Abstract
Acute lung injury (ALI) and acute respiratory distress syndrome (ARDS) are common and complicated inflammatory lung diseases. Circ RNAs have emerged as a novel class of gene regulatory molecules that play vital roles in multiple complex diseases, including ALI. In this study, we aimed to identify potential regulators of Circ 0001434 on acute lung injury (ALI) and to explore their roles in lipopolysaccharide (LPS)-induced ALI. In a mouse ALI model, Circ 0001434 expression was effectively down-regulated, compared with the control group. Up-regulation of Circ 0001434 effectively decreased the inflammation of ALI in an in vitro model. Down-regulation of Circ 0001434 effectively promoted inflammation in ALI in an in vitro model. Over-expression of Circ 0001434 induced Wnt and β-catenin protein expression, and suppressed NF-κB p65 protein expression in the ALI in vitro model by miR-625-5p. Down-regulation of Circ 0001434 significantly suppressed Wnt and β-catenin, and induced NF-κB p65 protein expression in the ALI in vitro model by miR-625-5p. Wnt reduced the function of Circ 0001434 on inflammation in ALI in an in vitro model. The inhibition of miR-625-5p reversed the function of anti-Circ 0001434 on inflammation in ALI vitro model. Taken together, Circ 0001434 mediates ALI-induced lung inflammation through Wnt/β-catenin and NF-κB activation by miR-625-5p.
Keywords: Circ 0001434, ALI, inflammation, NF-κB, Wnt/β-catenin, miR-625-5p
Introduction
Acute lung injury (ALI) is defined as the damage caused to alveolar epithelial cells and pulmonary capillary endothelial cells during serious infection, shock, trauma and burns, subsequently causing alveolar capillary permeability in the vesicle cavity [1]. The accumulation of edema fluid containing protein could lead to diffuse pulmonary interstitial edema and a further clinical syndrome characterized by progressive hypoxemia and respiratory distress [2]. In terms of disease stage, ALI is considered as the early stage of ARDS [3].
An inflammatory factor release-induced lung inflammatory response is one of the causes of increased capillary permeability of ALI alveoli and a clearance disorder of alveolar fluid [4]. NF-κB, an important transcription factor, regulates TNF-α, IL-1 and IL-6 to play important roles in the production of pro-inflammatory cytokines [5]. Under the stimulation of LPS, NF-κB is tranlocated from the cytoplasm to the nucleus, thereby initiating transcription of genes such as inflammatory cytokines, inflammatory mediators, and chemokines. Therefore, it is urgent to use interventions targeting NF-κB activating sites [6].
The canonical Wnt signaling pathway is the Wnt/β-catenin pathway [7]. The basic process of signal transduction is as follows: Wnt signaling protein binds to transmembrane receptor to activate the Wnt signaling pathway, which subsequently activates Frizzled protein and GSK-β binding protein [8]. GSK-β binding protein can recognize and inhibit the phosphorylation activity of GSK-3β, rendering it unable to phosphorylate β-catenin, which causes an inability of β-catenin to be recognized by ubiquitin ligase and it is not degraded by protease complexes [9]. The amount of β-catenin is dramatically increased in the nucleus, eventually triggering transcription of the target genes. Recent studies have shown that abnormal activation of the Wnt signaling pathway plays an important role in the development of organ fibrosis, including liver, kidney, lung, heart, and skin [9].
The expression changes of circ RNA are associated with immune responses, inflammatory signaling pathways, and the pathogenesis of inflammatory lung diseases including ALI. Therefore, circ RNAs are promising novel therapeutic targets. Although the changes of relevant gene expression caused by circ RNAs are generally modest, the outcomes may affect the expression of a large number of subsequent genes, which in turn affect a variety of biologic processes. Therefore, it is feasible to use Circ RNAs as markers.
It has been confirmed that the expression of miRNA is dynamic, reflecting changes in the environment and signaling inside and outside the cells [10]. Therefore, there is potential to apply miRNA as a biomarker to represent a specific disease state or its deeper pathophysiologic processes in different stages of development [11]. In addition, miRNAs can be detected in a variety of specimens, including tissue, blood and other body fluids. miRNAs are also stable and almost unaffected by sample processing errors, which make miRNAs more attractive as biomarkers [12]. Therefore, in this study, we aimed to identify potential regulators of Circ 0001434 on acute lung injury (ALI) and to explore their roles in lipopolysaccharide (LPS)-induced ALI.
Materials and methods
Animal experiments
A total of 20 C57BL/6 mice (5-6 weeks old, 18-20 g) were used in this study and randomly assigned to the following two groups (n = 10 for each), control PBS and ALI model group. The mice were anesthetized and intratracheally administered PBS or LPS (Sigma Aldrich; 1 mg/kg) for 1 day. All experiments involving animals were performed as per the approval by the Animal Care and Use Committee of The First Affiliated Hospital of Bengbu Medical College.
Quantitative real-time PCR analysis
Total RNA was isolated from lung tissue samples using TRIzol Reagent according to manufacturer’s instructions (Invitrogen, Carlsbad, CA, USA). Reverse transcription was performed using miRNA specific stem-loop primers (Applied Biosystems, Foster City, CA, USA). Mi-RNA was quantified using TaqMan® MicroRNA Assays (Thermo Fisher Scientific, Massachusetts, USA). The relative expression levels were calculated with the 2-ΔΔCt method.
Histologic assay and wet/dry weight ratio of lung tissues
The lung tissues were fixed in 10% formalin for 1 day, embedded in paraffin, and sectioned into 5 μM slices. Samples slices were then stained with haematoxylin-eosin (H&E) and captured using a light microscope (Nikon Eclipse TE2000-U, Nikon, Japan). The lung injury was scored according to the following principle. Each item was scored on a 5-point scale as follows: no damage or minimal damage = 0; mild damage = 1; moderate damage = 2; severe damage = 3; diffuse injury = 4.
The lung wet/dry (W/D) weight ratio was measured by dividing the wet weight by the dry weight. Subsequently, they were placed in an incubator for 24 h at 80°C to obtain the dry weight.
Cell culture, cell transfection, and LPS treatment
A549 cells were cultured in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal bovine serum (FBS) (Gibco, Carlsbad, CA, USA) 5% CO2 and 95% O2 at 37°C. Circ 0001434, anti-Circ 0001434 and negative mimics were obtained from Takara Biomedical Technology Co., Ltd. (Beijing, China). Circ 0001434, anti-Circ 0001434 and negative mimics (50 nM) were transfected using Lipofectamine 2000 reagent (Invitrogen). Cells were treated with LPS (1 μg/mL; Sigma-Aldrich, St. Louis, MO, USA) after transfection at 6 h.
Measurement of inflammatory cytokines by enzyme-linked immunosorbent assay (ELISA)
Cells after treatment with LPS for 48 h were used to measure inflammatory cytokines (IL-1β, IL-6, L-18 and TNF-α) using ELISA kits. Absorbancy was measured at 450 nm using a microplate reader (BioTek, Winooski, Vermont, USA).
Western blotting assay
Protein samples were isolated from the cells after being treated with LPS for 48 h using the protein extraction kit (Beyotime Biotechnology, Haimen, China) and protein content was determined using a BCA Protein Assay Kit (Beyotime Biotechnology, Haimen, China). The protein samples were separated electrophoretically to the 8-12% SDS-PAGE gel and transferred on to a PVDF membrane (Millipore, USA). The membrane was individually incubated overnight at 4°C with the primary antibodies: NF-κB, Wnt, β-catenin, and GAPDH (1:2000, Santa Cruz, CA, USA) at 4°C overnight after blocking nonspecific binding sites for 1 h at 37°C with 5% dried skim milk in TBST at 37°C. Then the membrane was incubated at room temperature for 2 h with horseradish peroxidase-conjugated antibody at 37°C and detected by the enhanced chemiluminescence (ECL) method.
Statistical analysis
Data are presented as the means ± standard deviation (SD). Statistical differences were analyzed using the Student’s t-test or one-way analysis of variance (ANOVA) with the Tukey’s test. P value < 0.05 was considered significant.
Results
Circ 0001434 expression in mice of the ALI model
To explore the mechanism of microRNAs in the ALI model, we analyzed the changes and effects of Circ 0001434 in the ALI model. As shown in Figure 1A, 1B, the lung tissues were subjected to H&E staining. The LPS-induced ALI group showed severe histopathologic changes, including alveolar wall thickening, and pulmonary congestion. Lung injury score was increased in the ALI model, compared with a sham group. Lung wet/dry weight ratio was increased in the ALI model, compared with a sham group (Figure 1C). IL-1β, IL-6, L-18, and TNF-α levels were enhanced in the ALI model, compared with a sham group (Figure 1D-G). Circ 0001434 expression was down-regulated in the ALI model, compared with a sham group (Figure 1H, 1I).
Figure 1.
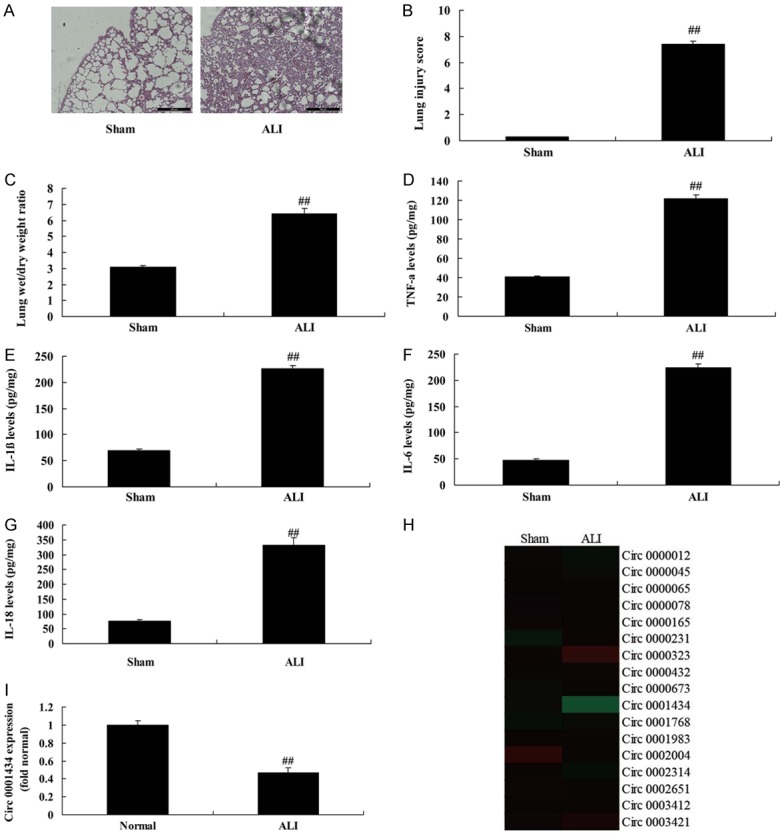
Circ 0001434 expression in mice after ALI. H&E staining (A), lung injury score (B), Lung wet/dry weight ratio (C), TNF-α (D), IL-1β (E), IL-6 (F), L-18 (G) levels, Circ 0001434 expression by gene chip (H) and QPCR (I). Sham, normal control group; ALI, ALI model group. Acute lung injury (ALI). Data are presented as the means ± standard deviation (n = 3). ##P < 0.01 versus normal control group.
Down-regulation of Circ 0001434 accelerated inflammation in an vitro model of ALI by miR-625-5p
We investigated the function and mechanism of Circ 0001434 on inflammation in an ALI in vitro model. The expression of Circ 0001434 inhibitor was effectively decreased using Circ 0001434 inhibitor mimics, compared with the negative group (Figure 2A). Then, up-regulation of Circ 0001434 promoted the increase of IL-1β, IL-6, L-18 and TNF-α levels in the ALI in vitro model, compared with the negative group (Figure 2B-E). We found that miR-625-5p expression was increased in the in vivo model, compared with control group (Figure 2F). Over-expression of Circ 0001434 downregulated miR-625-5p expression in the in vitro model, compared with the negative group (Figure 2G). Analysis results showed that miR-625-5p may be a common focus (Figure 2H). The 3’UTR region of Circ 0001434 showed potential alignment with miR-625-5p sequence and luciferase reporter activity levels were reduced by over-expression of Circ 0001434, compared with the negative group (Figure 2I, 2J). miR-625-5p expression was decreased by up-regulation of miR-625-5p expression. miR-625-5p expression was increased by down-regulation of miR-625-5p expression, compared with the negative group (Figure 2K, 2L).
Figure 2.
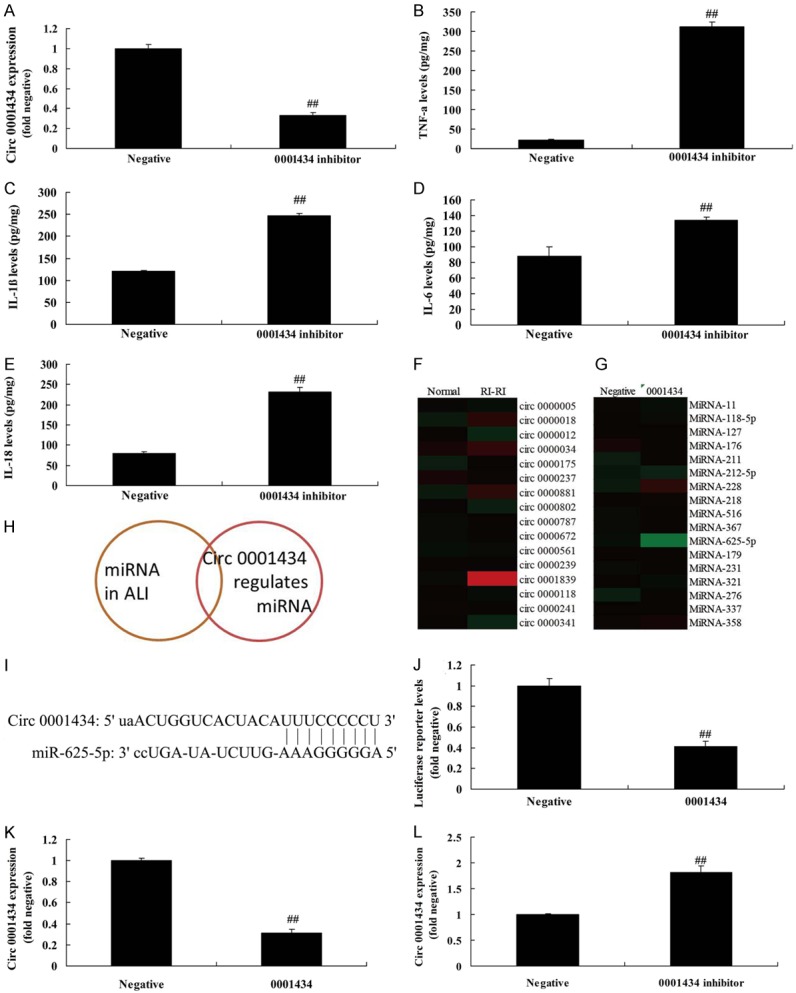
Down-regulation of Circ 0001434 accelerated inflammation in the in vitro model of ALI through miR-625-5p. Circ 0001434 expression (A), TNF-α (B), IL-1β (C), IL-6 (D), L-18 (E) levels, Gene chip (F and G), analysis f results (H), 3’UTR region of Circ 0001434 showed potential alignment with miR-625-5p sequence (I), luciferase reporter activity levels (J), miR-625-5p expression (K and L). Negative, control negative group; miRNA-376c-3p, Up-regulation of Circ 0001434 group. ##P < 0.01 versus control negative group.
miR-625-5p regulates inflammation in the in vitro model of ALI by Wnt/β-catenin/NF-κB
We investigated the function and mechanism of miR-625-5p on inflammation in the ALI in vitro model. Up-regulation of miR-625-5p induced the Wnt/β-catenin/NF-κB signaling pathway in the ALI in vitro model, compared with the negative group (Figure 3A). Figure 3B, 3C shows that the 3’UTR region of Wnt showed potential alignment with the miR-625-5p sequence and luciferase reporter activity levels were reduced by over-expression of miR-625-5p, compared with the negative group. Over-expression of miR-625-5p suppressed Wnt/β-catenin protein expressions and induced NF-κB protein expression in the ALI in vitro model, compared with the negative group (Figure 3D-G). Over-expression of miR-625-5p reduced Wnt protein expression in ALI in vitro model, compared with the negative group (Figure 3H). These results showed that Circ 0001434 expression was up-regulated in ALI, which induced inflammation in ALI, and its mechanism needs to be investigated.
Figure 3.
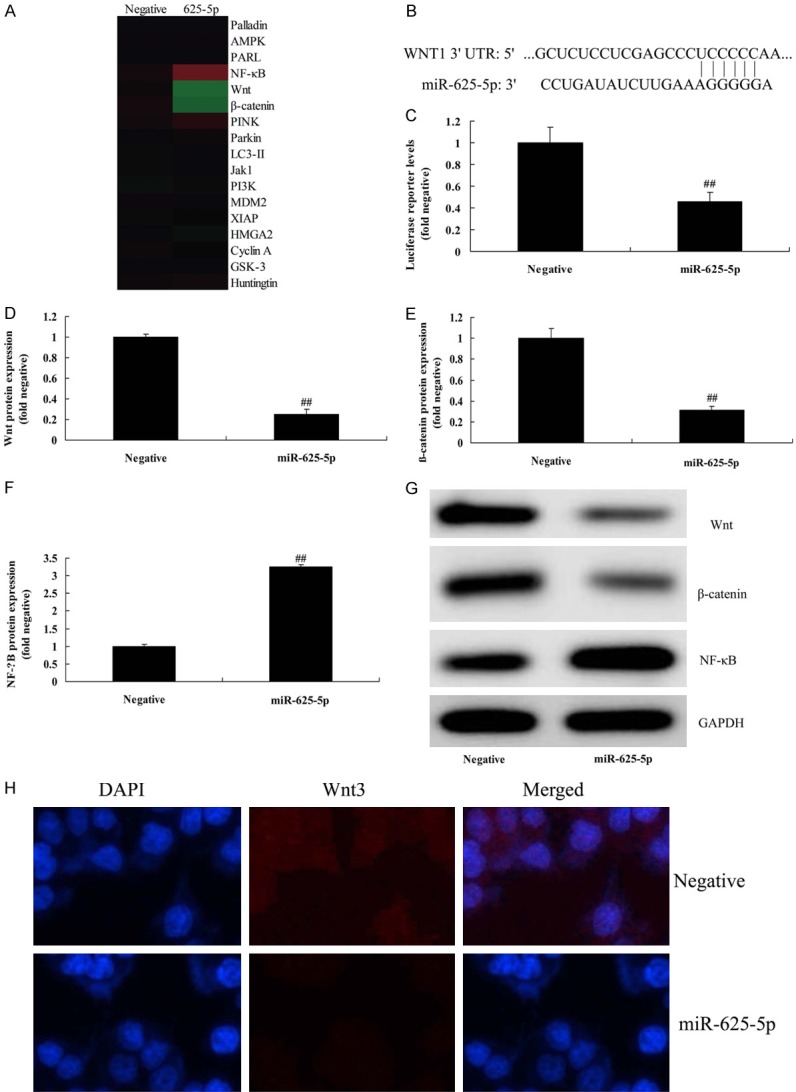
miR-625-5p regulates inflammation in the in vitro model of ALI through Wnt/β-catenin/NF-κB. Gene chip (A), 3’UTR region of Wnt showed potential alignment with miR-625-5p sequence (B), luciferase reporter activity levels (C), Wnt, β-catenin and p65 protein expression using statistical analysis (D-F) and western blot analysis (G), Wnt protein expression using IF (H). Negative, control negative group; miRNA-376c-3p, Up-regulation of Circ 0001434 group. ##P < 0.01 versus control negative group.
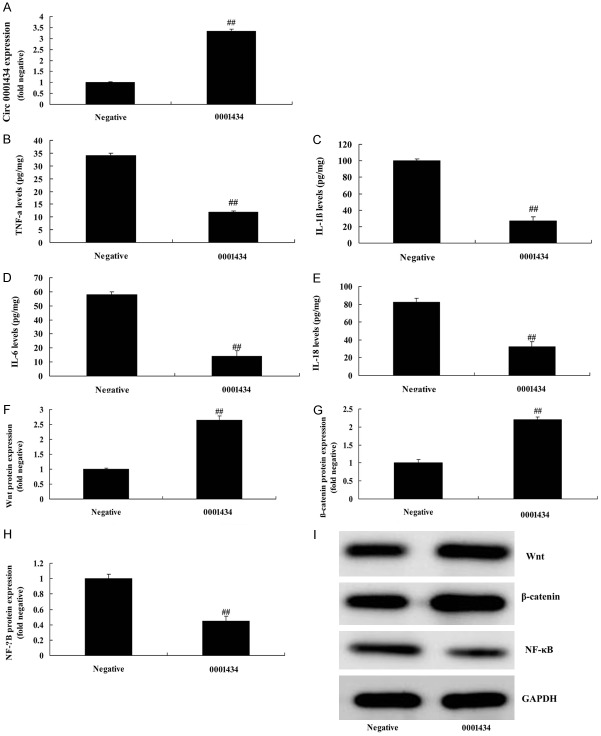
Up-regulation of Circ 0001434 reduced inflammation in the in vitro model of ALI through Wnt/β-catenin/NF-κB
We investigated the function of Circ 0001434 on inflammation in ALI in an in vitro model. The expression of Circ 0001434 effectively increased using Circ 0001434 mimics, compared with a negative group (Figure 4A). Then, up-regulation of Circ 0001434 reduced IL-1β, IL-6, IL-18, and TNF-α levels in the ALI in vitro model, compared with the negative group (Figure 4B-E). However, up-regulation of Circ 0001434 regulated the signaling pathway, induced Wnt/β-catenin protein expression, and suppressed the NF-κB signaling pathway in an ALI in vitro model, compared with the negative group (Figure 4F-I).
Figure 4.
Down-regulation of Circ 0001434 reduced inflammation in ALI in the in vitro model. Circ 0001434 expression (A), TNF-α (B), IL-1β (C), IL-6 (D), L-18 (E) levels, Wnt, β-catenin and p65 protein expression using statistical analysis (F-H) and western blot analysis (I). Negative, control negative group; 0001434, Up-regulation of Circ 0001434 group. ##P < 0.01 versus control negative group.
miR-625-5p reduced the effect of Circ 0001434 on inflammation in an ALI in vitro model
To further investigate the function of miR-625-5p in the effect of Circ 0001434 on inflammation of ALI, the ALI in vitro model was co-transfected with circ 0001434 inhibitor and anti-miR-625-5p mimics. As shown in Figure 5A, miR-625-5p expression was reduced using anti-miR-625-5p mimics in the ALI in vitro model with circ 0001434 inhibitor, compared with the circ 0001434 inhibitor group. Next, we found that down-regulation of Circ miR-625-5p induced Wnt, and β-catenin, and suppressed NF-κB protein expressions in the ALI in vitro model following circ 0001434 inhibitor, compared with the circ 0001434 inhibitor group (Figure 5B-E). Down-regulation of miR-625-5p expression reduced IL-1β, IL-6, IL-18 and TNF-α levels in the ALI in vitro model following circ 0001434 inhibitor, compared with the circ 0001434 inhibitor group (Figure 5F-I).
Figure 5.
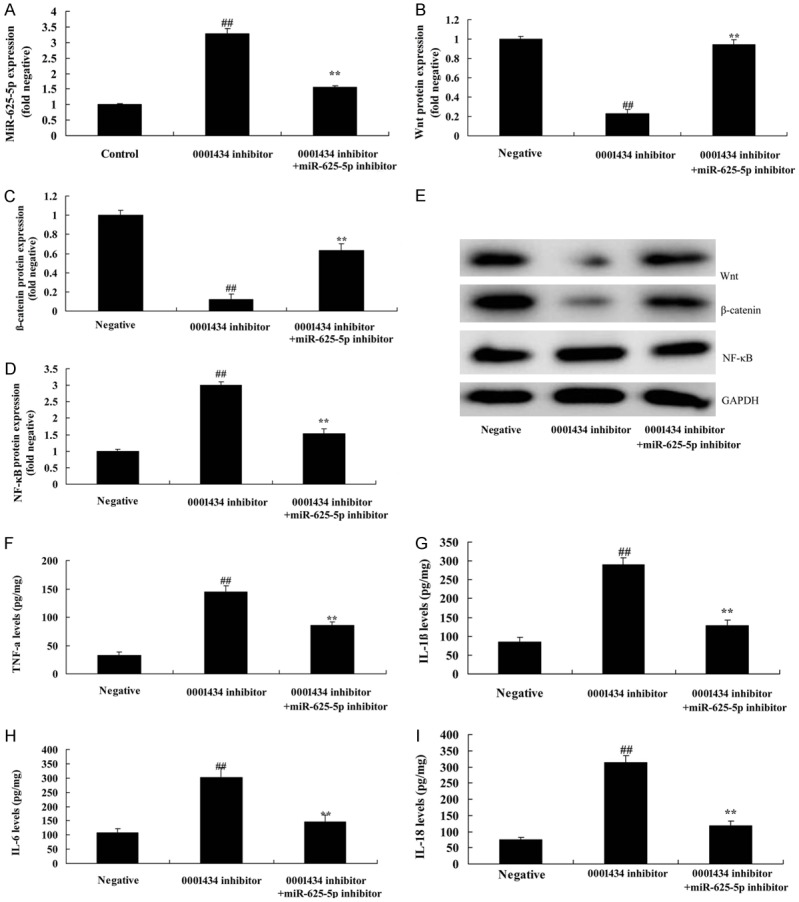
miR-625-5p reduced the effect of Circ 0001434 on inflammation in ALI in an in vitro model. miR-625-5p expression (A), Wnt, β-catenin and p65 protein expression using statistical analysis (B-D) and western blot analysis (E), TNF-α (F), IL-1β (G), IL-6 (H), L-18 (I) levels. Negative, control negative group; 0001434 inhibitor, down-regulation of Circ 0001434 group; 0001434 inhibitor + si-miR-625-5p, down-regulation of Circ 0001434 and miR-625-5p group. ##P < 0.01 versus control negative group, **P < 0.01 versus down-regulation of Circ 0001434 group.
Wnt reduced the function of anti-Circ 0001434 on inflammation in ALI in an in vitro model
To further investigate the role of Wnt in the function of Circ 0001434 on cell apoptosis of ALI, we used Wnt plasmid, andinduced Wnt and β-catenin protein expression in the ALI in vitro model. Figure 6 shows that Wnt plasmid induced Wnt and β-catenin protein expression and suppressed NF-κB protein expression, and reduced IL-1β, IL-6, L-18 and TNF-α levels following anti-Circ 0001434, compared with the anti-Circ 0001434 group.
Figure 6.
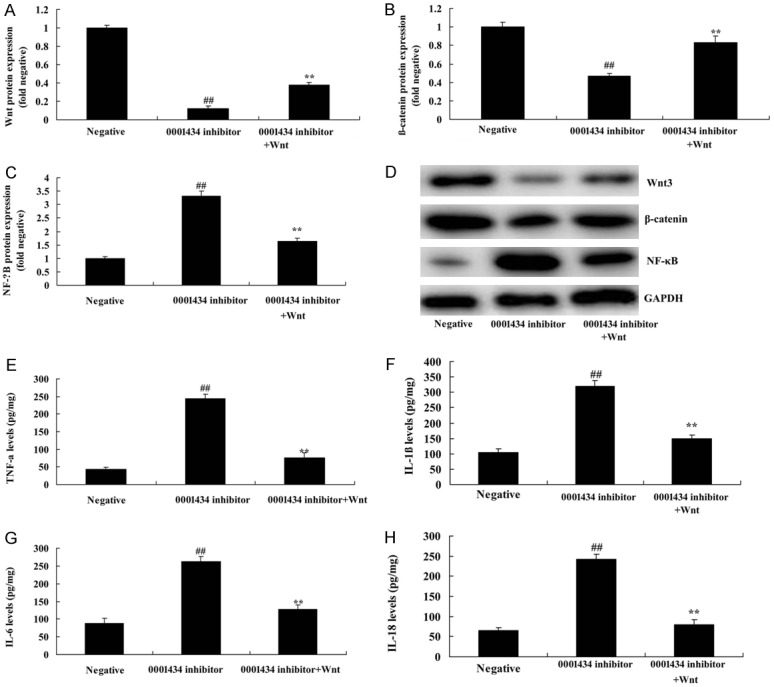
Wnt reduced the function of anti-Circ 0001434 on inflammation in the ALI in vitro model. Wnt, β-catenin, and p65 protein expression using statistical analysis (A-C) and western blot analysis (D), TNF-α (E), IL-1β (F), IL-6 (G), IL-18 (H) levels. Negative, control negative group; 0001434 inhibitor, down-regulation of Circ 0001434 group; 0001434 inhibitor + Wnt, down-regulation of Circ 0001434 and over-expression of Wnt group. ##P < 0.01 versus control negative group, **P < 0.01 versus down-regulation of Circ 0001434 group.
Discussion
Acute lung injury (ALI) is caused by various non-cardiac factors (including infection, sepsis, shock, poisoning, trauma, etc.), resulting in damage to pulmonary capillary endothelial cells and alveolar epithelial cells, and inflammatory cell infiltration to increase the permeability of the alveolar capillary barrier system, causing diffuse pulmonary edema [13]. In addition, a variety of inflammatory mediators and effector cells involved in ALI, with cascade inflammation, lead to acute hypoxic respiratory failure, which is mainly characterized by refractory hypoxia, and respiratory distress [3]. ALI is one of the common emergencies and severe disease, whose main pathophysiological changes are inflammatory reactions [14]. The failure to control the development of inflammation could lead to the progression of ALI into ARDS, and eventually multiple organ dysfunction [14]. In this study, we demonstrated that circ 0001434 expression was down-regulated in an ALI model. Down-regulation of Circ 0001434 accelerated inflammation in an in vitro model of ALI through miR-625-5p. Dong et al. showed that miR-625-5p may impact the pathogenesis of dust mite-induced pediatric asthma [15].
The role of inflammatory factors in the ALI process has always been a hot topic worldwide, which is also considered to be a link affecting the prognosis of lung injury [16]. The pathogenesis of ALI is complex, and it is difficult to completely explain it with a single cellular pathway [16]. Instead, ALI is often the result of multi-factor and multi-link interactions, and is the pulmonary manifestation of systemic inflammatory response [17]. The pathophysiological changes of ALI are associated with the release of a large number of pro-inflammatory mediators, causing the aggregation of inflammatory cells (such as giant cells and neutrophils) in the lung tissue [17]. Furthermore, we found that up-regulation of Circ 0001434 regulated signaling pathways, and induced Wnt/β-catenin protein expression, and suppressed the NF-κB signaling pathway in an ALI in vitro model. miR-625-5p regulates inflammation in an in vitro model of ALI through Wnt/β-catenin/NF-κB. Sánchez-Jiménez et al. showed that miR-625-5p regulated Wnt signaling pathways in transiently TIA-depleted HeLa cells by genome-wide profiling [18].
NF-κB is an important signaling molecule in intracellular signal transduction. Under normal conditions, it is located in the cytoplasm in a non-activated form [19]. When stimulated by physical and chemical factors, it is activated and translocated into the nucleus to induce the expression of cytokines (IL-8 and TNF-α) and adhesion molecules (ICAM-1 and VCAM-1) [5]. NF-кB, a transcription factor widely distributed in multiple cell types, can regulate the stress response of diverse cells, including immune responses, inflammatory responses, and transcription of genes involved in anti-apoptotic effects of cells [19]. The dysregulation of NF-кB activation is directly associated with multiple human disorders (such as rheumatoid arthritis and cancer) [20]. Therefore, the regulatory mechanism of NF-κB activation has always been a hot topic in the field of cellular biology and immunology. In the present study, our results showed that the miR-625-5p reduced the effect of Circ 0001434 on inflammation in the ALI in vitro model. Shen et al. suggested that activation of the TLR4/NF-κB signaling pathway induces pro-inflammatory cytokines and up-regulates the expression of miR-625-5p in a model of human intervertebral disc degeneration [21].
Wnt/β-catenin signaling pathway interacts with the TGF-β1/Smads signaling pathway at the ligand level, and there are also interactions of transcription factors at both cytoplasmic and nuclear levels [22]. With further clarification of the relationship between the two signaling pathways, the combined effect of blocking the regulation of fibrosis would inevitably show extensive application value in multiple fields [7,23]. Expression of Smad2, Smad3 and β-catenin was significantly increased in pathological scars [24]. Moreover, TGF-β1 could significantly up-regulate the expression of β-catenin protein during the TGF-β1-induced differentiation of normal fibroblasts into myofibroblasts [7,22,23]. In this study, our results showed that Wnt reduced the function of anti-Circ 0001434 on inflammation in the ALI in vitro model. Sánchez-Jiménez et al. showed that miR-625-5p regulates Wnt in transiently TIA-depleted HeLa cells by genome-wide profiling [18].
In conclusion, Circ 0001434 mediates ALI-induced lung inflammation through Wnt/β-catenin/NF-κB activation by NF-κB by miR-625-5p. Anti-Circ 0001434 might be an effective agent for the treatment of ALI through anti-inflammatory effects through suppression of NF-κB and activation of the Wnt/β-catenin signaling pathway.
Acknowledgements
This study was supported by Major projects of Natural Science Research in Colleges and Universities of Anhui Province (No. KJ2018ZD023); The Key Project of Top-Notch Talent of Discipline (specialty) of the Higher Education Institute of Anhui Province 2016 (No. gxbjZD2016072); Anhui Province Education Key Projects (No. SK2018A1069).
Disclosure of conflict of interest
None.
References
- 1.Zhang Z, Chen L, Ni H. The effectiveness of corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: a secondary analysis. Sci Rep. 2015;5:17654. doi: 10.1038/srep17654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hoeboer SH, Groeneveld AB, van der Heijden M, Oudemans-van Straaten HM. Serial inflammatory biomarkers of the severity, course and outcome of late onset acute respiratory distress syndrome in critically ill patients with or at risk for the syndrome after new-onset fever. Biomark Med. 2015;9:605–616. doi: 10.2217/bmm.15.15. [DOI] [PubMed] [Google Scholar]
- 3.Feiner JR, Gropper MA, Toy P, Lieberman J, Twiford J, Weiskopf RB. A clinical trial to detect subclinical transfusion-induced lung injury during surgery. Anesthesiology. 2015;123:126–135. doi: 10.1097/ALN.0000000000000689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shao L, Meng D, Yang F, Song H, Tang D. Irisin-mediated protective effect on LPS-induced acute lung injury via suppressing inflammation and apoptosis of alveolar epithelial cells. Biochem Biophys Res Commun. 2017;487:194–200. doi: 10.1016/j.bbrc.2017.04.020. [DOI] [PubMed] [Google Scholar]
- 5.Tian L, Li W, Wang T. Therapeutic effects of silibinin on LPS-induced acute lung injury by inhibiting NLRP3 and NF-kappaB signaling pathways. Microb Pathog. 2017;108:104–108. doi: 10.1016/j.micpath.2017.05.011. [DOI] [PubMed] [Google Scholar]
- 6.Zhu G, Xin X, Liu Y, Huang Y, Li K, Wu C. Geraniin attenuates LPS-induced acute lung injury via inhibiting NF-kappaB and activating Nrf2 signaling pathways. Oncotarget. 2017;8:22835–22841. doi: 10.18632/oncotarget.15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang J, Shao Y, He D, Zhang L, Xu G, Shen J. Evidence that bone marrow-derived mesenchymal stem cells reduce epithelial permeability following phosgene-induced acute lung injury via activation of wnt3a protein-induced canonical wnt/beta-catenin signaling. Inhal Toxicol. 2016;28:572–579. doi: 10.1080/08958378.2016.1228720. [DOI] [PubMed] [Google Scholar]
- 8.Li B, Zhang H, Zeng M, He W, Li M, Huang X, Deng DY, Wu J. Bone marrow mesenchymal stem cells protect alveolar macrophages from lipopolysaccharide-induced apoptosis partially by inhibiting the Wnt/beta-catenin pathway. Cell Biol Int. 2015;39:192–200. doi: 10.1002/cbin.10359. [DOI] [PubMed] [Google Scholar]
- 9.Kuncewitch M, Yang WL, Jacob A, Khader A, Giangola M, Nicastro J, Coppa GF, Wang P. Stimulation of Wnt/beta-catenin signaling pathway with Wnt agonist reduces organ injury after hemorrhagic shock. J Trauma Acute Care Surg. 2015;78:793–800. doi: 10.1097/TA.0000000000000566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guo ZL, Ren T, Xu L, Zhang L, Yin Q, Wang JC, Liang YJ. The microRNAs expression changes rapidly in mice lung tissue during lipopolysaccharide-induced acute lung injury. Chin Med J (Engl) 2013;126:181–183. [PubMed] [Google Scholar]
- 11.Rajasekaran S, Pattarayan D, Rajaguru P, Sudhakar Gandhi PS, Thimmulappa RK. MicroRNA regulation of acute lung injury and acute respiratory distress syndrome. J Cell Physiol. 2016;231:2097–2106. doi: 10.1002/jcp.25316. [DOI] [PubMed] [Google Scholar]
- 12.Li W, Qiu X, Jiang H, Han Y, Wei D, Liu J. Downregulation of miR-181a protects mice from LPS-induced acute lung injury by targeting Bcl-2. Biomed Pharmacother. 2016;84:1375–1382. doi: 10.1016/j.biopha.2016.10.065. [DOI] [PubMed] [Google Scholar]
- 13.Craig TR, Duffy MJ, Shyamsundar M, McDowell C, O’Kane CM, Elborn JS, McAuley DF. A randomized clinical trial of hydroxymethylglutaryl- coenzyme a reductase inhibition for acute lung injury (The HARP Study) Am J Respir Crit Care Med. 2011;183:620–626. doi: 10.1164/rccm.201003-0423OC. [DOI] [PubMed] [Google Scholar]
- 14.Shinke H, Masuda S, Togashi Y, Ikemi Y, Ozawa A, Sato T, Kim YH, Mishima M, Ichimura T, Bonventre JV, Matsubara K. Urinary kidney injury molecule-1 and monocyte chemotactic protein-1 are noninvasive biomarkers of cisplatin-induced nephrotoxicity in lung cancer patients. Cancer Chemother Pharmacol. 2015;76:989–996. doi: 10.1007/s00280-015-2880-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dong X, Xu M, Ren Z, Gu J, Lu M, Lu Q, Zhong N. Regulation of CBL and ESR1 expression by microRNA-223p, 513a-5p and 625-5p may impact the pathogenesis of dust mite-induced pediatric asthma. Int J Mol Med. 2016;38:446–456. doi: 10.3892/ijmm.2016.2634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li Y, Chi G, Shen B, Tian Y, Feng H. Isorhamnetin ameliorates LPS-induced inflammatory response through downregulation of NF-kappaB signaling. Inflammation. 2016;39:1291–1301. doi: 10.1007/s10753-016-0361-z. [DOI] [PubMed] [Google Scholar]
- 17.Li Y, Xiao J, Tan Y, Wang J, Zhang Y, Deng X, Luo Y. Inhibition of PKR ameliorates lipopolysaccharide-induced acute lung injury by suppressing NF-kappaB pathway in mice. Immunopharmacol Immunotoxicol. 2017;39:165–172. doi: 10.1080/08923973.2017.1303839. [DOI] [PubMed] [Google Scholar]
- 18.Sanchez-Jimenez C, Carrascoso I, Barrero J, Izquierdo JM. Identification of a set of miRNAs differentially expressed in transiently TIA-depleted HeLa cells by genome-wide profiling. BMC Mol Biol. 2013;14:4. doi: 10.1186/1471-2199-14-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang Z, Sun D, Yan Z, Reynolds AB, Christman JW, Minshall RD, Malik AB, Zhang Y, Hu G. Differential role for p120-catenin in regulation of TLR4 signaling in macrophages. J Immunol. 2014;193:1931–1941. doi: 10.4049/jimmunol.1302863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen T, Guo Q, Wang H, Zhang H, Wang C, Zhang P, Meng S, Li Y, Ji H, Yan T. Effects of esculetin on lipopolysaccharide (LPS)-induced acute lung injury via regulation of RhoA/Rho Kinase/NF-small ka, CyrillicB pathways in vivo and in vitro. Free Radic Res. 2015;49:1459–1468. doi: 10.3109/10715762.2015.1087643. [DOI] [PubMed] [Google Scholar]
- 21.Zhang H, Tan X, Yang D, Lu J, Liu B, Baiyun R, Zhang Z. Dietary luteolin attenuates chronic liver injury induced by mercuric chloride via the Nrf2/NF-kappaB/P53 signaling pathway in rats. Oncotarget. 2017;8:40982–40993. doi: 10.18632/oncotarget.17334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li Y, Li JS, Li WW, Li SY, Tian YG, Lu XF, Jiang SL, Wang Y. Long-term effects of three Tiao-Bu Fei-Shen therapies on NF-kappaB/TGF-beta1/smad2 signaling in rats with chronic obstructive pulmonary disease. BMC Complement Altern Med. 2014;14:140. doi: 10.1186/1472-6882-14-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu F, Nie C, Zhao N, Wang Y, Liu Y, Li Y, Zeng Z, Ding C, Shao Q, Qing C, Xia L, Peng Z, Qian K. MiR-155 alleviates septic lung injury by inducing autophagy via inhibition of transforming growth factor-beta-activated binding protein 2. Shock. 2017;48:61–68. doi: 10.1097/SHK.0000000000000839. [DOI] [PubMed] [Google Scholar]
- 24.Du S, Li H, Cui Y, Yang L, Wu J, Huang H, Chen Y, Huang W, Zhang R, Yang J, Chen D, Li Y, Zhang S, Zhou J, Wei Z, Chow NT. Houttuynia cordata inhibits lipopolysaccharide-induced rapid pulmonary fibrosis by up-regulating IFN-gamma and inhibiting the TGF-beta1/Smad pathway. Int Immunopharmacol. 2012;13:331–340. doi: 10.1016/j.intimp.2012.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]