER-β expression but not with ER-α expression. In the normal cervical epithelium, ER-α and ER-β were expressed but the BORIS protein was not detected. In the LSIL samples, BORIS, ER-α and ER-β were expressed; however, in the HSIL samples, only the BORIS and ER-β expressions were detected, but ER-α expression was minimal or null. In the SC, only BORIS and ER-β were detected. In summary, the results show that the expressions of BORIS and ER-β increase while the expression of ER-α decreases according to the severity of the lesions. These results suggest synergistic roles for BORIS and ER-β during cervical cancer progression with a possible regulation of the estrogen receptors by BORIS in the development of cervical cancer; however, more detailed studies are needed to confirm this suggestion and to determine the precise role of BORIS in cervical cancer.
Keywords: Cervical cancer, BORIS, estrogen receptor alpha, estrogen receptor beta, expression
Introduction
Cervical cancer (CC) is a serious health problem. It is one of the most frequently diagnosed cancers and the fourth leading cause of cancer death in women worldwide, with an estimated 570,000 cases and 311,000 deaths in 2018 [1]. This neoplasia is a multifactorial disease in which human papillomavirus (HPV) infection is considered the main etiological factor. However, a high percentage of immunocompetent women infected with HPV do not develop cancer; therefore, other risk factors participate in the development of cancer, including dysregulation of tumor suppressors or proto-oncogenes [2], several genomic and genetic alterations, as well as risk factors associated with lifestyle or environment, such as smoking and alcoholism [3,4], multiple sexual partners, number of pregnancies, multiparty and use of hormonal contraceptives [5]. These latter factors are closely related to the response to sexual steroid hormones via the estrogen and progesterone receptors (ERs and PRs) in the human uterine cervix [6]. The ERs comprise two receptor subtypes, estrogen receptor alpha (ER-α) and beta (ER-β), which are differentially expressed in the cervical epithelium during the menstrual cycle; however, a low expression of the ER-α has been reported in CC by several groups. Interestingly, we have reported that the ER-β subtype could maintain its expression, suggesting that estrogens could perform physiologic or pathologic roles through ER-β in CC [7]. The expression of ER has been also reported in different hormone-dependent cancers such as ovarian, endometrial, prostate, and breast, where its role has been associated with the growth, progression, pathogenesis and response to a particular treatment.
Furthermore, the transcription factor CCCTC-binding factor-like (CTCFL/BORIS) is a transcriptional regulator and paralogue gene of the CCCTC-binding factor (CTCF) transcriptional repressor, which is involved in gene regulation, chromatin insulation and genomic imprinting. CTCF is expressed ubiquitously, whereas BORIS (Brother of Regulator Imprinted Sites) is expressed only in the testis, but it is aberrantly expressed in several cancer tissues [8]. BORIS and CTCF proteins maintain a high grade of conservation in their DNA binding domains (DBD) but diverge in their N-terminal and C-terminal regions, for which both proteins could bind to the same promoters of target genes, but recruit different proteins. In this sense, BORIS acts as an antagonist of CTCF by competing for the same target sequences [9], and is considered as an oncogene. BORIS is located on the 20q13.3 chromosomal region [10], which is highly amplified in human cancer [11], including primary tumors and cell lines of CC [12]. Additionally, ER-α was down-regulated in a group of endometrial cancer patients expressing high CTCFL/BORIS mRNA levels [13]. Conversely, high BORIS levels correlate with high levels of the ER in breast tumors, and its expression is activated in vitro by CTCFL/BORIS [14]. However, BORIS expression and its association with estrogen receptors has not been evaluated in invasive cervical tissues and premalignant lesions during cervical carcinogenesis.
Therefore, we decided to analyze the expression of BORIS and its relationship with the expression of ER-α and ER-β in cervical cancer cell lines and cervical cancer and premalignant lesions in order to understand their role in cervical carcinogenesis.
Materials and methods
Tissue samples and immunohistochemistry analysis
A group of fifty-four formalin-fixed paraffin-embedded (FFPE) cervical tissues were evaluated in this study. Eight normal cervical tissues without lesions (NC), seven low-grade squamous intraepithelial lesion (LSIL), 14 high-grade intraepithelial lesion (HSIL), and 25 invasive squamous carcinomas (SC) were collected from the Pathology Department at the Oncology Hospital, Centro Médico Nacional SXXI-IMSS, in Mexico City. The cervical tissue diagnoses was verified histopathologically, and all procedures were approved by the Comité Nacional de Investigación Científica (Scientific Research National Committee) at the Instituto Mexicano del Seguro Social (Mexican Institute for Social Security, IMSS, R-2014-3602-25).
A 4 µm tissue section was mounted on glass slides (Kling-On HIER Slides, Biocare Medical, Concord, CA, USA), and then heated at 56°C for 30 min, deparaffinized with xylene and rehydrated in a series of alcohol solutions (100, 90, 70 and 30%) and water. The antigen exposition was performed in a microwave oven using an antigen retrieval solution (Vector Laboratories, Burlingame, CA, USA). The endogenous peroxidase was inactivated by incubation on 3% methanol/H2O2 for 30 min. Subsequently, the blocking was performed with 10% bovine serum albumin (BSA, Sigma-Aldrich, St. Louis, MO, USA) in PBS 1X for 30 min. Tissues were incubated separately with three primary antibodies: (1) anti-ER-α antibody (sc-8002, Santa Cruz Biotechnology, Santa Cruz, CA, USA), (2) anti-ERβ antibody (ab288 [14C8], Abcam, Cambridge, UK), and (3) anti-BORIS antibody (ab-187163, Abcam) at 4°C overnight. Tissue slides were washed in PBS 1X, and subsequently incubated at room temperature (RT) for 2 h with the Mouse/Rabbit ImmunoDetector HRP/DAB (Bio SB Inc. CA, USA), and rinsed with PBS 1X solution. The antigen-antibody interaction was developed with diaminobenzidine/H2O2 producing a brown precipitate in the reaction site. Tissues were counterstained with a hematoxylin solution, dehydrated and finally mounted with a mounting medium (Entellan, Merck & Co, New Jersey, USA). For negative controls, BSA/PBS 1X were used instead of the primary antibody.
Cell lines
Different human cervical cancer cell lines were used: C33A (HPV-), CaSki (HPV16), SiHa (HPV16), MS751 (HPV18) and HeLa (HPV18), which were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA). The cell lines ViBo (HPV-), RoVa (HPV16), CaLo (HPV18), INBL (HPV18), and ViPa (HPV18) were previously generated and validated from primary cervical cancer tissue cultures [15]. The cell lines were maintained in a RPMI-1640 medium (Sigma-Aldrich) supplemented with 15% fetal bovine serum (FBS, Gibco, Thermo Fisher Scientific, Waltham, MA, USA), 100 IU/ml penicillin, 100 μg/ml streptomycin (Gibco), and 5 mM of L-glutamine (Gibco) at 37°C with 5% CO2.
RNA extraction and RT-PCR
The total RNA extraction from the cervical cancer cell lines was performed using TRIzol (TRIzol RNA Isolation Reagent, Thermo Fisher Scientific) according to the manufacturer’s instructions. RNA quality was evaluated through the identification of the 28S and 18S ribosomal bands and its concentration was analyzed by spectrophotometry (NanoDrop® ND-1000, Thermo Fisher Scientific). All RNA samples were treated with the RNase-free DNase (TURBO DNA-free Kit, Ambion Co., Austin, TX, USA) to remove DNA contamination. The cDNA synthesis was performed with the High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA), and the amplification was performed by polymerase chain reaction (PCR) using AccessQuickTM Master Mix, (Promega, Madison, WI, USA), and specific primers to the ER-α, ER-β and BORIS genes in a Perkin Elmer 480 Thermocycler (PE, Waltham, MA, USA). The characteristics of the ER-α, ER-β and BORIS primers are presented in Table 1. The sizes of the amplicons to ER-α, ER-β and BORIS were 148 pb, 242 bp, and 271 bp, respectively, which were resolved in a 2% agarose gel stained with a nucleic acid dye (GelRed Nucleic Acid Gel Stain, Biotium Inc., Fremont, CA, USA) and visualized through UV light (Eagle Eye II, Stratagene, La Jolla, CA, USA). Distilled water was used as a negative control in the PCR reactions. The GAPDH gene was included as an amplification control.
Table 1.
Oligonucleotides characteristics
| Gene | Primers | Tm (°C) | Size (bp) | Reference |
|---|---|---|---|---|
| ER-α | Forward: TgTgCAATgACTATgCTTCA | 55 | 148 | [16] |
| Reverse: gCTCTTCCTCCTTgTTTTTA | ||||
| ER-β | Forward: gTCCATCgCCAgTTATCACATC | 57 | 242 | [16] |
| Reverse: gCCTTACATCCTTCACACgA | ||||
| BORIS | Forward: CAggCCCTACAAgTgTAACgACTgCAA | 62 | 271 | [17] |
| Reverse: gCATTCgTAAggCTTCTCACCTgAgTg | ||||
| GAPDH | Forward: CATCTCTgCCCCCTCTgCTgA | 60 | 205 | [18] |
| Reverse: ggATgACCTTgCCCACAgCCT |
Western-blot analysis
Cell cultures were allowed to grow until the cells reached a confluence of 5 × 105 cells. Subsequently, the cells were homogenized in a RIPA lysis buffer with protease inhibitors (1 mM EDTA, 2 μg/ml leupeptin, 2 μg/ml aprotinin, 1 mM PMSF). The proteins were collected by centrifugation at 20,000 × g, at 4°C for 15 min, and quantified by the Bradford method (Bio-Rad Laboratories, TX, USA). Afterwards, 50 µg of proteins were separated by SDS-PAGE at 20 mA and electrotransferred to nitrocellulose membranes (Amersham, NJ, USA) for 1 h at 60 mA at room temperature in semi-dry conditions. The membranes were blocked at room temperature with 3% nonfat dry milk and 1% bovine serum albumin for 2 h and then incubated with 0.8 μg/ml of anti-BORIS antibodies (ab18337, Abcam), anti-ER-α (sc-787, Santa Cruz Biotechnology), or anti-ER-β (sc-8974, Santa Cruz Biotechnology), at 4°C overnight. Finally, the blots were incubated with 400 μg/ml of secondary antibody conjugated to horseradish peroxidase (Santa Cruz, Biotechnology) for 45 min. To correct the differences in the amount of total protein loaded in each lane, the BORIS, ER-α, and ER-β protein content was normalized to that of α-tubulin. Blots were stripped with glycine (0.1 M, pH 2.5, 0.5% SDS) at 4°C overnight and at room temperature for 30 min and retested with 0.2 µg/ml of mouse anti-α-tubulin monoclonal antibody (sc-5286, Santa Cruz Biotechnology) at 4°C overnight. The blots were incubated with a 1:3000 dilution of goat anti-mouse IgG conjugated to horseradish peroxidase (Santa Cruz Biotechnology) at room temperature for 1 h. Chemiluminescence signals were detected exposing the membranes to Kodak Biomax Light Film (Sigma-Aldrich) using Supersignal West Femto as a peroxidase substrate (Thermo Fisher Scientific). The antigen-antibody complex was detected in a semi quantitative way as a band, the area of which is given in inches (with a default scale of 72 pixels/inch) using the HP Scanjet G3110 (Hewlett-Packard Company, Palo Alto, CA, USA) and the ImageJ 1.45S software (National Institutes of Health, USA). In order to minimize inter-assay variations, all western blots were carried out in parallel.
Densitometry
The densitometry analysis was performed using ImageJ software (National Institutes of Health). The images of the bands were transformed to an 8-bit resolution and the average intensity of the pixels of each band was measured.
Statistical analysis
The immunohistochemistry results were analyzed using a Chi-squared test (X2). The densitometry results from Western-blot and RT-PCR were subjected to a Shapiro-Wilks parametric test. As the data showed a non-parametric distribution, they were analyzed using the Spearman correlation. All the statistical analyzes were carried out using PRISM v7.0 software.
Results
BORIS, ER-α, and ER-β expressions in cervical cancer cells lines
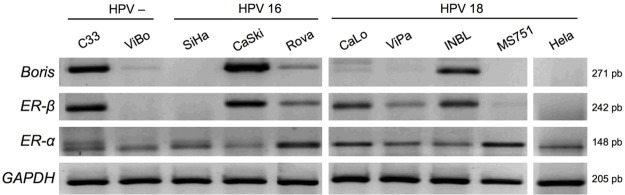
An RT-PCR analysis was performed to determine the mRNA expression of BORIS, ER-α and ER-β in ten cell lines derived from CC. ER-α was expressed in all the cell lines analyzed (10/10), but ER-β expression was found only in 6/10 cell lines. The highest ER-β expression was detected in the C33A (HPV-), CaSki (HPV16), CaLo (HPV18), and INBL (HPV18) cell lines (Figure 1). Similarly, a BORIS expression was found in 6/10 cell lines. Furthermore, a predominant association between BORIS and ER-β expression was detected in the C33A, CaSki, RoVa, and INBL cell lines (Figure 1). Interestingly, the ViBo, SiHa, MS751, and HeLa cell lines showed a minimal or null expression of ER-β and BORIS; but in the cell lines with marked expression of ER-α, the expression of BORIS and ER-β was minimal (RoVa and ViPa) or null (MS751 and HeLa). These results suggest a possible role of BORIS as an ER-β transcription activator due to the co-expression of both genes in the CC cell lines. Conversely, no association between BORIS and ER-α mRNA expression was found.
Figure 1.
Boris, ER-α, and ER-β mRNA expressions in cervical cancer cell lines by RT-PCR. Two HPV negative cell lines (C33 and ViBo), three HPV16 positive cell lines (SiHa, CaSki, and RoVa), and five HPV18 positive cell lines (CaLo, ViPa, INBL, MS751 and HeLa) were included. Evident expressions of Boris and ER-β are observed in the C33, CaSki, RoVa and INBL cell lines irrespective of the presence or HPV type. Amplification of GAPDH mRNA was used as an internal control.
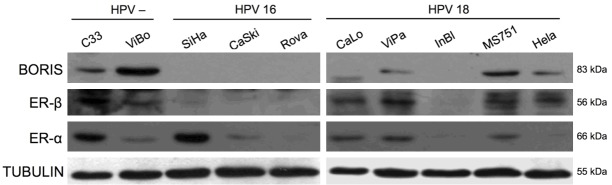
In order to determine any association between BORIS and ER-α/-β transcripts, a semi quantitative analysis was performed by gel densitometry (Figure 2A, 2C). Spearman’s correlation was performed for both receptors. ER-α showed a slight negative correlation (r=-0.28), which was not statistically significant (P=0.42) (Figure 2B). In contrast, ER-β showed a strong positive correlation with the BORIS transcript (r=0.82, P=0.003), suggesting that both genes could have important roles in the biology of cervical neoplastic cells (Figure 2D). Gene expression findings by RT-PCR were contrasted with the Western-blot (WB) results. As expected, the ER-β protein was detected in those cell lines that expressed the BORIS protein (C33A, ViBo, ViPa MS751, and HeLa) (Figure 3). The results showed that some cell lines expressed the transcript but not the protein (Caski, RoVa, and INBL); whereas other cell lines showed the opposite (ViBo, MS751, and HeLa). Therefore, post-transcriptional mechanisms as well as differences in translation rates for these genes in the transformed cells could be involved. Interestingly, in the lines that co-expressed BORIS and ER-β, a minimal (MS751, ViBo) or null (HeLa) presence of the ER-α protein was observed (Figure 3). Protein expression was determined through a semi quantitative analysis of BORIS and both estrogen receptors (Figure 4A and 4C). Similar to the RT-PCR results, no correlation between the ER-α and BORIS expression was observed (P=0.46) (Figure 4B); however, a strong correlation between ER-β and BORIS (r=0.78, P=0.001) was found (Figure 4D).
Figure 2.
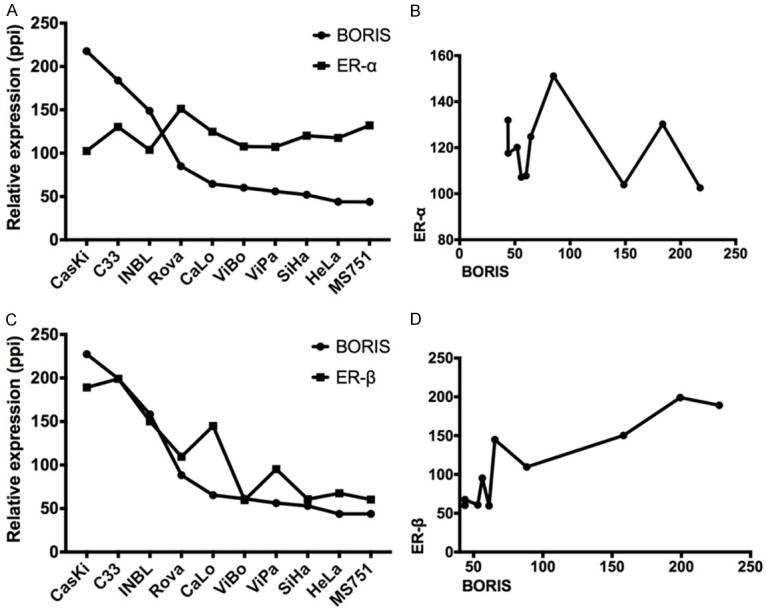
Relative quantification of BORIS, ER-α, and ER-β transcripts. A. Relative expression of ER-α and BORIS, showing that ER-α apparently has no changes in all the cell lines evaluated. B. XY correlation data between ER-α and BORIS, showing a slightly negative correlation between the two transcripts. C. Relative expressions of ER-β and BORIS, showing a clear tendency of both transcripts to have similarities in their expressions. D. XY correlation data between ER-β and BORIS, showing a strong correlation behavior. (ppi = pixels per inch).
Figure 3.
BORIS, ER-α, and ER-β protein expressions in cervical cancer cell lines by Western-blot. Two HPV negative cell lines (C33 and ViBo), three HPV16 cell lines (SiHa, CaSki and RoVa), and five HPV18 cell lines (CaLo, ViPa, InBl, MS751 and HeLa) were included. An association between BORIS and the ER-β protein was found in the C33, ViBo, ViPa, MS751, and HeLa cell lines. On the other hand, the absence of both proteins is observed in the SiHa, CaSki, RoVa and InBl cell lines. Tubulin was used as an internal control.
Figure 4.
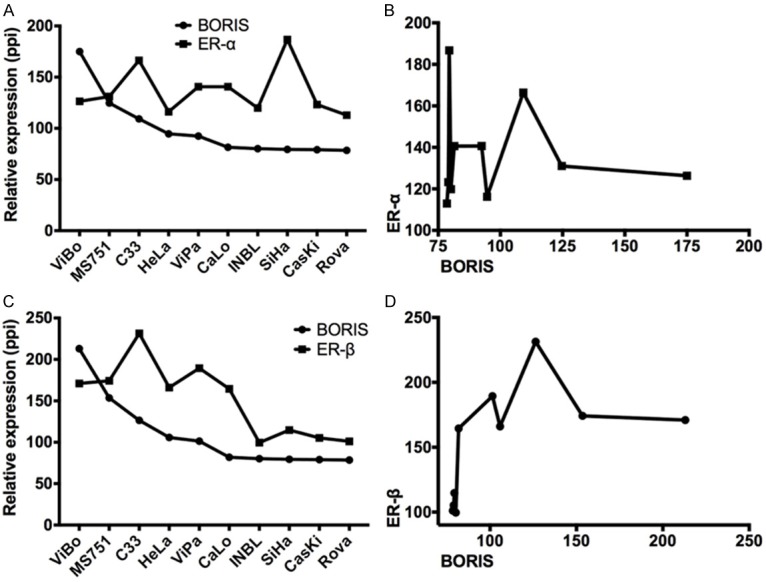
Relative quantification of the BORIS, ER-α, and ER-β proteins. A. Relative expression of ER-α and BORIS, showing that ER-α did not change in all the evaluated cell lines. B. XY data correlation between ER-α and BORIS did not show any correlation in the protein expression. C. Relative expression of ER-β and BORIS protein levels, showing a similar trend in their expression. D. XY data correlation between ER-β and BORIS showed a strong correlation between both proteins. (ppi = pixels per inch).
Additionally, the expression of ER-α, ER-β and BORIS at the RNA and protein levels was independent of HPV presence or type. These results in vitro suggest a probable synergistic role of BORIS and ER-β in both the cervical neoplastic cells and the human cervical pathophysiology.
BORIS, ER-α and ER-β expressions in cervical tissues
In order to determine the ER-α, ER-β, and BORIS expression patterns in cervical tissue, a group of normal cervical tissues (NC), premalignant cervical lesions (LSIL and HSIL), and invasive squamous carcinomas (SC) were analyzed by immunohistochemical (IHC) assays. The results of the expressions in the cervical tissues are summarized in Table 2.
Table 2.
Expressions of BORIS and estrogen receptors in cervical tissues by immunohistochemistry
| BORIS | ER-β | ER-α | ||||
|---|---|---|---|---|---|---|
|
|
|
|
||||
| + (%) | - (%) | + (%) | - (%) | + (%) | - (%) | |
| NC n=8 | 0 (0.0) | 8 (100) | 6 (75.0) | 2 (25.0) | 8 (100) | 0 (0.0) |
| LSIL n=7 | 4 (57.0) | 3 (43.0) | 4 (57.0) | 3 (43.0) | 5 (71.5) | 2 (28.5) |
| HSIL n=14 | 10 (71.5) | 4 (28.5) | 8 (57.0) | 7 (43.0) | 2 (14.0) | 12 (86.0) |
| SC n=25 | 20 (80.0) | 5 (20.0) | 15 (60.0) | 10 (40.0) | 2 (8.0) | 23 (92.0) |
NC = normal cervix, LSIL = Low-grade squamous intraepithelial lesion, HSIL = High-grade squamous intraepithelial lesion, SC = squamous carcinoma. + = positive cases, - = negative cases, % = percent of cases.
In the normal cervix epithelium, the BORIS protein was not detected (Figure 5A). Conversely, the nuclear immunoreactions of ER-α (Figure 5I) and ER-β (Figure 5E) were observed in the normal tissue, mainly in parabasal and basal cells, but the apical epithelial cells were negative for both receptors. In the LSIL, nuclear and cytoplasmic BORIS expressions were found in these cervical lesions (Figure 5B), but the nuclear expressions of ER-α (Figure 5J) and ER-β (Figure 5F) were generally identified in the first third of the epithelium. In the case of HSIL, BORIS (Figure 5C) and ER-β (Figure 5G) were expressed at the nucleus and cytoplasm in at least two thirds of the cervical epithelium, but the expression of ER-α was minimal or null in this type of lesion (Figure 5K). In the SC, ER-α expression in epithelial cells was not observed (Figure 5L), and only two cases showed a weak expression, and limited cases showed nuclear expression only in some stromal cells (data not shown). Furthermore, nuclear and cytoplasmic immunoreactions of BORIS (Figure 5D) and ER-β (Figure 5H) were observed in CC tissues.
Figure 5.
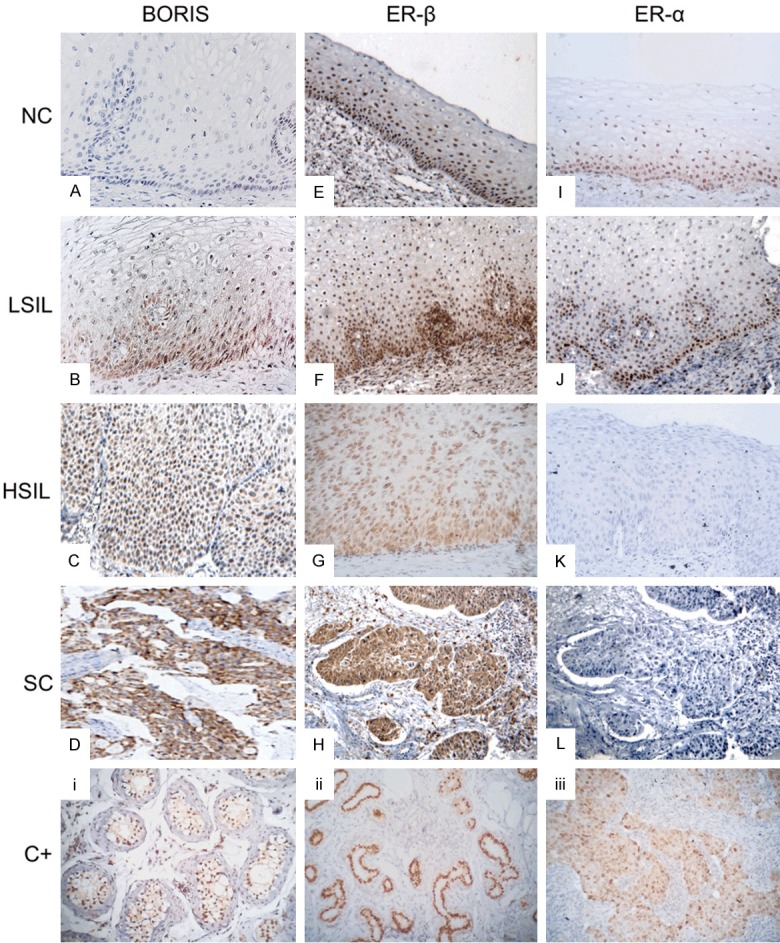
Representative microphotographs of the expressions of BORIS, ER-α, and ER-β in cervical tissues. (A) BORIS is not expressed in the normal cervical epithelium. (B) The nuclear and cytoplasmic expressions of BORIS in the first third of the cervical epithelium in the LSIL, and in the HSIL, its expression is mainly nuclear (C). (D) Strong nuclear and cytoplasmic immunoreactions of BORIS in SC. (E) Nuclear immunoreaction of ER-β in the normal cervical epithelium mainly in the basal and parabasal cells. (F) Nuclear expression of ER-β in the first third of the cervical epithelium in the LSIL. (G) Nuclear immunoreaction of ER-β in HSIL. (H) Nuclear and citoplasmic immunoreaction of ER-β in the epithelial invasion zones of the SC. (I) Nuclear expression of ER-α mainly in the basal and parabasal cells of the normal cervical epithelium. (J) Nuclear expression of ER-α in the basal cells and some parabasal cells of the epithelium of LSIL. (K, L) The HSIL and SC do not express the ER-α protein. Testis (i), breast (ii), and breast cancer (iii) tissues served as positive controls (C+) for BORIS, ER-β, and ER-α, respectively. A negative control for all cases consisted of H2O instead of the primary antibody (data not shown).
BORIS showed a positive association with CC (P=0.0008) due to the protein’s absence in normal cervical epithelia and a strong expression in SC where BORIS expression showed a directly proportional increase with malignant progression (Figure 6A), suggesting that as BORIS is not expressed under normal conditions, its expression could be associated with cervical carcinogenesis.
Figure 6.
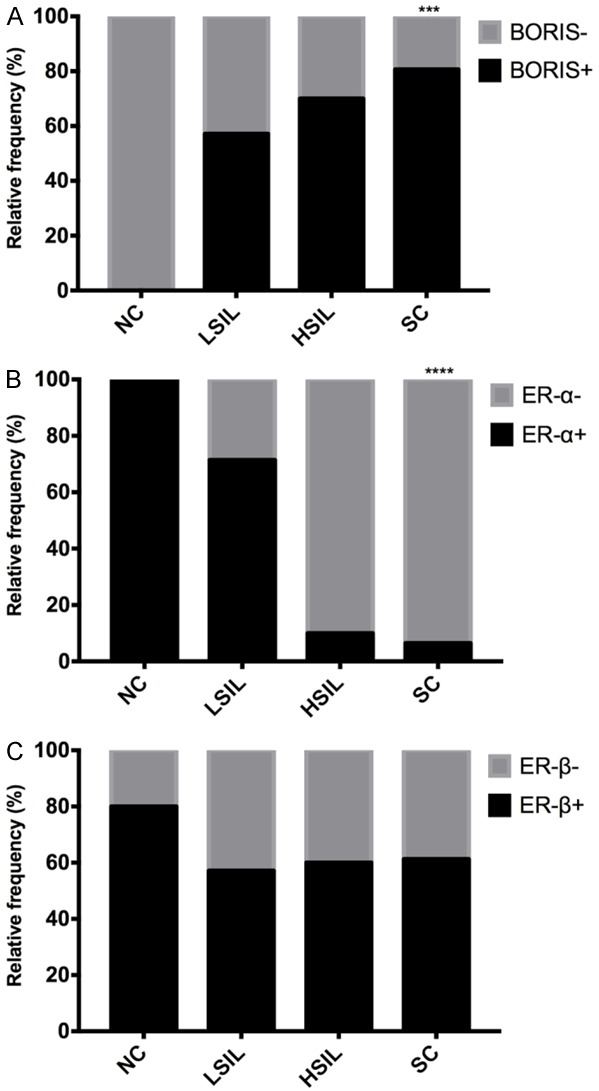
The relative frequency of the positive cases analyzed for BORIS, ER-α and ER-β. A. BORIS is positively associated (p=0.0008) as the cervical lesions progress. B. Unlike BORIS, ER-α showed a negative association as the cervical lesion progresses. C. ER-β showed no significant association with cervical lesions. (NC = normal cervix, LSIL = low-grade squamous intraepithelial lesion, HSIL = high-grade squamous intraepithelial lesion, SC = invasive squamous carcinoma. ***P=0.0008; ****P<0.0001).
A negative association between ER-α and CC (P=0.0001) was found, suggesting that this receptor is highly expressed in normal tissues, but its reduced expression could be related to malignant progression (Figure 6B). No statistical association (P>0.05) between ER-β expression and both LSIL and HSIL was observed (Figure 6C).
A Fisher’s exact test was performed to determine the association between BORIS and ER-α or ER-β. The data showed a negative association for ER-α (P=0.0002, Figure 7A), supporting the hypothesis that BORIS should not be expressed under normal conditions, whereas ER-α is expressed, displaying an inverse expression as the cervical lesion advances, and suggesting that the expression pattern of both proteins could be associated with malignant progression. In contrast to the results obtained in cervical cancer cell lines, no association between ER-β and BORIS expression was shown (P=0.10, Figure 7B); however, the expression of both genes could play a synergistic role as the severity of the lesions increases. Further studies are needed to confirm these observations.
Figure 7.
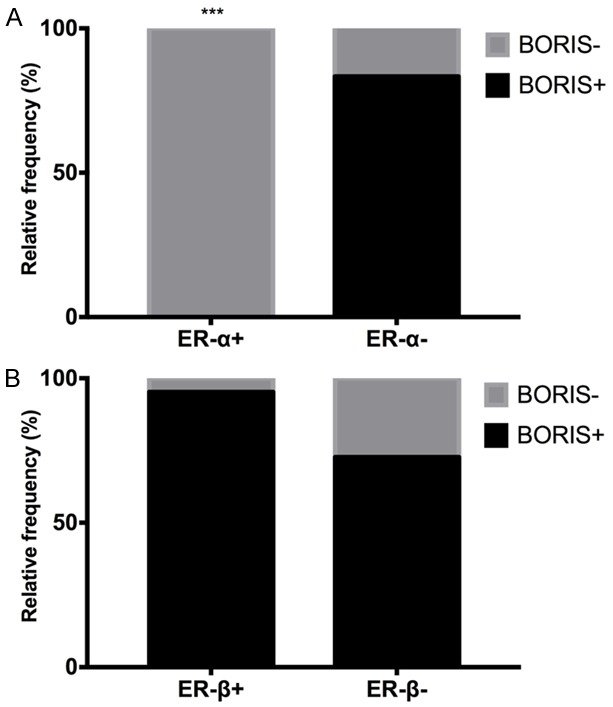
Association between the BORIS protein and both estrogen receptors in the cervical tissues analyzed by IHC. A. The relative frequencies of ER-α and BORIS showed a negative association of ER-α and BORIS (P=0.0002). B. The relative frequency of ER-β and BORIS in which there was no significant association; nevertheless, a similar tendency of expression of both proteins was observed.
Discussion
In this work, BORIS expression and its association with estrogen receptors ER-α and ER-β were determined during CC development to evaluate their possible roles in cervical carcinogenesis. BORIS, ER-α, and ER-β expression were analyzed in CC derived cell-lines by RT-PCR and Western-blot assays, in which an association between BORIS and ER-β mRNA expression was observed in C33A (HPV-), CaSki (HPV16), and INBL (HPV18) cell-lines, but with a minimal ER-α expression. In contrast, cell lines with a high expression of ER-α showed a low (RoVa, HPV16) or null (MS751, HPV18) expression of BORIS and ER-β. At the protein level, the SiHa cells strongly expressed ER-α but not BORIS or ER-β. On the contrary, lines with minimal (ViBo, ViPa, MS751) or null (HeLa) expression of ER-α showed high levels of BORIS and ER-β. These findings show a co-expression pattern of BORIS and ER-β in cervical neoplastic cells and suggest a role in their pathobiology. This co-expression pattern between BORIS and ER-β remained at the RNA and protein levels independently of the presence of HPV. Some cell lines expressed the mRNA but not the protein. This could be explicated by epigenetic [19] or post-transcriptional [7] regulation mechanisms.
Subsequently, we investigated whether this coordinated expression of BORIS and ER-β was maintained in cervical carcinomas where heterogeneity and cellular interrelation are constant.
BORIS and ER expression were evaluated in normal cervical tissues, low- and high-grade premalignant lesions, and primary cervical tumors by IHC to determine their expressions during the development of CC. For that, we used a specific anti-BORIS monoclonal antibody. BORIS expression was not detected in the normal cervical tissues, only in the basal and parabasal epithelial cells of LSIL. BORIS expression increased in HSIL and was spread in the invasion zones in the SC. These results are according with Vázquez et al. [20], who reported BORIS mRNA overexpression in squamous intraepithelial lesions and cervical cancer by RT-PCR, although they also found BORIS expressed in one normal cervical sample. However, this result could be due to the high sensitivity of the technique (RT-PCR), but it may lack some biological significance in normal tissue. The present results highlight an expression of BORIS in invasive carcinomas; however, the molecular mechanism of its activation in this neoplasia is still unknown.
It has been reported that BORIS expression is tightly regulated by the hypomethylation of its promoter in ovarian [21] and endometrial cancer [22]. However, BORIS copy number variations could be involved in its overexpression in CC. Several findings indicate that genomic amplification is a major mechanism underlying the activation of genes during tumor development. Amplification of the 20q13 chromosomic region has been widely reported in several neoplasm types and is considered one of the most commonly amplified regions in cancer [23]. Moreover, it has been suggested that this region harbors an important oncogene or dominant immortalizing genes that promote genetic instability [24]. Interestingly, the 20q13 region harbors BORIS [10], which showed a progressive increase from LSIL, through HSIL to CC (21, 74 and 100%, respectively) [25]. Our group also reported genomic amplification and gain in the number of copies of 20q13 in more than 50% of primary tumors and cervical cancer cell lines [12], whereas Lunh et al. reported this observation in premalignant lesions [26]. Therefore, the aberrant expression of BORIS in CC, premalignant lesions, and neoplastic cervical cell lines could be due to an increase in the number of copies of the 20q13.31 region, as already has been suggested in colorectal cancer [27].
The role of BORIS in CC is currently unknown. However, Asano et al. [28] have reported that a high level expression of BORIS is related to poor prognosis for patients with FIGO stages III/IV cervical cancer. Additionally, they reported the expression of BORIS sf6 (a BORIS subfamily) in cervical cancer stem-like cells (CSCs)/cancer-initiating cells (CICs), and suggested that BORIS is involved in cervical cancer stemness and might be associated with stem cell-related transcription factors. Interestingly, one of these transcription factors associated with stem cells in the breast is the estrogen receptor [29]. Darcy et al. [14] previously reported that high levels of BORIS correlated with high levels of ER in breast cancer, and that BORIS can regulate ER expression in vitro. This ability of BORIS to activate hormonal receptors has also been reported for the androgen receptor [30]. Consequently, BORIS could be involved in the growth and development of hormone-dependent tumors. In this sense, we detected the expression of BORIS and ER-β but not ER-α in CC. Therefore, our data could suggest an activation of ER-β by BORIS in the CC context [31]. Further studies are needed to validate this hypothesis.
We identified a strong nuclear expression of ER-α in the cervical squamous epithelium, but not in the HSIL or SC, coinciding with previous reports [6,7,32-34], except for some cases of CC with ER-α expression in stromal cells but absent in epithelial cells [35] (data not shown). Thus, a downregulation of ER expression has been claimed to be an early event in the transition of normal epithelium to SIL [36]. As the promoter of ER-α is not frequently methylated in cervical cancer, a possibility of its negative status in premalignant lesions and cervical cancer could be due to a mechanism of post-transcriptional regulation of its gene [7]. We cannot rule out that this inactivation of ER-α could also be due to a negative regulation by BORIS, since a deregulation of the ER-α receptor in patients with endometrial cancer that express high levels of BORIS has been reported [37]. Future research into a possible role of BORIS in hormone-dependent gene regulation in CC is necessary to support this hypothesis.
Limited studies on ER-β in CC have been published with contrasting results. It has been reported that ER-β is not expressed in the normal epithelium of the human cervix or in cervical cancer [38], but Fadiel et al. reported that ER-β is only expressed in the normal epithelium of the human cervix but not in cervical cancer [34]. Conversely, our group and others have reported ER-β expression in the normal cervical epithelium and in cervical cancer [7]. The discrepancy in these studies may be due to differences in immunostaining protocols, sample processing, and the primary antibodies used. Contrasting results in the ER-β expression have also been reported in different types of cancer.
Because of these discrepancies, various research groups evaluated and compared 7 [39], 8 [40] and 11 [41] different ER-β antibodies to determine those best at detecting ER-β through IHC. The 14C8 antibody proved to be one of the best antibodies for immunohistochemistry in paraffin-embedded tissues, with good immunoreaction and specificity [42]. Based on these reports, we used the mouse monoclonal antibody 14C8 to determine ER-β protein expression in cervical tissues. We identified ER-β nuclear expression in normal cervical tissue, suggesting that the effect of estrogens on the cervix might be regulated by the relative expression of both estrogen receptors with a specific role in cervical epithelium physiology [43].
Additionally, we found that ER-β is expressed in LSIL, and its expression is conserved in HSIL and SC. So, our data demonstrate that neoplastic and pre-neoplastic epithelial cells of the cervix express ER-β.
Although ER-β is currently not considered for clinical management, our data suggest that anti-estrogen therapy could benefit a subgroup of patients with cervical cancer that expresses ER-β. Previous reports have shown that ER-β can regulate the expression of several genes in the cell cycle and in apoptosis [44] and potentially constitute a probable tumor suppressor anti-proliferative gene when it is activated by anti-estrogens [45]. Interestingly, the anti-estrogens tamoxifen and raloxifene appear to be specific agonists of ER-β [46], for which ER-β positive cervical cancer patients could be considered as candidates for hormonal therapy [47]. The results show that ER-α is the main estrogen receptor subtype expressed in the cervical epithelium and suggest that ER-β is the predominant estrogen receptor subtype expressed in the neoplastic cervical epithelium, and its expression could be regulated by the BORIS transcription factor.
Conclusion
We demonstrated a decreasing expression of ER-α and a continuous expression of ER-β coordinated with BORIS expression during the development of cervical cancer in premalignant lesions and invasive carcinomas. To the best of our knowledge, this is the first study that shows such an association in cervical cancer and premalignant lesions. Our results suggest that BORIS might directly exert its action through ER-β during the development of the cervical cancer and has a potential role in cervical cancer. Further studies are needed to confirm this hypothesis and understand BORIS downstream mechanisms in cervical cancer. Additionally, BORIS and ER-β could be potential targets for future cervical cancer therapy.
Acknowledgements
We wish to thank Julia Segura Uribe from the Unidad de Investigación Médica en Enfermedades Neurológicas, Hospital de Especialidades, CMN SXXI-IMSS, for her technical help. The present work was supported by the Instituto Mexicano del Seguro Social, grant FIS/IMSS/PROT/G15/1409.
Disclosure of conflict of interest
None.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Anton M, Horký M, Bláha O. The role of tumour suppressors and viral oncoproteins in cervical carcinogenesis. Ceska Gynekol. 2000;65:275–8. [PubMed] [Google Scholar]
- 3.Haverkos HW. Multifactorial etiology of cervical cancer: a hypothesis. MedGenMed. 2005;7:57. [PMC free article] [PubMed] [Google Scholar]
- 4.Licciardone JC, Wilkins JR 3rd, Brownson RC, Chang JC. Cigarette smoking and alcohol consumption in the aetiology of uterine cervical cancer. Int J Epidemiol. 1989;18:533–7. doi: 10.1093/ije/18.3.533. [DOI] [PubMed] [Google Scholar]
- 5.Roura E, Travier N, Waterboer T, de Sanjosé S, Bosch FX, Pawlita M, Pala V, Weiderpass E, Margall N, Dillner J, Gram IT, Tjønneland A, Munk C, Palli D, Khaw KT, Overvad K, Clavel-Chapelon F, Mesrine S, Fournier A, Fortner RT, Ose J, Steffen A, Trichopoulou A, Lagiou P, Orfanos P, Masala G, Tumino R, Sacerdote C, Polidoro S, Mattiello A, Lund E, Peeters PH, Bueno-de-Mesquita HB, Quirós JR, Sánchez MJ, Navarro C, Barricarte A, Larrañaga N, Ekström J, Lindquist D, Idahl A, Travis RC, Merritt MA, Gunter MJ, Rinaldi S, Tommasino M, Franceschi S, Riboli E, Castellsagué X. The influence of hormonal factors on the risk of developing cervical cancer and pre-cancer: results from the EPIC cohort. PLoS One. 2016;11:e0147029. doi: 10.1371/journal.pone.0147029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kwasniewska A, Postawski K, Gozdzicka-Jozefiak A, Kwasniewski W, Grywalska E, Zdunek M, Korobowicz E. Estrogen and progesterone receptor expression in HPV-positive and HPV-negative cervical carcinomas. Oncol Rep. 2011;26:153–60. doi: 10.3892/or.2011.1256. [DOI] [PubMed] [Google Scholar]
- 7.López R, Garrido E, Rangel A, Manuel L, Piña P, Lazos M, Mantilla A, Bandala C, Salcedo M. The cervical malignant cells display a down regulation of ER-α but retain the ER-β expression. Int J Clin Exp Pathol. 2013;6:1594–602. [PMC free article] [PubMed] [Google Scholar]
- 8.Pugacheva EM, Suzuki T, Pack SD, Kosaka-Suzuki N, Yoon J, Vostrov AA, Barsov E, Strunnikov AV, Morse HC 3rd, Loukinov D, Lobanenkov V. The structural complexity of the human BORIS gene in gametogenesis and cancer. PLoS One. 2010;5:e13872. doi: 10.1371/journal.pone.0013872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin I. BORIS in human cancers -- a review. Eur J Cancer. 2012;48:929–35. doi: 10.1016/j.ejca.2011.09.009. [DOI] [PubMed] [Google Scholar]
- 10.Loukinov DI, Pugacheva E, Vatolin S, Pack SD, Moon H, Chernukhin I, Mannan P, Larsson E, Kanduri C, Vostrov AA, Cui H, Niemitz EL, Rasko JE, Docquier FM, Kistler M, Breen JJ, Zhuang Z, Quitschke WW, Renkawitz R, Klenova EM, Feinberg AP, Ohlsson R, Morse HC 3rd, Lobanenkov VV. BORIS, a novel male germ-line-specific protein associated with epigenetic reprogramming events, shares the same 11-zinc-finger domain with CTCF, the insulator protein involved in reading imprinting marks in the soma. Proc Natl Acad Sci U S A. 2002;99:6806–11. doi: 10.1073/pnas.092123699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tabach Y, Kogan-Sakin I, Buganim Y, Solomon H, Goldfinger N, Hovland R, Ke XS, Oyan AM, Kalland KH, Rotter V, Domany E. Amplification of the 20q chromosomal arm occurs early in tumorigenic transformation and may initiate cancer. PLoS One. 2011;6:e14632. doi: 10.1371/journal.pone.0014632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hidalgo A, Schewe C, Petersen S, Salcedo M, Gariglio P, Schlüns K, Dietel M, Petersen I. Human papilloma virus status and chromosomal imbalances in primary cervical carcinomas and tumour cell lines. Eur J Cancer. 2000;36:542–8. doi: 10.1016/s0959-8049(99)00323-8. [DOI] [PubMed] [Google Scholar]
- 13.Hoivik EA, Kusonmano K, Halle MK, Berg A, Wik E, Werner HM, Petersen K, Oyan AM, Kalland KH, Krakstad C, Trovik J, Widschwendter M, Salvesen HB. Hypomethylation of the CTCFL/BORIS promoter and aberrant expression during endometrial cancer progression suggests a role as an Epi-driver gene. Oncotarget. 2014;5:1052–61. doi: 10.18632/oncotarget.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D’Arcy V, Pore N, Docquier F, Abdullaev ZK, Chernukhin I, Kita GX, Rai S, Smart M, Farrar D, Pack S, Lobanenkov V, Klenova E. BORIS, a paralogue of the transcription factor, CTCF, is aberrantly expressed in breast tumours. Br J Cancer. 2008;98:571–9. doi: 10.1038/sj.bjc.6604181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mora ML, Ávila LR, García R, Weiss B, Hernández J, Don CA, Gutiérrez V, Titla IJ, Fuentes MC, Monroy A, Jave LF, Chacón R, Vallejo L, Pérez SM, Monroy A. Cervical cancer cells suppress effector functions of cytotoxic T cells through the adenosinergic pathway. Cell Immunol. 2017;320:46–55. doi: 10.1016/j.cellimm.2017.09.002. [DOI] [PubMed] [Google Scholar]
- 16.Leygue E, Dotzlaw H, Watson PH, Murphy LC. Altered estrogen receptor alpha and beta messenger RNA expression during human breast tumorigenesis. Cancer Res. 1998;58:3197–201. [PubMed] [Google Scholar]
- 17.Hong JA, Kang Y, Abdullaev Z, Flanagan PT, Pack SD, Fischette MR, Adnani MT, Loukinov DI, Vatolin S, Risinger JI, Custer M, Chen GA, Zhao M, Nguyen DM, Barrett JC, Lobanenkov VV, Schrump DS. Reciprocal binding of CTCF and BORIS to the NY-ESO-1 promoter coincides with derepression of this cancer-testis gene in lung cancer cells. Cancer Res. 2005;65:7763–74. doi: 10.1158/0008-5472.CAN-05-0823. [DOI] [PubMed] [Google Scholar]
- 18.Hsu EM, McNicol PJ, Guijon FB, Paraskevas M. Quantification of HPV-16 E6-E7 transcription in cervical intraepithelial neoplasia by reverse transcriptase polymerase chain reaction. Int J Cancer. 1993;55:397–401. doi: 10.1002/ijc.2910550311. [DOI] [PubMed] [Google Scholar]
- 19.Hua H, Zhang H, Kong Q, Jiang Y. Mechanisms for estrogen receptor expression in human cancer. Exp Hematol Oncol. 2018;7:24. doi: 10.1186/s40164-018-0116-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velázquez N, Reyes MA, Barragán M, Guerrero F, Rodríguez M, Aguilar M, Lazalde Medina B. BORIS and CTCF are overexpressed in squamous intraepithelial lesions and cervical cancer. Genet Mol Res. 2015;14:6094–100. doi: 10.4238/2015.June.8.7. [DOI] [PubMed] [Google Scholar]
- 21.Woloszynska-Read A, Zhang W, Yu J, Link PA, Mhawech-Fauceglia P, Collamat G, Akers SN, Ostler KR, Godley LA, Odunsi K, Karpf AR. Coordinated cancer germline antigen promoter and global DNA hypomethylation in ovarian cancer: association with the BORIS/CTCF expression ratio and advanced stage. Clin Cancer Res. 2011;17:2170–80. doi: 10.1158/1078-0432.CCR-10-2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoivik EA, Kusonmano K, Halle MK, Berg A, Wik E, Werner HM, Petersen K, Oyan AM, Kalland KH, Krakstad C, Trovik J, Widschwendter M, Salvesen HB. Hypomethylation of the CTCFL/BORIS promoter and aberrant expression during endometrial cancer progression suggests a role as an Epi-driver gene. Oncotarget. 2014;5:1052–61. doi: 10.18632/oncotarget.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tabach Y, Kogan-Sakin I, Buganim Y, Solomon H, Goldfinger N, Hovland R, Ke XS, Oyan AM, Kalland KH, Rotter V, Domany E. Amplification of the 20q chromosomal arm occurs early in tumorigenic transformation and may initiate cancer. PLoS One. 2011;6:e14632. doi: 10.1371/journal.pone.0014632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Klenova EM, Morse HC 3rd, Ohlsson R, Lobanenkov VV. The novel BORIS + CTCF gene family is uniquely involved in the epigenetics of normal biology and cancer. Semin Cancer Biol. 2002;12:399–414. doi: 10.1016/s1044-579x(02)00060-3. [DOI] [PubMed] [Google Scholar]
- 25.Policht FA, Song M, Sitailo S, O’Hare A, Ashfaq R, Muller CY, Morrison LE, King W, Sokolova IA. Analysis of genetic copy number changes in cervical disease progression. BMC Cancer. 2010;10:432. doi: 10.1186/1471-2407-10-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luhn P, Houldsworth J, Cahill L, Schiffman M, Castle PE, Zuna RE, Dunn ST, Gold MA, Walker J, Wentzensen N. Chromosomal gains measured in cytology samples from women with abnormal cervical cancer screening results. Gynecol Oncol. 2013;130:595–600. doi: 10.1016/j.ygyno.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eldai H, Periyasamy S, Al Qarni S, Al Rodayyan M, Muhammed Mustafa S, Deeb A, Al Sheikh E, Afzal M, Johani M, Yousef Z, Aziz MA. Novel genes associated with colorectal cancer are revealed by high resolution cytogenetic analysis in a patient specific manner. PLoS One. 2013;8:e76251. doi: 10.1371/journal.pone.0076251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Asano T, Hirohashi Y, Torigoe T, Mariya T, Horibe R, Kuroda T, Tabuchi Y, Saijo H, Yasuda K, Mizuuchi M, Takahashi A, Asanuma H, Hasegawa T, Saito T, Sato N. Brother of the regulator of the imprinted site (BORIS) variant subfamily 6 is involved in cervical cancer stemness and can be a target of immunotherapy. Oncotarget. 2016;7:11223–37. doi: 10.18632/oncotarget.7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Keymeulen A, Fioramonti M, Centonze A, Bouvencourt G, Achouri Y, Blanpain C. Lineage-restricted mammary stem cells sustain the development, homeostasis, and regeneration of the estrogen receptor positive lineage. Cell Rep. 2017;20:1525–1532. doi: 10.1016/j.celrep.2017.07.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheema Z, Hari-Gupta Y, Kita GX, Farrar D, Seddon I, Corr J, Klenova E. Expression of the cancer-testis antigen BORIS correlates with prostate cancer. Prostate. 2014;74:164–76. doi: 10.1002/pros.22738. [DOI] [PubMed] [Google Scholar]
- 31.Dey P, Barros RP, Warner M, Ström A, Gustafsson JÅ. Insight into the mechanisms of action of estrogen receptor β in the breast, prostate, colon, and CNS. J Mol Endocrinol. 2013;51:T61–74. doi: 10.1530/JME-13-0150. [DOI] [PubMed] [Google Scholar]
- 32.Shen K, Yueng W, Ngan H. Estrogen and progesterone receptors in normal cervix and primary cervical carcinoma. Chin Med J. 1994;107:648–52. [PubMed] [Google Scholar]
- 33.Kanai M, Shiozawa T, Xin L, Nikaido T, Fujii S. Immunohistochemical detection of sex steroid receptor, cyclins, and cyclin-dependent kinases in the normal and neoplastic squamous epithelia of the uterini cervix. Cancer. 1998;82:1709–1719. doi: 10.1002/(sici)1097-0142(19980501)82:9<1709::aid-cncr18>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 34.Fadiel A, Choi SD, Park B, Kim TH, Buldo-Licciardi J, Ahmadi M, Arslan A, Mittal K, Naftolin F. Expression of ezrin and estrogen receptors during cervical carcinogenesis. Reprod Sci. 2017;24:706–712. doi: 10.1177/1933719116667222. [DOI] [PubMed] [Google Scholar]
- 35.Chung SH, Shin MK, Korach KS, Lambert PF. Requirement for stromal estrogen receptor alpha in cervical neoplasia. Horm Cancer. 2013;4:50–9. doi: 10.1007/s12672-012-0125-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bekkers RL, van der Avoort IA, Melchers WJ, Bulten J, de Wilde PC, Massuger LF. Down regulation of estrogen receptor expression is an early event in human papillomavirus infected cervical dysplasia. Eur J Gynaecol Oncol. 2005;26:376–82. [PubMed] [Google Scholar]
- 37.Hoivik EA, Kusonmano K, Halle MK, Berg A, Wik E, Werner HM, Petersen K, Oyan AM, Kalland KH, Krakstad C, Trovik J, Widschwendter M, Salvesen HB. Hypomethylation of the CTCFL/BORIS promoter and aberrant expression during endometrial cancer progression suggests a role as an Epi-driver gene. Oncotarget. 2014;5:1052–61. doi: 10.18632/oncotarget.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chung SH, Wiedmeyer K, Shai A, Korach KS, Lambert PF. Requirement for estrogen receptor alpha in a mouse model for human papillomavirus-associated cervical cancer. Cancer Res. 2008;68:9928–34. doi: 10.1158/0008-5472.CAN-08-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Skliris GP, Parkes AT, Limer JL, Burdall SE, Carder PJ, Speirs V. Evaluation of seven oestrogen receptor beta antibodies for immunohistochemistry, western blotting, and flow cytometry in human breast tissue. J Pathol. 2002;197:155–62. doi: 10.1002/path.1077. [DOI] [PubMed] [Google Scholar]
- 40.Nelson AW, Groen AJ, Miller JL, Warren AY, Holmes KA, Tarulli GA, Tilley WD, Katzenellenbogen BS, Hawse JR, Gnanapragasam VJ, Carroll JS. Comprehensive assessment of estrogen receptor beta antibodies in cancer cell line models and tissue reveals critical limitations in reagent specificity. Mol Cell Endocrinol. 2017;440:138–150. doi: 10.1016/j.mce.2016.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weitsman GE, Skliris G, Ung K, Peng B, Younes M, Watson PH, Murphy LC. Assessment of multiple different estrogen receptor-beta antibodies for their ability to immunoprecipitate under chromatin immunoprecipitation conditions. Breast Cancer Res Treat. 2006;100:23–31. doi: 10.1007/s10549-006-9229-5. [DOI] [PubMed] [Google Scholar]
- 42.Carder PJ, Murphy CE, Dervan P, Kennedy M, McCann A, Saunders PT, Shaaban AM, Foster CS, Witton CJ, Bartlett JM, Walker RA, Speirs V. A multi-centre investigation towards reaching a consensus on the immunohistochemical detection of ERbeta in archival formalin-fixed paraffin embedded human breast tissue. Breast Cancer Res Treat. 2005;92:287–93. doi: 10.1007/s10549-004-4262-8. [DOI] [PubMed] [Google Scholar]
- 43.Böttner M, Thelen P, Jarry H. Estrogen receptor beta: tissue distribution and the still largely enigmatic physiological function. J Steroid Biochem Mol Biol. 2014;139:245–51. doi: 10.1016/j.jsbmb.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 44.Kyriakidis I, Papaioannidou P. Estrogen receptor beta and ovarian cancer: a key to pathogenesis and response to therapy. Arch Gynecol Obstet. 2016;293:1161–8. doi: 10.1007/s00404-016-4027-8. [DOI] [PubMed] [Google Scholar]
- 45.Hodges-Gallagher L, Valentine CD, El Bader S, Kushner PJ. Estrogen receptor beta increases the efficacy of antiestrogens by effects on apoptosis and cell cycling in breast cancer cells. Breast Cancer Res Treat. 2008;109:241–50. doi: 10.1007/s10549-007-9640-6. [DOI] [PubMed] [Google Scholar]
- 46.Saville B, Wormke M, Wang F, Nguyen T, Enmark E, Kuiper G, Gustafsson JA, Safe S. Ligand-, cell-, and estrogen receptor subtype (alpha/beta)-dependent activation at GC-rich (Sp1) promoter elements. J Biol Chem. 2000;275:5379–87. doi: 10.1074/jbc.275.8.5379. [DOI] [PubMed] [Google Scholar]
- 47.Chung SH. Targeting female hormone receptors as cervical cancer therapy. Trends Endocrinol Metab. 2015;26:399. doi: 10.1016/j.tem.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]