Abstract
Although many publications have evaluated the correlation between dopamine receptor D2 (DRD2) TaqIA polymorphism and Parkinson disease (PD), the results remain inconclusive. In order to further address the association between DRD2 TaqIA polymorphism and PD risk, we performed a meta-analysis of all eligible studies from more databases. Related studies were identified from six databases involving PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) through Octorber 2018. Pooled odds ratios (ORs) and 95% confidence intervals (CIs) were used to assess the strength of the associations. A total of 13 studies including 3558 PD patients and 10186 controls were involved in this meta-analysis. Overall, no significant association was found between DRD2 TaqIA polymorphism and PD risk in the total population. A further subgroup study by ethnicity showed a significant association between DRD2 TaqIA polymorphism and PD in Caucasians (for A1 vs. A2: P=0.02, OR=1.14, 95% CI: 1.02-1.27; for (A1A1 + A1A2) vs. A2A2: P=0.03, OR=1.16, 95% CI: 1.02-1.33). No significant results were observed in Asians. In conclusion, this meta-analysis provides evidence that DRD2 TaqIA polymorphism may contribute to the PD development in Caucasians, and large-scale well-designed studies are required in future to confirm this conclusion.
Keywords: Meta-analysis, dopamine receptor D2, polymorphism, Parkinson disease
Introduction
Parkinson disease (PD) is the second most prevalent neurodegenerative disease, with a complex etiology. Previous studies have indicated that exposure to environmental agents is the main causative factor for sporadic PD [1,2]. However, a recent study has shown that environmental triggers in association with genetic changes can alter individual’s susceptibility to this disease [3]. The exact etiology of PD still remains poorly understood, but it is increasingly recognized that genetic factors play an important role. Many common low-penetrance genes have been identified as potential PD susceptibility genes. Among these, dopamine receptors have been considered as possible factors in the pathophysiology of PD. The dopamine receptor D2 (DRD2) gene is located on chromosome 11 at q22-q23 [4], and several polymorphisms of the gene have been described. The DRD2 gene has a TaqI A restriction fragment length polymorphism (RFLP) that is situated in the untranslated region, approximately 10 kilobases from the 3’ end of the gene. This polymorphism creates the two alleles A1 (variant) and A2 [4]. An association between DRD2 polymorphisms and PD was first reported by Costa-Mallen and co-workers in 2000 in a Caucasian population [5]. As a consequence, many studies have attempted to clarify this relationship, but there has been no definite consensus to date. A meta-analysis published in 2014 had found a borderline association between DRD2 TaqIA polymorphism and PD in Europeans (P=0.05, OR=1.13, 95% CI: 1.00-1.27) [6]. In order to further address the association between DRD2 TaqIA polymorphism and PD risk, we performed a meta-analysis of all eligible studies from more databases.
Materials and methods
Search strategy and selection criteria
All eligible literature that assessed the relation between DRD2 TaqIA polymorphism and PD published before October 2018 was considered in this study. Six databases involving PubMed, Springer Link, Ovid, Chinese Wanfang Data Knowledge Service Platform, Chinese National Knowledge Infrastructure (CNKI), and Chinese Biology Medicine (CBM) were used for literature searching. A combination of keywords (“DRD2” OR “dopamine receptor D2 gene”) AND (“PD” OR “Parkinson disease”) was used. Additional studies were identified by a hand search of references of original studies or review articles. No publication date or language restrictions were imposed.
Inclusion criteria: (1) case-control or cohort studies describing the association of DRD2 TaqIA polymorphism and PD, (2) provides the distribution of DRD2 TaqIA polymorphism in patients and controls. The exclusion criteria were defined as follows: (1) overlapped literature, (2) unextractable data, (3) study design is not a case-control study, (4) abstracts or reviews.
Data extraction
Two investigators independently extracted data from all included publications and reached a consensus by discussion. Titles and abstracts of all potentially relevant articles were screened to determine their relevance. Full articles were scrutinized if the title and abstract were ambiguous. Data were recorded as follows: first author’s surname, year of publication, geographic areas, ethnicity, sample size, and the number of subjects with DRD2 TaqIA genotypes.
Statistical analysis
The odds ratios (ORs) and 95% confidence intervals (CIs) were generated for DRD2 TaqIA polymorphism and PD risk. The model of A1 versus A2, A1A1 versus A2A2, A1A1 versus (A1A2 + A2A2) and (A1A1 + A1A2) versus A2A2 were examined with the PD risk, respectively. Heterogeneity of pooled results as well as Hardy-Weinberg equilibrium (HWE) in controls was assessed by I-squared statistic based on Q-test [7]. The random-effects model was applied to estimate the pooled ORs when Pheterogeneity < 0.1 or I2 > 50%; otherwise, the fixed-effects model was adopted. The statistical test of the whole calculated ORs was evaluated by Z-test. Both fixed-effects and random-effects model for each pooled ORs were computed to assess the sensitivity analysis results. Possible publication bias was evaluated by Egger’s test. We did all statistical analysis with Stata version 12 (StataCorp LP, College Station, TX), and a statistical test with a p-value less than 0.05 was considered significant. Furthermore, we also performed subgroup analysis by ethnicity to assess the relationship between DRD2 TaqIA and PD risk.
Results
Research characteristics
One hundred and fifty-three publications which assessed the relationship between DRD2 and PD were identified. In total, thirteen studies [5,8-19] were used in this report, which met our inclusion criteria. The publication year of included studies ranged from 2000 to 2013. The flowchart of Figure 1 reveals the detailed screening process of our analysis. At the end, 3558 PD patients and 10186 controls were included in the current study, which assessed the relation between DRD2 TaqIA polymorphism and PD risk. The main characteristics of the 13 articles are listed in Table 1.
Figure 1.
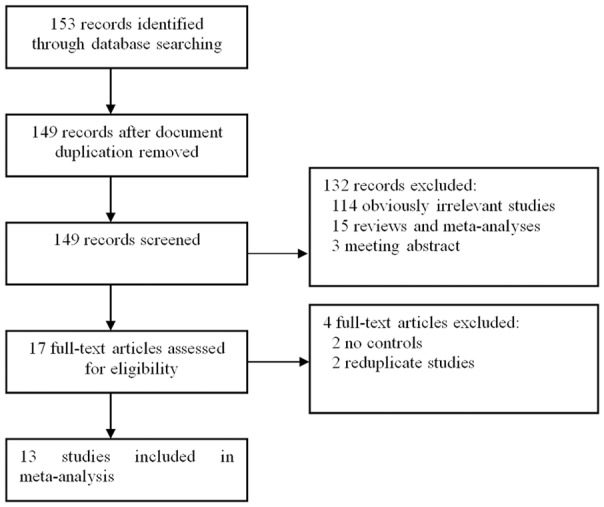
Flow diagram of the literature search.
Table 1.
Characteristics of studies included in the meta-analysis
| References | Country | Ethnicity | Cases number | Controls number | Cases | Controls | HWE | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| A2A2 | A1A2 | A1A1 | A2A2 | A1A2 | A1A1 | χ2 | P | |||||
| Grevle 2000 | Norway | Caucasian | 72 | 81 | 43 | 28 | 1 | 62 | 18 | 1 | 0.06 | 0.81 |
| Oliveri 2000 | Italy | Caucasian | 135 | 202 | 83 | 48 | 4 | 148 | 49 | 5 | 0.15 | 0.70 |
| Costa-Mallen 2000 | USA | Caucasian | 125 | 202 | 84 | 37 | 4 | 135 | 59 | 8 | 0.23 | 0.63 |
| Wang 2000 | China | Asians | 140 | 141 | 43 | 73 | 24 | 34 | 80 | 27 | 2.67 | 0.10 |
| Tan 2003 | Singapore | Asians | 204 | 216 | 74 | 94 | 36 | 81 | 100 | 35 | 0.20 | 0.66 |
| Chen 2006 | China | Asians | 180 | 387 | 64 | 80 | 36 | 141 | 184 | 62 | 0.02 | 0.88 |
| Singh 2008 | India | Asians | 70 | 100 | 38 | 28 | 4 | 49 | 37 | 14 | 2.46 | 0.12 |
| Lee 2009 | Korea | Asians | 402 | 558 | 130 | 203 | 69 | 196 | 271 | 91 | 0.03 | 0.87 |
| Li 2009 | China | Asians | 166 | 170 | 60 | 75 | 31 | 58 | 81 | 31 | 0.09 | 0.77 |
| Kiyohara 2011 | Japan | Asians | 238 | 369 | 92 | 117 | 29 | 125 | 192 | 52 | 2.54 | 0.11 |
| McGuirec 2011 | Canada | Caucasian | 1176 | 1443 | 729 | 378 | 69 | 916 | 461 | 66 | 0.67 | 0.41 |
| Lee 2011 | Korea | Asians | 500 | 559 | 164 | 250 | 86 | 196 | 272 | 91 | 0.04 | 0.84 |
| Kumudini 2013 | India | Caucasian | 150 | 186 | 60 | 75 | 15 | 91 | 74 | 21 | 0.99 | 0.32 |
Meta-analysis
As shown in Table 2, no significant associations were observed in the overall meta-analyses of five models. A further subgroup study by ethnicity showed a significant association between DRD2 TaqIA polymorphism and PD in Caucasians (for A1 vs. A2: P=0.02, OR=1.14, 95% CI: 1.02-1.27; for (A1A1 + A1A2) vs. A2A2: P=0.03, OR=1.16, 95% CI: 1.02-1.33; Table 2; Figures 2 and 3). No significant results were observed in Asians. There was no publication bias for the meta-analyses (t=0.08, P=0.939; Figures 4 and 5). In order to compare the difference and evaluate the sensitivity of the meta-analysis, we used both models (the fixed-effects model and the random-effects model) to evaluate the stability of the meta-analysis. All the results were not materially altered in total and subgroup analyses (Table 2). Hence, results of the sensitivity analysis suggest that the data in this meta-analysis are relatively stable and credible.
Table 2.
Association of the DRD2 TaqIA polymorphism on PD susceptibility
| Analysis model | n | ORr (95% CI) | ORf (95% CI) | Ph | |
|---|---|---|---|---|---|
| A1 vs. A2 | Total analysis | 13 | 1.05 (0.97-1.14) | 1.05 (0.98-1.13) | 0.253 |
| Caucasian | 5 | 1.18 (1.00-1.40) | 1.14 (1.02-1.27) | 0.221 | |
| Asians | 8 | 1.01 (0.92-1.10) | 1.01 (0.92-1.10) | 0.518 | |
| A1A1 vs. A2A2 | Total analysis | 13 | 1.08 (0.92-1.26) | 1.07 (0.92-1.25) | 0.735 |
| Caucasian | 5 | 1.24 (0.92-1.67) | 1.24 (0.92-1.67) | 0.943 | |
| Asians | 8 | 1.02 (0.85-1.23) | 1.02 (0.85-1.22) | 0.465 | |
| A1A1 vs. A1A2 | Total analysis | 13 | 1.04 (0.90-1.21) | 1.04 (0.90-1.21) | 0.876 |
| Caucasian | 5 | 1.09 (0.80-1.48) | 1.08 (0.80-1.47) | 0.627 | |
| Asians | 8 | 1.03 (0.87-1.22) | 1.03 (0.86-1.22) | 0.776 | |
| A1A1 vs. (A2A2 + A1A2) | Total analysis | 13 | 1.06 (0.92-1.23) | 1.06 (0.92-1.22) | 0.850 |
| Caucasian | 5 | 1.17 (0.88-1.57) | 1.17 (0.88-1.57) | 0.844 | |
| Asians | 8 | 1.03 (0.88-1.21) | 1.03 (0.87-1.21) | 0.645 | |
| (A1A1 + A1A2) vs. A2A2 | Total analysis | 13 | 1.07 (0.96-1.21) | 1.07 (0.98-1.18) | 0.181 |
| Caucasian | 5 | 1.31 (1.01-1.69) | 1.16 (1.02-1.33) | 0.077 | |
| Asians | 8 | 1.00 (0.88-1.13) | 1.00 (0.88-1.13) | 0.648 | |
ORr: Odds ratio for random-effect model; ORf: Odds ratio for fixed-effect model; Ph: P value for heterogeneity test.
Figure 2.
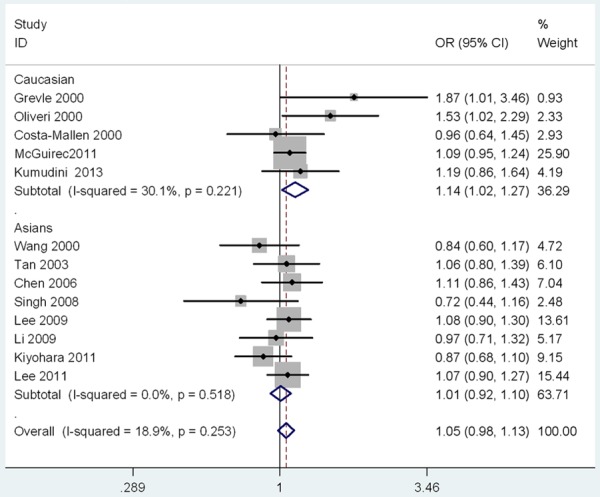
The Forest plots of the association between DRD2 TaqIA polymorphism and PD under allele model.
Figure 3.
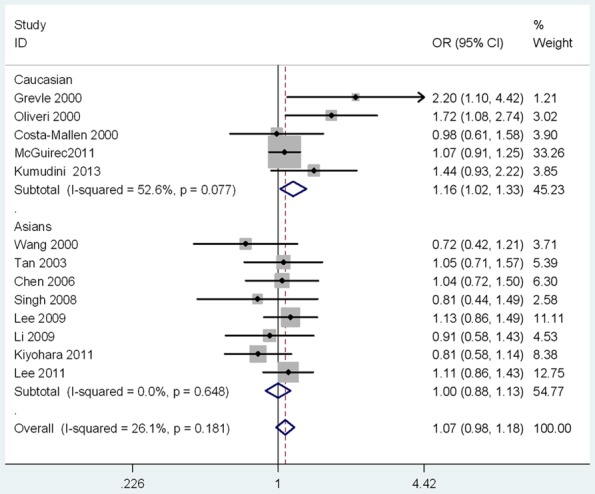
The Forest plots of the association between DRD2 TaqIA polymorphism and PD under dominant model.
Figure 4.
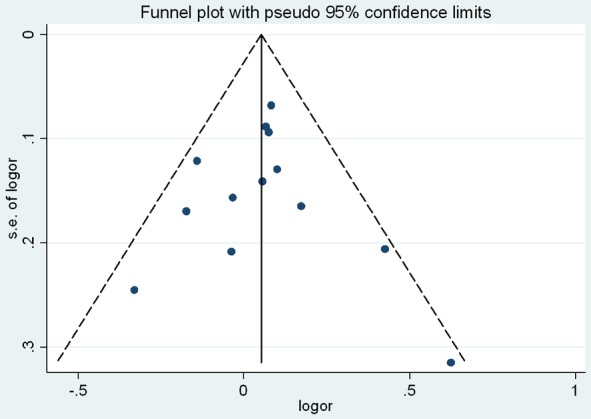
The Begg’s funnel plot for publication bias assessment.
Figure 5.
The Egger’s linear regression for publication bias assessment.
Discussion
Convincing evidence has emerged that individual susceptibility to PD is partially determined by genetic predisposition. The relationship between DRD2 TaqIA polymorphism and PD risk attracted the attention of both doctors and researchers. There are two previously published meta-analyses regarding DRD2 polymorphisms and PD risk [6,20]. Of these, one meta-analysis reported that there was a borderline association between DRD2 TaqIA polymorphism and PD in Europeans [6], but no significant difference was found in another meta-analysis [20]. A limited number of studies included in two meta-analyses is one likely reason for the conflict results. Therefore, we conducted this updated meta-analysis to derive a more precise estimation of DRD2 TaqIA polymorphism and susceptibility to PD. Our meta-analysis involved 13 studies, including 3558 PD patients and 10186 controls. In the total analysis, no significant association between the DRD2 TaqIA polymorphism and PD was found in each model. To further explain the ethnic difference between Asians and Caucasians, a subgroup analysis stratified by ethnicity was performed. We found that the variants of DRD2 TaqIA significantly increases the risk of PD in Caucasians, but not in Asians. This result suggested the relationship between DRD2 TaqIA polymorphism and PD might be susceptible in different ethnicity. We did not perform subgroup analysis on other ethnicity history, because of the lack of sufficient data.
Compared to the previous meta-analysis by Dai et al. [6], the current study included more PD patients and controls. Our study has performed four genetype models of A1 versus A2, A1A1 versus A2A2, A1A1 versus (A1A2 + A2A2) and (A1A1 + A1A2) versus A2A2 while only allele model for DRD2 TaqIA polymorphism in the Dai et al. study [6]. The effects of ethnic difference with respect to PD risk were also conducted by a subgroup analysis. The sensitivity analysis confirmed the reliability and stability of the meta-analysis. The genotype distributions in the controls of 13 studies were in agreement with HWE. A funnel plot and Egger’s linear regression test was used to assess publication bias. Therefore, our meta-analysis indicated a significant association between DRD2 TaqIA polymorphism and PD in caucasians.
Nevertheless, several limitations should be considered in our meta-analysis. First, only English and Chinese databases were used for literature searching in this meta-analysis; other language articles/databases were not included. Therefore, further studies are needed to assess the association in other population. Second, the etiology of PD is complex and is mediated by the activities of multiple genes. The effect of any single gene might have a limited impact on PD risk than have been anticipated so far. Finally, due to insufficient information, we could not conduct subgroup analyses stratified by other factors, such as gender, and smoking status.
In conclusion, this meta-analysis indicated a significantly increased association between DRD2 TaqIA polymorphism and PD in Caucasians not in Asians. Ethnicity seems to play an important role in the genetic association of the disease, and large-scale well-designed studies are required in future to confirm this conclusion.
Disclosure of conflict of interest
None.
References
- 1.Semchuk KM, Love EJ, Lee RG. Parkinson’s disease and exposure to agricultural work and pesticide chemicals. Neurology. 1992;42:1328–1335. doi: 10.1212/wnl.42.7.1328. [DOI] [PubMed] [Google Scholar]
- 2.Priyadarshi A, Khuder SA, Schaub EA, Priyadarshi SS. Environmental risk factors and parkinson’s disease: a metaanalysis. Environ Res. 2001;86:122–127. doi: 10.1006/enrs.2001.4264. [DOI] [PubMed] [Google Scholar]
- 3.Dauer W, Przedborski S. Parkinson’s disease: mechanisms and models. Neuron. 2003;39:889–909. doi: 10.1016/s0896-6273(03)00568-3. [DOI] [PubMed] [Google Scholar]
- 4.Grandy DK, Litt M, Allen L, Bunzow JR, Marchionni M, Makam H, Reed L, Magenis RE, Civelli O. The human dopamine D2 receptor gene is located on chromosome 11 at q22-q23 and identifies a TaqI RFLP. Am J Human Genet. 1989;45:778–785. [PMC free article] [PubMed] [Google Scholar]
- 5.Costa-Mallen P, Costa LG, Smith-Weller T, Franklin GM, Swanson PD, Checkoway H. Genetic polymorphism of dopamine D2 receptors in Parkinson’s disease and interactions with cigarette smoking and MAO-B intron 13 polymorphism. J Neurol Neurosurg Psychiatry. 2000;69:535–537. doi: 10.1136/jnnp.69.4.535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dai D, Wang Y, Wang L, Li J, Ma Q, Tao J, Zhou X, Zhou H, Jiang Y, Pan G, Xu L, Ru P, Lin D, Pan J, Xu L, Ye M, Duan S. Polymorphisms of DRD2 and DRD3 genes and Parkinson’s disease: a meta-analysis. Biomed Rep. 2014;2:275–281. doi: 10.3892/br.2014.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hoaglin DC. Assessment of heterogeneity in meta-analyses. JAMA. 2014;312:2286–2287. doi: 10.1001/jama.2014.14346. [DOI] [PubMed] [Google Scholar]
- 8.Grevle L, Güzey C, Hadidi H, Brennersted R, Idle JR, Aasly J. Allelic association between the DRD2 TaqI A polymorphism and Parkinson’s disease. Mov Disord. 2000;15:1070–1074. doi: 10.1002/1531-8257(200011)15:6<1070::aid-mds1003>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 9.Oliveri RL, Annesi G, Zappia M, Civitelli D, De Marco EV, Pasqua AA, Annesi F, Spadafora P, Gambardella A, Nicoletti G, Branca D, Caracciolo M, Aguglia U, Quattrone A. The dopamine D2 receptor gene is a susceptibility locus for Parkinson’s disease. Mov Disord. 2000;15:127–131. doi: 10.1002/1531-8257(200001)15:1<120::aid-mds1019>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 10.Wang J, Liu ZL, Chen B, Lin JR. Relationship between dopamine receptor gene polymorphism and genetic susceptibility to Parkinson’s disease. Chin J Neurol. 2000;33:13. [Google Scholar]
- 11.Tan EK, Tan Y, Chai A, Tan C, Shen H, Lum SY, Fook-Cheong SM, Teoh ML, Yih Y, Wong MC, Zhao Y. Dopamine D2 receptor TaqIA and TaqIB polymorphisms in Parkinson’s disease. Mov Disord. 2003;18:593–595. doi: 10.1002/mds.10406. [DOI] [PubMed] [Google Scholar]
- 12.Chen X. The association between cigarette smoking and genes and Parkinson’s disease. Master Thesis of Capital Medical University. 2006 [Google Scholar]
- 13.Singh M, Khan AJ, Shah PP, Shukla R, Khanna VK, Parmar D. Polymorphism in environment responsive genes and association with Parkinson disease. Mol Cell Biochem. 2008;312:131–138. doi: 10.1007/s11010-008-9728-2. [DOI] [PubMed] [Google Scholar]
- 14.Lee JY, Lee EK, Park SS, Lim JY, Kim HJ, Kim JS, Jeon BS. Association of DRD3 and GRIN2B with impulse control and related behaviors in Parkinson’s disease. Mov Disord. 2009;24:1803–1810. doi: 10.1002/mds.22678. [DOI] [PubMed] [Google Scholar]
- 15.Li W, Sun MM, Lin XX, Jiang BZ, Xia CL. The relationship between genetric polymorphism of dopamine receptor-D2 gene and Parkinson’s disease in chinese. Suzhou University J Med Sci. 2009;29:303–306. [Google Scholar]
- 16.Kiyohara C, Miyake Y, Koyanagi M, Fujimoto T, Shirasawa S, Tanaka K, Fukushima W, Sasaki S, Tsuboi Y, Yamada T, Oeda T, Shimada H, Kawamura N, Sakae N, Fukuyama H, Hirota Y, Nagai M Fukuoka Kinki Parkinson’s Disease Study Group. Genetic polymorphisms involved in dopaminergic neurotransmission and risk for Parkinson’s disease in a Japanese population. BMC Neurol. 2011;11:89. doi: 10.1186/1471-2377-11-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGuire V, Van Den Eeden SK, Tanner CM, Kamel F, Umbach DM, Marder K, Mayeux R, Ritz B, Ross GW, Petrovitch H, Topol B, Popat RA, Costello S, Manthripragada AD, Southwick A, Myers RM, Nelson LM. Association of DRD2 and DRD3 polymorphisms with Parkinson’s disease in a multiethnic consortium. J Neurol Sci. 2011;307:22–29. doi: 10.1016/j.jns.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JY, Cho J, Lee EK, Park SS, Jeon BS. Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Mov Disord. 2011;26:73–9. doi: 10.1002/mds.23400. [DOI] [PubMed] [Google Scholar]
- 19.Kumudini N, Umai A, Devi YP, Naushad SM, Mridula R, Borgohain R, Kutala VK. Impact of COMT H108L, MAOB int 13 A>G and DRD2 haplotype on the susceptibility to Parkinson’s disease in South Indian subjects. Indian J Biochem Biophys. 2013;50:436–441. [PubMed] [Google Scholar]
- 20.Tan EK, Khajavi M, Thornby JI, Nagamitsu S, Jankovic J, Ashizawa T. Variability and validity of polymorphism association studies in Parkinson’s disease. Neurology. 2000;55:533–538. doi: 10.1212/wnl.55.4.533. [DOI] [PubMed] [Google Scholar]


