Abstract
Pulmonary fibrosis is a serious interstitial disease characterized by initial diffuse alveolar inflammation, fibroblast proliferation, ECM accumulation, and the destruction of normal pulmonary tissues, whose etiology remains unknown and therapeutic options remain limited. The prevalence of Vitamin D deficiency is increasing and has been linked to pulmonary fibrosis. In recent years, many studies focused on the mechanistic pathway of Vitamin D in the prevention of fibrosis. This review highlights the current evidence on the molecular mechanisms of Vitamin D in pulmonary fibrosis. We want to provide new clues to the clinical management of pulmonary fibrosis.
Keywords: Vitamin D, vitamin D receptor, VDR, pulmonary fibrosis
Introduction
Pulmonary fibrosis describes a progressive and irreversible lung disease, with a mean life expectancy of only 3-5 years [1-5], posing a big threat to public health. Common symptoms caused by pulmonary fibrosis such as fatigue, cough, and dyspnea have a major impact on the quality of life (QOL) of patients [6]. Its etiology remains elusive and therapeutic options remain limited. Until now, there is no pharmacological therapy but lung transplantation to change the natural course of the disease.
Pulmonary fibrosis is a wound healing response caused by lung injury and infection. However, chronic exposure to the injury factor such as allergens, toxic chemicals, and radiation leads to dysregulated wound healing response, overlapping inflammation and subsequent pulmonary fibrosis [2]. Finally the scar tissues take the place of normal lung architecture [7]. To date, the potential molecular mechanism of pulmonary fibrosis is still unknown. But it is closely related to the regulation of collagen-secreting myofibroblasts proliferation, activation, and differentiation, and inflammation.
Vitamin D has long been regarded as a key player in calcium homeostasis, bone health, electrolyte and blood pressure regulation and immune response [8,9]. It is provided by the kidney and parathyroid gland endocrine system. To achieve full biologic activity, Vitamin D must be metabolized to the hormonal form 1,25-dihydroxy Vitamin D by the activating hydroxylase the Vitamin D 25-hydroxylase, and 1-αhydroxylase [10]. As is known to all, Vitamin D is distributed not only to the liver but also to all tissues in the human body. For the moment, many of these tissues are now found to contain many hydroxylases that alters Vitamin D into 1,25-dihydroxy Vitamin D, thus the autocrine production of 1,25-dihydroxy Vitamin D in those tissues occurs [11-14]. The biologically active metabolite of 1,25-dihydroxy Vitamin D is thought to exert its principal actions by binding to the Vitamin D receptor (VDR, a ligand-dependent transcription factor) [15].
However, vitamin D is not just a vitamin; the pleiotropic roles of Vitamin D have been highlighted in various diseases. Vitamin D is related to cell proliferation, differentiation, apoptosis, intercellular adhesion, oxidative stress, matrix homeostasis, and regulation of inflammatory response [16-18]. The association between serum Vitamin D and pulmonary fibrosis has become a hot topic in recent years. Vitamin D affects the progress of pulmonary fibrosisa variety of ways. This review gives a more detailed and integrated elaboration on the mechanistic pathway of Vitamin D in prevention of pulmonary fibrosis.
The distinct stages of pulmonary fibrosis and vitamin D
Pulmonary fibrosis is an abnormal wound healing response caused by lung injury and infection, which has four distinct stages: a clotting/coagulation phase, an inflammatory phase, a fibroblast migration/proliferation/activation phase, and a tissue remodeling and resolution phase [7]; pulmonary fibrosis usually occurs if any stage in the tissue repair process is dysregulated.
A clotting/coagulation phase
When epithelial cells are stimulated by injury factors, they release inflammatory mediators and then activate an antifibrinolytic coagulation cascade. Unfortunately, the excessive activation of the coagulation cascade has been seen throughout the process of pulmonary fibrosis [19,20]. Recent studies implicated that the coagulation factors are predominantly mediated by protease-activated receptors (PARs), and PARs play a significant role in the pathogenesis of lung fibrosis [19,21-23]. This family comprises four members (PAR1, PAR2, PAR3, and PAR4) but current evidence suggests PAR1 play a major role in the context of lung injury. PARs mediates tissue factor (TF) [24,25], then TF together with activated factor VIIa (FVIIa) forms TF/FVIIa complex. The TF/FVIIa is the primary initiator of the coagulation cascade, which activates factor IX, but is blocked by the TF pathway inhibitor (TFPI), a protease inhibitor.
Vitamin D can exert anticoagulant effects by two predominant ways (Figure 1): 1) Some experimental work has shown that the Vitamin D had a potent capacity to suppress TF expression, by tumor necrosis factor-α (TNFα, a key activator of TF) [26-29]. Other studies demonstrated a significant positive correlation between Vitamin D and TFPI levels [26], hence Vitamin D could exert anticoagulant effects.
Figure 1.
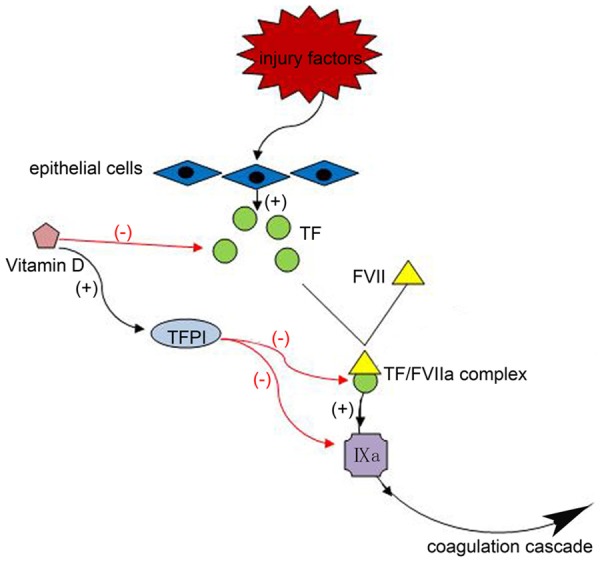
When epithelial cells get stimulated by injury factors, they will release TF, and then together with activated factor VIIa (FVIIa) formed TF/FVIIa complex, which activate factor IX, this is the primary initiator of coagulation cascade. Vitamin D can exert anticoagulant effects in two ways: (1) Vitamin D suppresses TF expression, (2) Vitamin D increases the levels of TFPI.
An inflammatory phase
The next phase of wound healing is inflammation: the injured epithelial or endothelial cells release excessive inflammatory mediators, inducing the sequential infiltration of inflammatory cells (neutrophils, macrophages and lymphocytes). Macrophages can release cytokines IL-13, IL-1, growth factors such as active transforming growth factor β (TGF-β), platelet-derived growth factor (PDGF), fibroblast growth factor (FGF) and TNF-α, which promote the inflammatory response and fibrosis [5,30-33]. These intricate and poorly understood interactions ultimately result in myofibroblast activation and collagen expression, especially TGF-β, a multifunctional cytokine that is causally linked to pulmonary fibrosis.
Past research suggests that inflammatory mediators play a role in the initiation and progression of pulmonary fibrosis [34,35]. However, now more studies are showing that pulmonary fibrosis is the consequence of multiple repeated injuries to the lung epithelium [5,30]. The concept of pathogenesis for lung fibrosis has been transited inflammatory-driven into an epithelial-driven, and inflammation is not a cause, but a consequence, of pulmonary fibrosis [5,30].
Vitamin D has been shown to cause decline in serum inflammatory cytokine levels (Figure 2), including IL-13 [36], IL-17 [36,37], IL-1, IL-6, IL-8, and TNF-α [38], and may also act directly on CD4+ T cells to promote T-regulatory cells (Tregs) that secrete the anti-inflammatory cytokine IL-10 [36,37,39,40], and prevent the further expansion of the inflammatory response.
Figure 2.
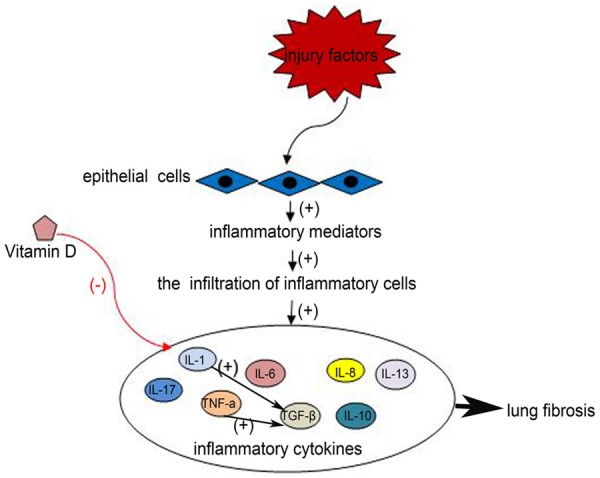
The injured epithelial cells release excessive inflammatory mediators, inducing the sequential infiltration of inflammatory cells. Then inflammatory cells release cytokines (IL-1, IL-6, IL-8, IL-10, IL-13, IL-17, TGF-β, TNF-α), which promote the inflammatory response and fibrosis. Vitamin D can reduce the levels of inflammatory cytokines, preventing the further expansion of the inflammatory response.
Several studies have supported the notion that VD3 could markedly inhibit activation of TGF-β signaling pathways, diminish the up-regulation of fibronectin and collagen expression, and also inhibit the trans-differentiation of TGF-β1 and stimulated lung epithelial cells into myofibroblasts [41,42], and we will elaborate on this in the next section.
Fibroblast migration/proliferation/activation phase
After the inflammation, the wound healing process enters the next phase, where fibroblast hyperplasia and exaggerated ECM deposition is initiated, and more than one mechanism is involved in the fibrosis process.
TGF-β/SMAD signaling pathways
At the very beginning of lung damage, the injured epithelial or endothelial cells release excessive inflammatory mediators that start an antifibrinolytic-coagulation cascade that triggers clotting and creates an interim ECM. Then it enters the next phase, characterized by a fibroblast migration/proliferation/activation phase, and myofibroblasts plays a significant role in this phase. Myofibroblasts are converted from a variety of sources including settled mesenchymal cells, bone marrow progenitors (also entitled fibrocytes). Epithelial cells go through epithelial-mesenchymal transition (EMT) [7]. EMT of epithelial cells is a major step toward pulmonary fibrosis, and TGF-β is an accepted activator of EMT, and also plays a central role in the proliferation, differentiation, and migration of cells [41,43-46].
TGF-β expression can be induced by proinflammatory cytokines, such as IL-1β and TNF-α, and IL-1β triggers TGF-β gene expression by activating NF-B and AP-1 pathways [47]. TGF-β attaches to cell surface type I and II serine/threonine receptor kinases, leading to phosphorylation of SMAD2 and SMAD3, then the phosphorylation of receptor kinases released into the cytosol, and make up a complex with SMAD4. Followed by into the nucleus, the activated Smad complexes in conjunction with SMAD-binding elements within the genome to play its role, such as regulating the expression of profibrotics [48,49].
Studies have revealed that Vitamin D can inhibits the TGF-β-SMAD signaling pathway [41,50-52], and the details of this process is 1,25(OH)2D3 binds a complex with VDR, then the complex directly interacts with SMAD3 and decreased binding of SMAD3 to DNA, at last resulting in the inhibited TGF-β-SMAD signal transduction [42,53].
Ang II-AT1 receptor signaling
The activation of renin-angiotensin system (RAS) has been implicated to induce lung fibrosis both in transgenic animals and in disease models [54-56], and is recognized one important pathogenic factor in the pathogenesis of lung fibrosis [57]. However, the induction of lung fibrosis by RAS is not due to hypertension, although hypertension is an independent risk factor for lung fibrosis [58]. The RAS consists of angiotensinogen (AGT), an aspartyl protease such as renin or cathepsin D, angiotensin-converting enzyme (ACE). Renin cleaves angiotensinogen to angiotensin (Ang) I, which is further converted to Ang II by angiotensin-converting enzyme (ACE). Ang II plays an important role in lung fibrogenesis by both AT1 and AT2 receptors, which are mediated mainly by the AT1 receptor [57,59,60]. Ang II activated by AT1 receptor, directly stimulates ECM protein production, and expression of TGF-β and connective tissue growth factor (CTGF) [61,62], both of which could activate the fibrotic cascade and contribute to the development of lung fibrosis. Importantly, Ang II can activate SMAD2/3 directly by the ERK/p38 pathway [63] (64), and regulate TGF-β/SMAD3 signaling at multiple levels.
Vitamin D plays its antifibrotic role through a negative regulation of the RAS (Figure 3). Research has shown that hypovitaminosis D has been the other face of RAS activation [64], and VDR knockout mice caused the over-expression of renin, resulting in more angiotensinogen transformed into angiotensin II. In summary, vitamin D could reduce over-activation of RAS, causing an antifibrotic effect. Besides, 1,25(OH)2D3 binds a complex with VDR, and the complex directly interacts with SMAD3, which restrains the ERK/p38 pathway. Vitamin D plays its negative regulation of the RAS both of these two ways.
Figure 3.
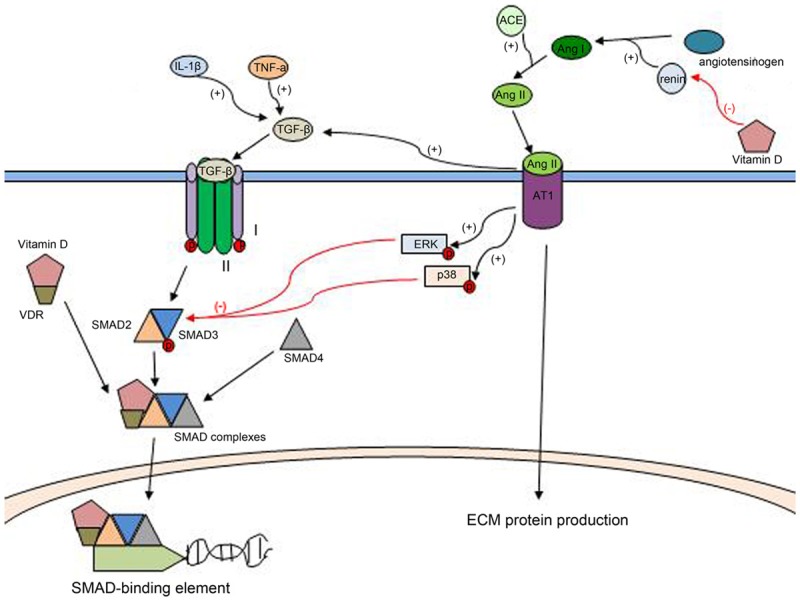
(1) TNF-α, and IL-1β can induce the expression of TGF-β. TGF-β attaches to cell surface type I and II serine/threonine receptor kinases, leading to phosphorylation of SMAD2 and SMAD3, then makes up a complex with SMAD4. Followed by translocation into the nucleus, the activated SMAD complexes in conjunction with SMAD-binding elements and plays its role. Vitamin D binds a complex with VDR, then the complex directly interacts with SMAD3 and decreases binding of SMAD3 to DNA, at last resulting in the inhibited TGF-β-SMAD signaling pathways. (2) Renin cleaves angiotensinogen to angiotensin (Ang) I, which is further converted to Ang II by ACE. Ang II plays an important role in lung fibrogenesis by the AT1 receptor. Ang II is activated by AT1 receptor, directly stimulates ECM protein production, and elevates the expression of TGF-β, meanwhile it activates SMAD2/3 directly by the ERK/p38 pathway. Vitamin D can reduce the expression of rennin, and regulate TGF-β SMAD3 signaling, Vitamin D causes negative regulation of the RASboth of these two ways.
Discussion
A large number of studies have displayed the prominent role Vitamin D has in fibrosis disease. Vitamin D affects the progress of clotting/coagulation phase, inflammation phase, and fibroblast migration/proliferation/activation phase of pulmonary fibrosis, in many ways, and then plays its antifibrotic role.
However, the progress of pulmonary fibrosis is complex and results from different factors. More importantly, a variety of pathways could be responsible for lung fibrosis, not only TGF-β/SMAD signaling and Ang II-AT1 receptor signaling, but also other ways, such as NF-κB signaling [65,66], WNT and β-catenin signaling pathways [67], and so on; which limits the antifibrotic role of Vitamin D.
Nevertheless, the evidence reviewed in this paper indicates a crucial role of Vitamin D in lung fibrosis.
Acknowledgements
This work was supported by State Key Development Program of Shandong Province, China, under Grant number 2017GSF221003.
Disclosure of conflict of interest
None.
References
- 1.Raghu G, Collard HR, Egan JJ, Martinez FJ, Behr J, Brown KK, Colby TV, Cordier JF, Flaherty KR, Lasky JA, Lynch DA, Ryu JH, Swigris JJ, Wells AU, Ancochea J, Bouros D, Carvalho C, Costabel U, Ebina M, Hansell DM, Johkoh T, Kim DS, King TE Jr, Kondoh Y, Myers J, Müller NL, Nicholson AG, Richeldi L, Selman M, Dudden RF, Griss BS, Protzko SL, Schünemann HJ ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med. 2011;183:788–824. doi: 10.1164/rccm.2009-040GL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sundarakrishnan A, Chen Y, Black LD, Aldridge BB, Kaplan DL. Engineered cell and tissue models of pulmonary fibrosis. Adv Drug Deliv Rev. 2018;129:78–94. doi: 10.1016/j.addr.2017.12.013. [DOI] [PubMed] [Google Scholar]
- 3.Ahluwalia N, Shea BS, Tager AM. New therapeutic targets in idiopathic pulmonary fibrosis. Aiming to rein in runaway wound-healing responses. Am J Respir Crit Care Med. 2014;190:867–78. doi: 10.1164/rccm.201403-0509PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wolters PJ, Blackwell TS, Eickelberg O, Loyd JE, Kaminski N, Jenkins G, Maher TM, Molina-Molina M, Noble PW, Raghu G, Richeldi L, Schwarz MI, Selman M, Wuyts WA, Schwartz DA. Time for a change: is idiopathic pulmonary fibrosis still idiopathic and only fibrotic? Lancet Respir Med. 2018;6:154–160. doi: 10.1016/S2213-2600(18)30007-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu YM, Nepali K, Liou JP. Idiopathic pulmonary fibrosis: current status, recent progress, and emerging targets. J Med Chem. 2017;60:527–553. doi: 10.1021/acs.jmedchem.6b00935. [DOI] [PubMed] [Google Scholar]
- 6.Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134:136–51. doi: 10.7326/0003-4819-134-2-200101160-00015. [DOI] [PubMed] [Google Scholar]
- 7.Wynn TA. Integrating mechanisms of pulmonary fibrosis. J Exp Med. 2011;208:1339–50. doi: 10.1084/jem.20110551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demay MB. Mechanism of vitamin D receptor action. Ann N Y Acad Sci. 2006;1068:204–13. doi: 10.1196/annals.1346.026. [DOI] [PubMed] [Google Scholar]
- 9.Reid IR, Bolland MJ, Grey A. Effects of vitamin D supplements on bone mineral density: a systematic review and meta-analysis. Lancet. 2014;383:146–155. doi: 10.1016/S0140-6736(13)61647-5. [DOI] [PubMed] [Google Scholar]
- 10.Hollis BW, Wagner CL. Clinical review: the role of the parent compound vitamin D with respect to metabolism and function: why clinical dose intervals can affect clinical outcomes. J Clin Endocrinol Metab. 2013;98:4619–28. doi: 10.1210/jc.2013-2653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flanagan JN, Young MV, Persons KS, Wang L, Mathieu JS, Whitlatch LW, Holick MF, Chen TC. Vitamin D metabolism in human prostate cells: implications for prostate cancer chemoprevention by vitamin D. Anticancer Res. 2006;26:2567–2572. [PubMed] [Google Scholar]
- 12.Hosseinpour F, Wikvall K. Porcine microsomal vitamin D(3) 25-hydroxylase (CYP2D25). Catalytic properties, tissue distribution, and comparison with human CYP2D6. J Biol Chem. 2000;275:34650–5. doi: 10.1074/jbc.M004185200. [DOI] [PubMed] [Google Scholar]
- 13.Schuessler M, Astecker N, Herzig G, Vorisek G, Schuster I. Skin is an autonomous organ in synthesis, two-step activation and degradation of vitamin D(3): CYP27 in epidermis completes the set of essential vitamin D(3)-hydroxylases. Steroids. 2001;66:399–408. doi: 10.1016/s0039-128x(00)00229-4. [DOI] [PubMed] [Google Scholar]
- 14.Zhu J, DeLuca HF. Vitamin D 25-hydroxylase-four decades of searching, are we there yet? Arch Biochem Biophys. 2012;523:30–6. doi: 10.1016/j.abb.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Haussler MR, Whitfield GK, Haussler CA, Hsieh JC, Thompson PD, Selznick SH, Dominguez CE, Jurutka PW. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. J Bone Miner Res. 1998;13:325–349. doi: 10.1359/jbmr.1998.13.3.325. [DOI] [PubMed] [Google Scholar]
- 16.Prietl B, Treiber G, Pieber TR, Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–21. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Samuel S, Sitrin MD. Vitamin D’s role in cell proliferation and differentiation. Nutr Rev. 2008;66:S116–24. doi: 10.1111/j.1753-4887.2008.00094.x. [DOI] [PubMed] [Google Scholar]
- 18.Norman PE, Powell JT. Vitamin D and cardiovascular disease. Circ Res. 2014;114:379–93. doi: 10.1161/CIRCRESAHA.113.301241. [DOI] [PubMed] [Google Scholar]
- 19.Park YS, Park CM, Lee HJ, Goo JM, Chung DH, Lee SM, Yim JJ, Kim YW, Han SK, Yoo CG. Clinical implication of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Respir Med. 2013;107:256–62. doi: 10.1016/j.rmed.2012.10.011. [DOI] [PubMed] [Google Scholar]
- 20.Imokawa S, Sato A, Hayakawa H, Kotani M, Urano T, Takada A. Tissue factor expression and fibrin deposition in the lungs of patients with idiopathic pulmonary fibrosis and systemic sclerosis. Am J Respir Crit Care Med. 1997;156:631–6. doi: 10.1164/ajrccm.156.2.9608094. [DOI] [PubMed] [Google Scholar]
- 21.Lin C, Borensztajn K, Spek CA. Targeting coagulation factor receptors-protease-activated receptors in idiopathic pulmonary fibrosis. J Thromb Haemost. 2017;15:597–607. doi: 10.1111/jth.13623. [DOI] [PubMed] [Google Scholar]
- 22.Borensztajn K, Stiekema J, Nijmeijer S, Reitsma PH, Peppelenbosch MP, Spek CA. Factor Xa stimulates proinflammatory and profibrotic responses in fibroblasts via protease-activated receptor-2 activation. Am J Pathol. 2008;172:309–20. doi: 10.2353/ajpath.2008.070347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wygrecka M, Kwapiszewska G, Jablonska E, von Gerlach S, Henneke I, Zakrzewicz D, Guenther A, Preissner KT, Markart P. Role of protease-activated receptor-2 in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2011;183:1703–14. doi: 10.1164/rccm.201009-1479OC. [DOI] [PubMed] [Google Scholar]
- 24.Ruf W, Mueller BM. Tissue factor signaling. Thromb Haemost. 1999;82:175–182. [PubMed] [Google Scholar]
- 25.Schaffner F, Ruf W. Tissue factor and protease-activated receptor signaling in cancer. Semin Thromb Hemost. 2008;34:147–53. doi: 10.1055/s-2008-1079254. [DOI] [PubMed] [Google Scholar]
- 26.Kasthuri RS, Glover SL, Boles J, Mackman N. Tissue factor and tissue factor pathway inhibitor as key regulators of global hemostasis: measurement of their levels in coagulation assays. Semin Thromb Hemost. 2010;36:764–71. doi: 10.1055/s-0030-1265293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koyama T, Shibakura M, Ohsawa M, Kamiyama R, Hirosawa S. Anticoagulant effects of 1alpha,25-dihydroxyVitamin D3 on human myelogenous leukemia cells and monocytes. Blood. 1998;92:160–167. [PubMed] [Google Scholar]
- 28.Ohsawa M, Koyama T, Yamamoto K, Hirosawa S, Kamei S, Kamiyama R. 1alpha,25-dihydroxyvitamin D(3) and its potent synthetic analogs downregulate tissue factor and upregulate thrombomodulin expression in monocytic cells, counteracting the effects of tumor necrosis factor and oxidized LDL. Circulation. 2000;102:2867–2872. doi: 10.1161/01.cir.102.23.2867. [DOI] [PubMed] [Google Scholar]
- 29.Martinez-Moreno JM, Herencia C, Montes de Oca A, Munoz-Castaneda JR, Rodriguez-Ortiz ME, Diaz-Tocados JM, Peralbo-Santaella E, Camargo A, Canalejo A, Rodriguez M, Velasco-Gimena F, Almaden Y. Vitamin D modulates tissue factor and protease-activated receptor 2 expression in vascular smooth muscle cells. FASEB J. 2016;30:1367–76. doi: 10.1096/fj.15-272872. [DOI] [PubMed] [Google Scholar]
- 30.Noble PW. Idiopathic pulmonary fibrosis. New insights into classification and pathogenesis usher in a new era therapeutic approaches. Am J Respir Cell Mol Biol. 2003;29:S27–31. [PubMed] [Google Scholar]
- 31.Khalil N, O’Connor R. Idiopathic pulmonary fibrosis: current understanding of the pathogenesis and the status of treatment. CMAJ. 2004;171:153–60. doi: 10.1503/cmaj.1030055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Selman M, Pardo A. The epithelial/fibroblastic pathway in the pathogenesis of idiopathic pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29:S93–7. [PubMed] [Google Scholar]
- 33.Plataki M, Koutsopoulos AV, Darivianaki K, Delides G, Siafakas NM, Bouros D. Expression of apoptotic and antiapoptotic markers in epithelial cells in idiopathic pulmonary fibrosis. Chest. 2005;127:266–74. doi: 10.1378/chest.127.1.266. [DOI] [PubMed] [Google Scholar]
- 34.Bringardner BD, Baran CP, Eubank TD, Marsh CB. The role of inflammation in the pathogenesis of idiopathic pulmonary fibrosis. Antioxid Redox Signal. 2008;10:287–301. doi: 10.1089/ars.2007.1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mura M, Belmonte G, Fanti S, Contini P, Pacilli AM, Fasano L, Zompatori M, Schiavina M, Fabbri M. Inflammatory activity is still present in the advanced stages of idiopathic pulmonary fibrosis. Respirology. 2005;10:609–14. doi: 10.1111/j.1440-1843.2005.00757.x. [DOI] [PubMed] [Google Scholar]
- 36.Brown SD, Calvert HH, Fitzpatrick AM. Vitamin D and asthma. Dermatoendocrinol. 2012;4:137–45. doi: 10.4161/derm.20434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paul G, Brehm JM, Alcorn JF, Holguin F, Aujla SJ, Celedon JC. Vitamin D and asthma. Am J Respir Crit Care Med. 2012;185:124–32. doi: 10.1164/rccm.201108-1502CI. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Stubbs JR, Idiculla A, Slusser J, Menard R, Quarles LD. Cholecalciferol supplementation alters calcitriol-responsive monocyte proteins and decreases inflammatory cytokines in ESRD. J Am Soc Nephrol. 2010;21:353–61. doi: 10.1681/ASN.2009040451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Herr C, Greulich T, Koczulla RA, Meyer S, Zakharkina T, Branscheidt M, Eschmann R, Bals R. The role of vitamin D in pulmonary disease: COPD, asthma, infection, and cancer. Respir Res. 2011;12:31. doi: 10.1186/1465-9921-12-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Finklea JD, Grossmann RE, Tangpricha V. Vitamin D and chronic lung disease: a review of molecular mechanisms and clinical studies. Adv Nutr. 2011;2:244–53. doi: 10.3945/an.111.000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fischer KD, Agrawal DK. Erratum to: vitamin D regulating TGF-β induced epithelial-mesenchymal transition. Respir Res. 2015;16:139. doi: 10.1186/s12931-015-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jiang F, Yang Y, Xue L, Li B, Zhang Z. 1α,25-dihydroxyvitamin D3 attenuates TGF-β-induced pro-fibrotic effects in human lung epithelial cells through inhibition of epithelial-mesenchymal transition. Nutrients. 2017;9 doi: 10.3390/nu9090980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun X, Chen E, Dong R, Chen W, Hu Y. Nuclear factor (NF)-κB p65 regulates differentiation of human and mouse lung fibroblasts mediated by TGF-β. Life Sci. 2015;122:8–14. doi: 10.1016/j.lfs.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 44.Abramovitch S, Sharvit E, Weisman Y, Bentov A, Brazowski E, Cohen G, Volovelsky O, Reif S. Vitamin D inhibits development of liver fibrosis in an animal model but cannot ameliorate established cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2015;308:G112–20. doi: 10.1152/ajpgi.00132.2013. [DOI] [PubMed] [Google Scholar]
- 45.Sporn MB, Roberts AB, Wakefield LM, Assoian RK. Transforming growth factor-beta: biological function and chemical structure. Science. 1986;233:532–4. doi: 10.1126/science.3487831. [DOI] [PubMed] [Google Scholar]
- 46.Massague J. The transforming growth factor-beta family. Annu Rev Cell Biol. 1990;6:597–641. doi: 10.1146/annurev.cb.06.110190.003121. [DOI] [PubMed] [Google Scholar]
- 47.Lee KY, Ito K, Hayashi R, Jazrawi EP, Barnes PJ, Adcock IM. NF-kappaB and activator protein 1 response elements and the role of histone modifications in IL-1beta-induced TGF-beta1 gene transcription. J Immunol. 2006;176:603–15. doi: 10.4049/jimmunol.176.1.603. [DOI] [PubMed] [Google Scholar]
- 48.Bonventre JV. Antifibrotic vitamin D analogs. J Clin Invest. 2013;123:4570–3. doi: 10.1172/JCI72748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Huang LS, Natarajan V. Sphingolipids in pulmonary fibrosis. Adv Biol Regul. 2015;57:55–63. doi: 10.1016/j.jbior.2014.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Artaza JN, Norris KC. Vitamin D reduces the expression of collagen and key profibrotic factors by inducing an antifibrotic phenotype in mesenchymal multipotent cells. J Endocrinol. 2009;200:207–21. doi: 10.1677/JOE-08-0241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Yuan T, Du G, Zhao Q, Ma L, Zhu J. The impact of 1,25-dihydroxyvitamin D3 on the expression of connective tissue growth factor and transforming growth factor-beta1 in the myocardium of rats with diabetes. Diabetes Res Clin Pract. 2014;104:226–33. doi: 10.1016/j.diabres.2014.01.031. [DOI] [PubMed] [Google Scholar]
- 52.Kabel AM, Abd Elmaaboud MA, Atef A, Baali MH. Ameliorative potential of linagliptin and/or calcipotriol on bleomycin-induced lung fibrosis: in vivo and in vitro study. Environ Toxicol Pharmacol. 2017;50:216–226. doi: 10.1016/j.etap.2017.02.008. [DOI] [PubMed] [Google Scholar]
- 53.Ito I, Waku T, Aoki M, Abe R, Nagai Y, Watanabe T, Nakajima Y, Ohkido I, Yokoyama K, Miyachi H, Shimizu T, Murayama A, Kishimoto H, Nagasawa K, Yanagisawa J. A nonclassical vitamin D receptor pathway suppresses renal fibrosis. J Clin Invest. 2013;123:4579–94. doi: 10.1172/JCI67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang J, Chen L, Chen B, Meliton A, Liu SQ, Shi Y, Liu T, Deb DK, Solway J, Li YC. Chronic activation of the renin-angiotensin system induces lung fibrosis. Sci Rep. 2015;5:15561. doi: 10.1038/srep15561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jiang JS, Lang YD, Chou HC, Shih CM, Wu MY, Chen CM, Wang LF. Activation of the renin-angiotensin system in hyperoxia-induced lung fibrosis in neonatal rats. Neonatology. 2012;101:47–54. doi: 10.1159/000329451. [DOI] [PubMed] [Google Scholar]
- 56.Lang YD, Hung CL, Wu TY, Wang LF, Chen CM. The renin-angiotensin system mediates hyperoxia-induced collagen production in human lung fibroblasts. Free Radic Biol Med. 2010;49:88–95. doi: 10.1016/j.freeradbiomed.2010.03.022. [DOI] [PubMed] [Google Scholar]
- 57.Uhal BD, Li X, Piasecki CC, Molina-Molina M. Angiotensin signalling in pulmonary fibrosis. Int J Biochem Cell Biol. 2012;44:465–8. doi: 10.1016/j.biocel.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shi Y, Liu T, Yao L, Xing Y, Zhao X, Fu J, Xue X. Chronic vitamin D deficiency induces lung fibrosis through activation of the renin-angiotensin system. Sci Rep. 2017;7:3312. doi: 10.1038/s41598-017-03474-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Konigshoff M, Wilhelm A, Jahn A, Sedding D, Amarie OV, Eul B, Seeger W, Fink L, Gunther A, Eickelberg O, Rose F. The angiotensin II receptor 2 is expressed and mediates angiotensin II signaling in lung fibrosis. Am J Respir Cell Mol Biol. 2007;37:640–50. doi: 10.1165/rcmb.2006-0379TR. [DOI] [PubMed] [Google Scholar]
- 60.Marshall RP, McAnulty RJ, Laurent GJ. Angiotensin II is mitogenic for human lung fibroblasts via activation of the type 1 receptor. Am J Respir Crit Care Med. 2000;161:1999–2004. doi: 10.1164/ajrccm.161.6.9907004. [DOI] [PubMed] [Google Scholar]
- 61.Finckenberg P, Inkinen K, Ahonen J, Merasto S, Louhelainen M, Vapaatalo H, Müller D, Ganten D, Luft F, Mervaala E. Angiotensin II induces connective tissue growth factor gene expression via calcineurin-dependent pathways. Am J Pathol. 2003;163:355–66. doi: 10.1016/S0002-9440(10)63659-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Marshall RP, Gohlke P, Chambers RC, Howell DC, Bottoms SE, Unger T, McAnulty RJ, Laurent GJ. Angiotensin II and the fibroproliferative response to acute lung injury. Am J Physiol Lung Cell Mol Physiol. 2004;286:L156–64. doi: 10.1152/ajplung.00313.2002. [DOI] [PubMed] [Google Scholar]
- 63.Mirkovic K, de Borst MH. Beyond the RAAS: dissecting the antifibrotic effects of vitamin D analogues. Lab Invest. 2012;92:1666–9. doi: 10.1038/labinvest.2012.150. [DOI] [PubMed] [Google Scholar]
- 64.Ferder M, Inserra F, Manucha W, Ferder L. The world pandemic of vitamin D deficiency could possibly be explained by cellular inflammatory response activity induced by the renin-angiotensin system. Am J Physiol Cell Physiol. 2013;304:C1027–39. doi: 10.1152/ajpcell.00403.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou J, Ma T, Cao H, Chen Y, Wang C, Chen X, Xiang Z, Han X. TNF-alpha-induced NF-kappaB activation promotes myofibroblast differentiation of LR-MSCs and exacerbates bleomycin-induced pulmonary fibrosis. J Cell Physiol. 2018;233:2409–2419. doi: 10.1002/jcp.26112. [DOI] [PubMed] [Google Scholar]
- 66.Kandhare AD, Bodhankar SL, Mohan V, Thakurdesai PA. Effect of glycosides based standardized fenugreek seed extract in bleomycin-induced pulmonary fibrosis in rats: decisive role of Bax, Nrf2, NF-kappaB, Muc5ac, TNF-alpha and IL-1beta. Chem Biol Interact. 2015;237:151–65. doi: 10.1016/j.cbi.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 67.Moon RT, Kohn AD, De Ferrari GV, Kaykas A. WNT and beta-catenin signalling: diseases and therapies. Nat Rev Genet. 2004;5:691–701. doi: 10.1038/nrg1427. [DOI] [PubMed] [Google Scholar]


