Abstract
Aortic aneurysm (AA) is a complex and dangerous vascular disease, featuring progressive and irreversible vessel dilatation. AA is typically detected either by screening, or identified incidentally through imaging studies. To date, no effective pharmacological therapies have been identified for clinical AA management, and either endovascular repair or open surgery remains the only option capable of preventing aneurysm rupture. In recent years, multiple research groups have endeavored to both identify noncoding RNAs and to clarify their function in vascular diseases, including aneurysmal pathologies. Notably, the molecular roles of noncoding RNAs in AA development appear to vary significantly between thoracic aortic aneurysms (TAAs) and abdominal aortic aneurysms (AAAs). Some microRNAs (miRNA - a non-coding RNA subspecies) appear to contribute to AA pathophysiology, with some showing major potential for use as biomarkers or as therapeutic targets. Studies of long noncoding RNAs (lncRNAs) are more limited, and their specific contributions to disease development and progression largely remain unexplored. This review aims to summarize and discuss the most current data on lncRNAs and their mediation of AA pathophysiology.
Keywords: Long noncoding RNA, Aortic aneurysm, Smooth muscle cells, Vascular disease, Non-coding RNA
Graphical abstract
Highlights
-
•
This current review covers studies that have identified long non-coding RNAs in aortic aneurysm development and progression.
-
•
We separately discuss transcripts and mechanisms of importance to thoracic as well as abdominal aortic aneurysms.
-
•
Functional data on lncRNAs being identified are highlighted.
-
•
Some have been studied in human as well as experimental models of the disease pathology.
1. Aortic aneurysm disease
A true aneurysm is defined as a permanent dilatation of the vessel wall, involving all layers. Enlargement >1.5 times the expected diameter is considered an aneurysm [1]. Close to 90% of aortic aneurysms (AAs) are found in the abdominal aorta, where the typical clinical diameter threshold for an aneurysm is 30 mm, although individual anatomy may vary, and women can have smaller baseline diameters [2]. The aneurysmal size criteria in thoracic aorta differ somewhat from those in abdominal aorta, and are dependent on the site of the lesion [3,4]. The prevalence of abdominal AA (AAA) is lower than 4% for people (≥65 years old) in Europe [5]. In contrast, the incidence of thoracic AA (TAA) is about 7.6 per 100,000, with familial TAA accounting for 21.5% of cases [6,7]. As most aortic aneurysms remain asymptomatic until they rupture or dissect, they are typically detected either by targeted screening, or by chance when imaging is conducted for other reasons [8,9]. (see Table 1)
Table 1.
Long non-coding RNAs in thoracic aortic aneurysms (TAA) and abdominal aortic aneurysms (AAA).
| AA | lncRNA | Regulation | Targets | Functions | References |
|---|---|---|---|---|---|
| TAA | HIF1A-AS1 | ↑ | Casp3/8, BRG1, BCL-2 | Promotes VSMC apoptosis and inhibits proliferation | [32,33] |
| LincRNA-p21 | ↑ | p53, p300, hnRNP-K, TGFβ pathway | Promotes VSMC apoptosis and inhibits proliferation | [[34], [35], [36], [37]] | |
| MALAT1 | ↑ | HDAC9, BRG1 | Mediates cellular phenotypes; promotes EC proliferation | [38,39] | |
| HOTAIR | ↓ | Collagen types I/III | Knockdown of HOTAIR promotes VSMC apoptosis and inhibits proliferation; mediates ECM remodeling | [40] | |
| MIAT | ↑ | BCL-2, BCL-xl, miR-145, miR-150-5p | Promotes VSMC proliferation and inhibits apoptosis promotes EC proliferation | [41,42] | |
| AK056155 | ↑ | – | Involved in Loeys-Dietz syndrome | [43] | |
| AAA | H19 | ↑ | miR-675, HIF1α, miR-148b, let-7a, MCP-1, Sp1, | Generates miRNA; promotes VSMC proliferation; induces VSMC apoptosis; promotes vascular inflammation | [[49], [50], [51], [52], [53]] |
| PVT1 | ↑ | MMP-2/9, | Overexpression of PVT1 promotes apoptosis and phenotype switching | [55] | |
| GAS5 | ↓ | TGFβ/SMAD3 signaling; miR-223; | VSMC differentiation; knockdown of GAS5 inhibits endothelial progenitor cells proliferation and promotes senescence | [[56], [57], [58]] | |
| SENCR | ↑ | FIL1, CKAP4 | Mediates cellular phenotypes, migration, EC adherens junctions | [[59], [60], [61]] | |
| MYOSLID | – | MKL1/SRF, TGFβ-1/SMAD pathways | VSMC phenotypes; promotes VSMC differentiation and attenuates proliferation | [62] | |
| SMILR | ↑ | HAS2 | Knockdown of SMILR reduces VSMC proliferation | [63] | |
| NEAT | ↑ | WDR5 | VSMC phenotypes; promotes VSMC proliferation and migration | [64] | |
| Lnc-ang362 | ↑ | miR-221/222 | Promotes proliferation | [65] |
HIF1A-AS1, hypoxia-inducible factor 1α-antisense RNA 1; Casp3/8, caspase-3 and caspase-8; BRG1, Brahma-related gene 1; BCL-2, B cell lymphoma 2; VSMC, vascular smooth muscle cell; LincRNA-p21, long intergenic noncoding RNA-p21; hnRNP-K, heterogeneous nuclear ribonucleoprotein K; MALAT1, metastasis associated lung adenocarcinoma transcript 1; HDAC9, histone deacetylase 9; BRG1, Brahma-related gene 1; HOTAIR, HOX transcript antisense intergenic RNA; ECM, extracellular matrix; MIAT, myocardial infarction associated transcript; BCL-xl, B cell lymphoma extra large; EC, endothelial cell; H19, H19 imprinted maternally expressed transcript; MCP-1, macrophage chemoattractant protein-1; Sp1, specificity protein 1; PVT1, plasmacytoma variant translocation 1; MMP-2/9, metalloproteinase 2/9; GAS5, growth arrest–specific 5; SMAD3, SMAD family member 3; SENCR, smooth muscle and endothelial cell–enriched migration/differentiation-associated lncRNA; FIL1, friend Leukemia Integration virus 1; CKAP4, cytoskeletal-associated protein 4; MYOSLID, myocardin-induced smooth muscle lncRNA, inducer of differentiation; MKL1, MYOCD-related transcription factor A; SRF, serum response factor; SMILR, SMC-enriched long noncoding RNA; HAS2, hyaluronan synthase 2; NEAT, nuclear paraspeckle assembly transcript 1; WDR5, WD Repeat Domain 5.
While established medical treatments, such as statins, antiplatelet therapy and beta-blockers are able to reduce overall cardiovascular risk in AA patients, they only have little impact (angiotensin receptor and beta blockers in TAA) on rupture risk or aneurysm growth [8]. Currently, the main methods to prevent AA dissection or rupture include classic open surgical repair (OSR), and the more recent developed endovascular aortic repair (EVAR). In AAA, some studies show that EVAR is superior to OSR in terms of perioperative mortality and morbidity [10,11]. These benefits are largely lost in the long term, however, as patients treated endovascularly require more reinterventions and have a higher risk of secondary AAA rupture [12,13]. In TAA, while the rates of paraplegia and stroke with EVAR appear lower than those with OSR, other complications such as endoleaks raise concerns, particularly in the case of connective tissue syndromes where EVAR appears insufficient [4,14]. Further, many patients may not be surgical candidates due to co-morbidities. Accordingly, pharmacological agents capable of targeting AAs have been actively pursued for some time.
2. Non-coding RNAs
Non-coding RNAs (ncRNAs) are transcripts that are not translated into proteins. Over the past two decades, ncRNAs have experienced a dramatic shift. Initially perceived as “junk”, they are now thought of as significant regulators of both physiological and pathological processes. Several types of ncRNAs exist. The most common classification scheme is based on nucleotide (nt) length: small ncRNAs (<200 nt; including microRNAs-miRNAs) and long ncRNAs (lncRNAs; > 200 nt). MiRNAs have been extensively studied over the past 15 years. Their roles in regulating fundamental mechanisms of vascular disease development in general, and AA in particular, have suggested them as both promising therapeutic targets, and measurable biomarkers of disease progression [1,15,16]. Several miRNA-based clinical trials have been initiated [17,18]. In the realm of cardiovascular disease, antisense oligonucleotides (so called anti-miRs) and miRNA mimics that influence miR-21 and miR-29b expression [[19], [20], [21]] are of great interest as suitable therapeutic agents.
Compared with miRNAs, lncRNAs are globally less conserved across species. They have been shown to regulate the genome in a variety of ways at the transcriptional and post-transcriptional level. Functionally, lncRNAs can serve as decoys, mimicking transcription factor binding sites to bind to transcription factors. They can capture miRNAs to suppress target gene expression, compete with endogenous RNA molecules, or act as guides across intracellular compartments, among other functions [22,23]. LncRNAs can be further sub-classified into different categories according their regulatory functions and locations within the genome, or by biogenesis pathways, as well as by subcellular localization [[24], [25], [26]]. Recent advances in deep sequencing technologies have permitted more frequent discovery and annotation of novel lncRNAs. However, a detailed understanding of their specific functions and roles during disease development is mostly lacking. Here, we review recent studies that have identified dysregulated lncRNAs in TAA and AAA, and discuss to what extent these transcripts appear to be mechanistically involved in disease progression. Future studies are required to determine whether lncRNAs can be employed as therapeutic targets and/or biomarkers in treating AAs.
3. Role of lncRNAs in thoracic aortic aneurysm (TAA)
Some TAAs have common pathological features with AAA. However, unlike the vast majority of AAAs, TAAs are more typically associated with specific genetic factors, and can clinically manifest as part of syndromic connective tissue disorders, such as Marfan Syndrome, Ehlers-Danlos, and Loeys-Dietz Syndrome. Mutations in genes which encode for members of the TGF-β (Transforming growth factor-beta) pathway, components of the vascular smooth muscle cell (VSMC) actin-myosin cytoskeleton and contractile apparatus, and elements of the extracellular matrix (ECM) have been associated with TAA pathogenesis [27]. Dysfunctional endothelial cells (ECs) displaying abnormalities in signaling pathways and proteinases, such as Notch signaling, ADAM17 (a disintegrin and metalloproteinase-17), and endothelial nitric oxide synthetic signaling, are also found in TAA [[28], [29], [30]]. Relatively few lncRNAs have so far been studied with a proven biological relevance in TAA. These are summarized below.
3.1. HIF1A-AS1
Long noncoding-RNA HIF1α-AS1 (hypoxia-inducible factor 1α-antisense RNA 1) is involved in cancer development [31], but also in TAA pathogenesis. Elevated serum levels of HIF1α-AS1 were found in patients with TAA [32]. Knock-down of HIF1α-AS1 suppressed palmitic acid (PA)-induced apoptosis in VSMCs of TAA in vitro, and decreased expression levels of caspase-3 and caspase-8, while increasing the expression of the anti-apoptotic marker gene Bcl-2 (B cell lymphoma 2) [32]. Interactions between HIF1α-AS1 and BRG1 (Brahma-related gene 1) appeared to be important in TAA pathogenesis [33]. HIF1α-AS1 was positively regulated by BRG1, a major chromatin remodeling protein involved in vascular development. BRG1 was also found to be overexpressed in VSMCs of patients with TAA, promoting apoptosis and inhibiting proliferation through HIF1α-AS1. Evidence clarifying the contribution of the HIF1α-AS1/BRG1 axis to TAA disease in animal models is currently lacking.
3.2. LincRNA-p21
Another upregulated lncRNA described in TAAs is lincRNA-p21 (long intergenic noncoding RNA-p21)34, a transcriptional target of p53, playing a pivotal role in atherosclerosis [35]. LincRNA-p21 can enhance p53 transcriptional activity through positive feedback, repressing cell proliferation while inducing apoptosis in VSMCs [35]. LincRNA-p21 can also directly bind to MDM2 (Mouse double minute 2), a ubiquitin E3 ligase. Notably, decreasing the association of MDM2 and p53 triggers a p53-p300 interaction, leading to additional p53 histone acetylation and enhancing its transcription [35]. A second mechanism of action was through physical association with hnRNP-K (Heterogeneous nuclear ribonucleoprotein K), known to play various roles in the activation of the p53 pathway [36]. LincRNA-p21 interacted with hnRNP-K and modulated its localization, inducing p53-mediated apoptosis [36].
Recently an in vitro study indicated that overexpression of lincRNA-p21 can also promote apoptosis and inhibit proliferation in VSMCs of patients with TAA through the TGFβ-1 signaling pathway [37]. This work also showed that lincRNA-p21 was a sensitive (bio)marker in serum and aortic media of TAA patients. However, specificity issues remain a concern for lincRNA-p21, as changes in its expression have been linked to several diseases [37].
3.3. MALAT1
A ternary complex that mediates a critical mutual epigenetic pathway for VSMC dysfunction in TAA was discovered recently [38]. The lncRNA MALAT1 (Metastasis associated lung adenocarcinoma transcript 1) and BRG1 together formed a chromatin remodeling complex. HDAC9 (Histone deacetylase 9) was then recruited, forming a ternary complex [38]. The HDAC9-MALAT1-BRG1 complex can mediate cellular phenotypes by repressing expression of contractile protein-coding genes. Silencing the expression of MALAT1 can induce instability of the ternary complex and inhibit its nuclear co-localization with HDAC9, preventing phenotypic switching [38].
In addition, MALAT1 has also been found to regulate endothelial cell fate [39]. In vivo studies showed that genetic deletion of MALAT1 can inhibit proliferation of ECs in C57BL/6 mice [39]. However, the role of MALAT1 in the endothelium of TAAs remains to be explored.
3.4. HOTAIR
HOTAIR (HOX transcript antisense intergenic RNA) is an antisense lncRNA previously well studied in cancer, and also found to be decreased in TAA. A lncRNA:mRNA analysis revealed that HOTAIR levels correlated with the expression of several genes (matrix metalloproteinase-8,MMP8; keratocan, KERA; collagen and calcium binding EGF domains 1, CCBE1) that were up-regulated in the ECM of ascending aortic specimens of patients with sporadic TAA [40]. In vitro experiments further revealed that collagen type I and III were down-regulated upon knockdown of HOTAIR [40]. Lastly, knockdown of HOTAIR was found to further accelerate apoptosis in VSMCs from patients with sporadic TAAs [40].
3.5. MIAT
LncRNA MIAT (Myocardial infarction associated transcript) has been shown to function as a competing endogenous RNA in diabetes–induced microvascular dysfunction by sponging miR-150-5p, relieving the inhibitory effect of miR-150-5p on VEGF (Vascular endothelial growth factor) expression [41]. Knockdown of MIAT has also been shown to inhibit EC proliferation [41].
One recent study in TAA revealed that expression of MIAT was up-regulated in human VSMCs, and promoted the expression of Bcl-2 and Bcl-xl (B-cell lymphoma extra large), two proteins with anti-apoptotic properties [42]. Moreover, in vitro studies showed that MIAT can downregulate miR-145 through the PI3K/Akt (Phosphoinositide-3-kinase/Akt) pathway, promoting VSMC proliferation and inhibiting apoptosis upon activation [42].
3.6. AK056155
Another lncRNA potentially involved in TAA development is AK056155. Yu et al. [43] found that AK056155 was elevated in the circulation of patients with Loeys-Dietz Syndrome, and proposed ECs as the most likely source. They further revealed that the expression of AK056155 was regulated by TGFβ-1 via the PI3K/AKT pathway in HUVECs (Human umbilical vein endothelial cells).
4. Role of lncRNAs in abdominal aortic aneurysm (AAA)
The current understanding of the pathogenesis of AAA is largely based on studies of aneurysmal aortic tissue obtained during open AAA surgeries. As these interventions are usually conducted at advanced stages of the disease, minimal information about the initial stimuli or the early phases of development are available for humans. Questions remain as to whether the observed late-process changes are causal or a consequence of the disease [44].
Multiple vascular cell subtypes (ECs, SMCs and adventitial cells) are involved in the process of AAA formation [45], highlighted in Fig. 1. Dysfunctional ECs can trigger inflammation in the media and adventitia [46]. Also observed is a chronic T-cell-stimulated inflammatory reaction, which leads to proteolytic remodeling via macrophages and matrix metalloproteinases (MMPs), and thus to a structural weakening of the ECM. This is associated with a loss of elastic fibers, structural changes in the collagen fibers, and sprouting of new vessels (angiogenesis). In addition phenotypic switching, in which VSMC convert from a contractile/differentiated state into a synthetic/de-differentiated one, is involved in the process of AAA formation. VSMCs, the main cellular component of the aortic wall, de-differentiate and proliferate, initiating various signaling cascades that trigger further de-differentiation, proliferation, migration, and apoptosis. These processes are at least partly regulated by lncRNAs [47] as indicated in Fig. 2. Oxidative stress also seems to play an important role in developing aneurysms, as it activates MMPs, which subsequently degrade collagen fibers in the arterial wall [48]. The following section will highlight studies identifying lncRNAs that are involved in the above-mentioned mechanisms.
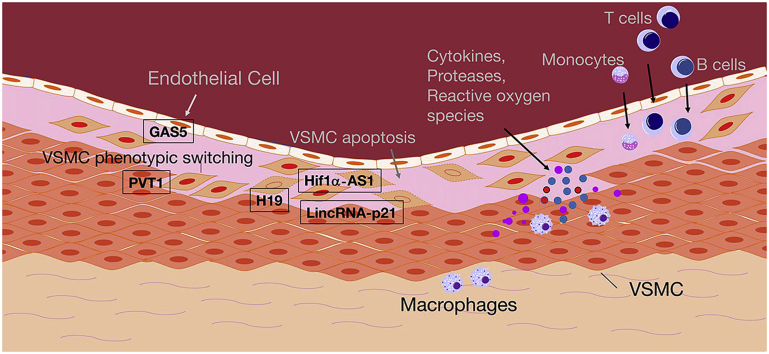
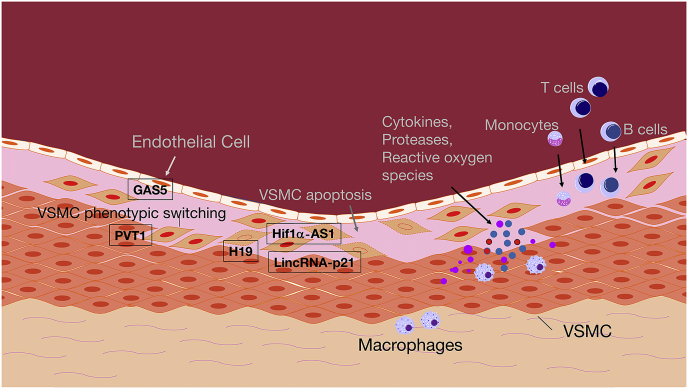
Fig. 1.
Schematic illustration of cellular processes involved in AAA formation. Inflammatory processes are mediated in a developing AA through T cells, monocytes, macrophages, and B cells, which all can infiltrate the media and adventitia of the aorta and release mediators that trigger the disease process (cytokines, proteases, reactive oxygen species, among others). Endothelial dysfunction, loss of VSMC contractility, phenotypic switching and apoptosis, as well as ECM degradation, further contribute to aneurysm formation. Few long non-coding RNAs with functional relevance in AA expansion have been described. H19, Hif1a-AS1 (hypoxia-inducible factor 1 alpha), and lincRNA-p21 regulate apoptosis in VSMCs, while PVT1 (plasmacytoma variant translocation 1) and GAS5 (growth arrest-specific 5) are involved in phenotypic switching of VSMCs and endothelial dysfunction. For further details please refer to the running text. AA: aortic aneurysm; VSMC: vascular smooth muscle cell; ECM: extracellular matrix.
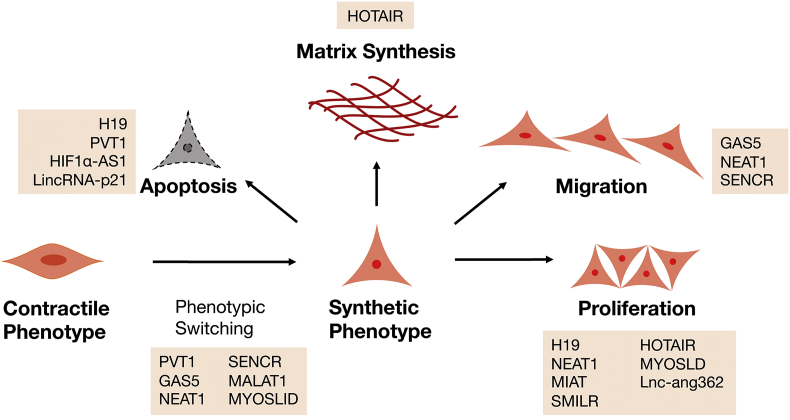
Fig. 2.
Schematic overview of long non-coding RNAs involved in smooth muscle cell dynamics during the development of AA. Mechanisms that alter the phenotype of vascular smooth muscle cells (VSMCs), or mediate their ability to migrate, proliferate, produce extracellular matrix components and essentially become apoptotic, are of great importance in AA formation. Various long non-coding RNAs have been shown to crucially regulate these processes.
4.1. H19
H19 (H19 imprinted maternally expressed transcript), one of the very first identified eukaryote lncRNAs, is highly expressed and well-conserved across mammals in embryonic and prenatal stages [25]. H19 seems to play diverse roles in vascular diseases [[49], [50], [51], [52], [53]]. It was discovered to stimulate VSMC proliferation in vitro, and H19-derived miR-675 was noted to directly target PTEN (Phosphatase and tensin homolog), a key regulator in the phenotypic transition of VSMCs [21,50]. However, as shown in our recent study in AAA [51], H19-mediated effects on VSMCs can occur in a miR-675-independent manner.
H19 has now been identified as having a key role in AAA development and progression, regulating VSMC apoptosis and aortic inflammation. H19 was upregulated in two independent murine AAA models (Angiotensin II and porcine pancreatic elastase - PPE), as well as aneurysmal human tissue specimens. Knockdown of H19 using locked nucleic acid (LNA)-GapmeRs repressed aneurysm growth in both of the murine AAA models. On the cellular level, VSMC apoptosis was induced by H19 via HIF1α. H19 promoted HIF1α expression by binding to the promoter region and recruiting the transcription factor Sp1 (Specificity protein 1) into the nucleus. In the cytoplasm, H19 retained HIF1α, which was then able to interact with MDM2 and mediate MDM2-p53 activity. Another potential pathway connecting H19 with VSMC apoptosis was described by Zhang et al. [49]. In their study, H19 directly interacted with and inhibited miR-148b expression, regulating the Wnt/β-catenin pathway in oxidized low-density lipoprotein-stimulated human aortic VSMCs.
Sun et al. have studied the role of H19 in regulating aortic inflammation during AAA formation [53]. They confirmed increased expression of H19 in two mouse AAA models (Angiotensin II and CaCl2-induced), as well as aneurysmal human tissue samples. In vitro and in vivo studies revealed that H19 can increase aortic IL-6 (Interleukin 6) levels by sponging let-7a in VSMCs and macrophages in Angiotensin–II–induced AAA (using C57BL/6 J mice). H19 also increased levels of MCP-1 (Macrophage chemoattractant protein-1), which enhanced macrophage infiltration. In ECs, H19 was repressed by aging, and was able to regulate inflammatory activation by inhibiting STAT3 (signal transducer and activator of transcription 3) signaling [54].
Taken together, these various publications suggest that H19 is central to the development and progression of AAA (see Fig. 3), making it a potential therapeutic target for future studies.
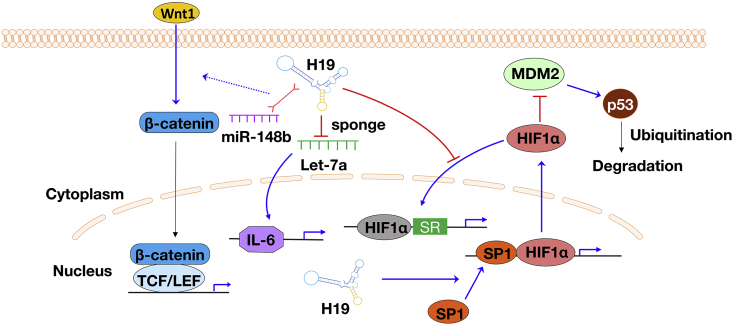
Fig. 3.
Schematic illustration of several pathways that H19 involved in the progression and development of AAA. SR: stress response; IL-6: interleukin-6; Wnt1: Wnt family number 1; HiF1α, hypoxia-inducible factor 1 alpha; SP1: specificity protein 1; MDM2: mouse double minute 2; TCF: T cell factor; LEF: lymphoid enhancer-binding factor; let-7a: microRNA let-7a.
4.2. PVT1
Zhang et al. [55] found that lncRNA PVT1 (Plasmacytoma variant translocation 1) was involved in the murine ApoE−/− Angiotensin-II induced AAA model. PVT1 upregulation was also confirmed in diseased human patient AAA tissue samples. Overexpression of PVT1 promoted the disease-triggering effects of Angiotensin-II on cultured VSMCs, facilitating apoptosis, elevating MMP-2 and MMP-9, and promoting VSMC phenotypic switching. Knockdown of PVT1 reversed these effects. PVT1 may function through sponging miRNAs in this process, however, further investigation will be required to elucidate mechanistic details.
4.3. GAS5
LncRNA GAS5 (Growth arrest–specific 5), a critical regulator in hypertension-induced vascular remodeling [56], is another potential lncRNA that has been linked to AAA. LncRNA GAS5 can negatively regulate TGFβ/SMAD3 (SMAD family member 3) signaling, known to play a critical role in VSMC differentiation [57]. SMAD3 protein was competitively bound by GAS5 via the RNA SMAD–binding elements, hindering it from binding to the SMAD–binding elements in the SM22α (smooth muscle protein 22-alpha) promoter [57]. Further, GAS5 may also sponge miR-223, decreasing the inhibitory effects of miR-223 on NAMPT (Nicotinamide phosphoribosyl-transferase), whose overexpression can reduce endothelial progenitor cell senescence [58].
4.4. SENCR
As a human vascular cell-enriched lncRNA, SENCR (Smooth muscle and Endothelial cell–enriched migration/differentiation-associated lncRNA) serves as a cis-acting element, acting in the cytoplasm [59]. Bell et al. [59] performed RNA sequencing in human coronary artery SMCs after SENCR silencing, and showed reduction in expression of VSMC contractile genes including MYOCD (Myocardin), while genes associated with VSMC migration were increased.
SENCR also correlated with the expression of the FIL1 (Friend Leukemia Integration virus 1) gene [59], a crucial regulator in EC function and specification. SENCR positively regulated angiogenic processes in HUVECs [60], and was further found to be induced by laminar shear stress in human ECs [61]. Knockdown of SENCR displaced CKAP4 (Cytoskeletal-associated protein 4), which then bound CDH5 (Cadherin 5), destabilizing the CDH5/CTNND1 (Catenin delta 1) complex and perturbing EC adherens junctions [61]. SENCR has not yet been directly linked to AA disease, but the proposed mechanisms in SMCs and ECs make it an intriguing lncRNA for further exploration in disease-relevant models.
4.5. MYOSLID
Another lncRNA involved in phenotypic switching is the VSMC-specific lncRNA MYOSLID (myocardin-induced smooth muscle lncRNA, inducer of differentiation), which promotes VSMC differentiation and attenuates proliferation [62]. MYOSLID may exert its function through two parallel pathways: MKL1 (MYOCD-related transcription factor A)/SRF (Serum response factor), and TGFβ-1/SMAD. Depletion of MYOSLID influenced F-actin assembly and then blocked MKL1 nuclear shuttling, which led to a down-regulation of VSMC contractile genes. MYOSLID also interacted with TGFβ-1/SMAD pathways in a positive feedback fashion. Though SENCR silencing can decrease expression of MYOCD, this effect was not observed on MYOSLID. Further, loss of MYOSLID did not alter SENCR expression.
4.6. SMILR
Ballantyne et al. [63] identified SMILR (SMC-enriched long noncoding RNA) through RNA sequencing in human saphenous SMCs treated with IL-1α (Interleukin-1α) and PDGF (Platelet-derived growth factor). Knockdown of SMILR reduced VSMC proliferation and decreased expression of HAS2 (Hyaluronan synthase 2). The protein-coding gene HAS2, is in close proximity to SMILR, and encodes an enzyme that synthesizes a component of the ECM, hyaluronic acid. SMILR may regulate HAS2 expression by acting as an enhancer or scaffold. This too needs to be further clarified as regards its contribution to AA disease.
4.7. NEAT1
The role of lncRNA NEAT1 (nuclear paraspeckle assembly transcript 1) in VSMC phenotypic switching was recently discovered by Ahmed et al. [64]. Both in vivo and in vitro studies showed increased levels of NEAT1 expression during phenotypic modulation in VSMCs. Functional tests revealed that NEAT1 can promote VSMC proliferation and migration. NEAT1 also decreased expression of SMC-specific contractile genes by binding WDR5 (WD Repeat Domain 5), a chromatin modifier, initiating an epigenetic ‘off-status’.
4.8. Lnc-Ang 362
An angiotensin II-upregulated lncRNA, lnc-Ang 362, was detected in rat VSMCs, where it served as a host transcript for miR-221/22,265. Knockdown of lnc-Ang 362 reduced the expression of Mcm7 (minichromosome maintenance complex component 7), a critical regulator during cell cycle progression. Lnc-Ang 362 inhibition can further attenuate proliferation of VSMCs, suggesting a functional role for this lncRNA in Angiotensin II-associated vascular diseases (like AAAs).
5. Summary and perspectives
AAs are “silent killers” for which current clinical diagnostic and therapeutic methods are still limited. New pharmacological approaches to slow aneurysm progression and limit the risk of (mostly fatal) acute ruptures are urgently needed. During the past decades, studies have accumulated demonstrating that ncRNAs, particularly microRNAs, serve as key regulators in the development and progression of AAs, including both TAAs and AAAs despite their differing mechanisms. MiRNAs have been studied in both animal models and human samples, with some having achieved clinical trial status to evaluate them as biomarkers or therapeutic targets.
Although some lncRNAs have been described as dysregulated in models or human tissue AA specimens, comparatively few studies exist to date that have established their functional roles in AA disease. The cellular specificity of lncRNAs is an intriguing feature of this ncRNA subclass, making them of particular interest for AA. Single cell RNA sequencing may assist in this regard, enabling transcript identification under high resolution conditions. Additional efforts are underway to identify other subclasses of ncRNAs that might have potential relevance in AA, but that remain largely unstudied (such as circular RNA). Future challenges include the establishment of optimized lncRNA modulation and targeting strategies to form the basis for future clinical trials, and more thoroughly performed in vivo studies.
Financial support
Zhiyuan Wu is supported by China Scholarship Council (201806210071). Lars Maegdefessel is funded through the German Center for Cardiovascular Research (DZHK), the European Research Council (ERC 679777 Starting Grant NORVAS), the Swedish Research Council (Vetenkapsrådet 2019-01577_3), and the Swedish Heart-Lung-Foundation (HLF 20180680). Lars Maegdefessel and Reinier A. Boon are Co-Principal Investigators in the DFG-sponsored TRR267 on ‘non-coding RNAs in the cardiovascular system’.
Declaration of competing interest
The authors declared they do not have anything to disclose regarding conflict of interest with respect to this manuscript.
References
- 1.Li Y., Maegdefessel L. Non-coding RNA contribution to thoracic and abdominal aortic aneurysm disease development and progression. Front. Physiol. 2017;8:429. doi: 10.3389/fphys.2017.00429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sakalihasan N., Michel J.-B., Katsargyris A. Abdominal aortic aneurysms. Nat. Rev. Dis. Prim. 2018;4:34. doi: 10.1038/s41572-018-0030-7. [DOI] [PubMed] [Google Scholar]
- 3.Czerny M., Schmidli J., Adler S. Current options and recommendations for the treatment of thoracic aortic pathologies involving the aortic arch: an expert consensus document of the European Association for Cardio-Thoracic surgery (EACTS) and the European Society for Vascular Surgery (ESVS) Eur. J. Cardiothorac. Surg. 2019;55:133–162. doi: 10.1093/ejcts/ezy313. [DOI] [PubMed] [Google Scholar]
- 4.Erbel R., Aboyans V., Boileau C. ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC) Eur. Heart J. 2014;35:2873–2926. doi: 10.1093/eurheartj/ehu281. 2014. [DOI] [PubMed] [Google Scholar]
- 5.Sprynger M., Willems M., Van Damme H. Screening program of abdominal aortic aneurysm. Angiology. 2019;70:407–413. doi: 10.1177/0003319718824940. [DOI] [PubMed] [Google Scholar]
- 6.McClure R.S., Brogly S.B., Lajkosz K. Epidemiology and management of thoracic aortic dissections and thoracic aortic aneurysms in Ontario, Canada: a population-based study. J. Thorac. Cardiovasc. Surg. 2018;155:2254–2264. doi: 10.1016/j.jtcvs.2017.11.105. e2254. [DOI] [PubMed] [Google Scholar]
- 7.Albornoz G., Coady M.A., Roberts M. Familial thoracic aortic aneurysms and dissections--incidence, modes of inheritance, and phenotypic patterns. Ann. Thorac. Surg. 2006;82:1400–1405. doi: 10.1016/j.athoracsur.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 8.Wanhainen A., Verzini F., Van Herzeele I. Editor's choice - European society for vascular surgery (ESVS) 2019 clinical practice guidelines on the management of abdominal aorto-iliac artery aneurysms. Eur. J. Vasc. Endovasc. Surg. 2019;57:8–93. doi: 10.1016/j.ejvs.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 9.Chaikof E.L., Dalman R.L., Eskandari M.K. The Society for Vascular Surgery practice guidelines on the care of patients with an abdominal aortic aneurysm. J. Vasc. Surg. 2018;67:2–77. doi: 10.1016/j.jvs.2017.10.044. e72. [DOI] [PubMed] [Google Scholar]
- 10.Powell J.T., Sweeting M.J., Ulug P. Meta-analysis of individual-patient data from EVAR-1, DREAM, OVER and ACE trials comparing outcomes of endovascular or open repair for abdominal aortic aneurysm over 5 years. Br. J. Surg. 2017;104:166–178. doi: 10.1002/bjs.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trenner M., Haller B., Storck M. Trends in patient safety of intact abdominal aortic aneurysm repair - German registry data on 36.594 procedures European journal of vascular and endovascular surgery. Off. J. Eur. Soc. Vasc. Surg. 2017;53:641–647. doi: 10.1016/j.ejvs.2016.12.024. [DOI] [PubMed] [Google Scholar]
- 12.Patel R., Sweeting M.J., Powell J.T. Endovascular versus open repair of abdominal aortic aneurysm in 15-years' follow-up of the UK endovascular aneurysm repair trial 1 (EVAR trial 1): a randomised controlled trial. Lancet. 2016;388:2366–2374. doi: 10.1016/S0140-6736(16)31135-7. [DOI] [PubMed] [Google Scholar]
- 13.Chen Z.G., Tan S.P., Diao Y.P. The long-term outcomes of open and endovascular repair for abdominal aortic aneurysm: a meta-analysis. Asian J. Surg. 2019;42:899–906. doi: 10.1016/j.asjsur.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 14.Parisi R., Secco G.G., Di Eusanio M. Endovascular repair of aortic dissection in marfan syndrome: current status and future perspectives. Diseases. 2015;3:159–166. doi: 10.3390/diseases3030159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumar S., Boon R.A., Maegdefessel L. Role of noncoding RNAs in the pathogenesis of abdominal aortic aneurysm. Circ. Res. 2019;124:619–630. doi: 10.1161/CIRCRESAHA.118.312438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Busch A., Eken S.M., Maegdefessel L. Prospective and therapeutic screening value of non-coding RNA as biomarkers in cardiovascular disease. Ann. Transl. Med. 2016;4:236. doi: 10.21037/atm.2016.06.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rupaimoole R., Slack F.J. MicroRNA therapeutics: towards a new era for the management of cancer and other diseases. Nat. Rev. Drug Discov. 2017;16:203–222. doi: 10.1038/nrd.2016.246. [DOI] [PubMed] [Google Scholar]
- 18.Janssen H.L., Reesink H.W., Lawitz E.J. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 19.Maegdefessel L., Spin J.M., Raaz U. miR-24 limits aortic vascular inflammation and murine abdominal aneurysm development. Nat. Commun. 2014;5:5214. doi: 10.1038/ncomms6214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maegdefessel L., Azuma J., Toh R. Inhibition of microRNA-29b reduces murine abdominal aortic aneurysm development. J. Clin. Investig. 2012;122:497–506. doi: 10.1172/JCI61598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maegdefessel L., Azuma J., Toh R. MicroRNA-21 blocks abdominal aortic aneurysm development and nicotine-augmented expansion. Sci. Transl. Med. 2012;4:122ra122. doi: 10.1126/scitranslmed.3003441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boon R.A., Jae N., Holdt L. Long noncoding RNAs: from clinical genetics to therapeutic targets? J. Am. Coll. Cardiol. 2016;67:1214–1226. doi: 10.1016/j.jacc.2015.12.051. [DOI] [PubMed] [Google Scholar]
- 23.Leeper N.J., Maegdefessel L. Non-coding RNAs: key regulators of smooth muscle cell fate in vascular disease. Cardiovasc. Res. 2018;114:611–621. doi: 10.1093/cvr/cvx249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.St Laurent G., Wahlestedt C., Kapranov P. The Landscape of long noncoding RNA classification. Trends Genet. 2015;31:239–251. doi: 10.1016/j.tig.2015.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarroux J., Morillon A., Pinskaya M. History, Discovery, and classification of lncRNAs. Adv. Exp. Med. Biol. 2017;1008:1–46. doi: 10.1007/978-981-10-5203-3_1. [DOI] [PubMed] [Google Scholar]
- 26.Ma L., Bajic V.B., Zhang Z. On the classification of long non-coding RNAs. RNA Biol. 2013;10:925–933. doi: 10.4161/rna.24604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isselbacher E.M., Lino Cardenas C.L., Lindsay M.E. Hereditary influence in thoracic aortic aneurysm and dissection. Circulation. 2016;133:2516–2528. doi: 10.1161/CIRCULATIONAHA.116.009762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kostina A., Bjork H., Ignatieva E. Notch, BMP and WNT/beta-catenin network is impaired in endothelial cells of the patients with thoracic aortic aneurysm. Atherosclerosis Suppl. 2018;35:e6–e13. doi: 10.1016/j.atherosclerosissup.2018.08.002. [DOI] [PubMed] [Google Scholar]
- 29.Shen M., Hu M., Fedak P.W.M. Cell-specific functions of ADAM17 regulate the progression of thoracic aortic aneurysm. Circ. Res. 2018;123:372–388. doi: 10.1161/CIRCRESAHA.118.313181. [DOI] [PubMed] [Google Scholar]
- 30.van de Pol V., Kurakula K., DeRuiter M.C. Thoracic aortic aneurysm development in patients with bicuspid aortic valve: what is the role of endothelial cells? Front. Physiol. 2017;8:938. doi: 10.3389/fphys.2017.00938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gong W., Tian M., Qiu H. Elevated serum level of lncRNA-HIF1A-AS1 as a novel diagnostic predictor for worse prognosis in colorectal carcinoma. Cancer Biomark. 2017;20:417–424. doi: 10.3233/CBM-170179. [DOI] [PubMed] [Google Scholar]
- 32.Zhao Y., Feng G., Wang Y. Regulation of apoptosis by long non-coding RNA HIF1A-AS1 in VSMCs: implications for TAA pathogenesis. Int. J. Clin. Exp. Pathol. 2014;7:7643–7652. [PMC free article] [PubMed] [Google Scholar]
- 33.Wang S., Zhang X., Yuan Y. BRG1 expression is increased in thoracic aortic aneurysms and regulates proliferation and apoptosis of vascular smooth muscle cells through the long non-coding RNA HIF1A-AS1 in vitro. Eur. J. Cardiothorac. Surg. 2015;47:439–446. doi: 10.1093/ejcts/ezu215. [DOI] [PubMed] [Google Scholar]
- 34.Mantella L., Singh K., Sandhu P. LinRNA‐P21 is regulated by cyclic mechanical stretch and upregulated in aortic aneurysms. Can. J. Cardiol. 2017;33:S208. [Google Scholar]
- 35.Wu G., Cai J., Han Y. LincRNA-p21 regulates neointima formation, vascular smooth muscle cell proliferation, apoptosis, and atherosclerosis by enhancing p53 activity. Circulation. 2014;130:1452–1465. doi: 10.1161/CIRCULATIONAHA.114.011675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huarte M., Guttman M., Feldser D. A large intergenic noncoding RNA induced by p53 mediates global gene repression in the p53 response. Cell. 2010;142:409–419. doi: 10.1016/j.cell.2010.06.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu W., Wang Z., Li Q. vol.120. 2019. pp. 4113–4120. (Upregulation of lincRNA-P21 in Thoracic Aortic Aneurysms Is Involved in the Regulation of Proliferation and Apoptosis of Vascular Smooth Muscle Cells by Activating TGF-Beta1 Signaling Pathway). [DOI] [PubMed] [Google Scholar]
- 38.Lino Cardenas C.L., Kessinger C.W., Cheng Y. An HDAC9-MALAT1-BRG1 complex mediates smooth muscle dysfunction in thoracic aortic aneurysm. Nat. Commun. 2018;9:1009. doi: 10.1038/s41467-018-03394-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Michalik K.M., You X., Manavski Y. Long noncoding RNA MALAT1 regulates endothelial cell function and vessel growth. Circ. Res. 2014;114:1389–1397. doi: 10.1161/CIRCRESAHA.114.303265. [DOI] [PubMed] [Google Scholar]
- 40.Guo X., Chang Q., Pei H. Long non-coding RNA-mRNA correlation analysis reveals the potential role of HOTAIR in pathogenesis of sporadic thoracic aortic aneurysm. Eur. J. Vasc. Endovasc. Surg. 2017;54:303–314. doi: 10.1016/j.ejvs.2017.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Yan B., Yao J., Liu J.Y. lncRNA-MIAT regulates microvascular dysfunction by functioning as a competing endogenous RNA. Circ. Res. 2015;116:1143–1156. doi: 10.1161/CIRCRESAHA.116.305510. [DOI] [PubMed] [Google Scholar]
- 42.Chen S., Chen H., Yu C. Long noncoding RNA myocardial infarction associated transcript promotes the development of thoracic aortic by targeting microRNA-145 via the PI3K/Akt signaling pathway. J. Cell. Biochem. 2019:1–9. doi: 10.1002/jcb.28695. [DOI] [PubMed] [Google Scholar]
- 43.Yu B., Liu L., Sun H. Long noncoding RNA AK056155 involved in the development of Loeys-Dietz syndrome through AKT/PI3K signaling pathway. Int. J. Clin. Exp. Pathol. 2015;8:10768–10775. [PMC free article] [PubMed] [Google Scholar]
- 44.Curci J.A., Thompson R.W. Adaptive cellular immunity in aortic aneurysms: cause, consequence, or context? J. Clin. Investig. 2004;114:168–171. doi: 10.1172/JCI22309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maegdefessel L., Spin J.M., Adam M. Micromanaging abdominal aortic aneurysms. Int. J. Mol. Sci. 2013;14:14374–14394. doi: 10.3390/ijms140714374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Saito T., Hasegawa Y., Ishigaki Y. Importance of endothelial NF-kappaB signalling in vascular remodelling and aortic aneurysm formation. Cardiovasc. Res. 2013;97:106–114. doi: 10.1093/cvr/cvs298. [DOI] [PubMed] [Google Scholar]
- 47.Nordon I.M., Hinchliffe R.J., Loftus I.M. Pathophysiology and epidemiology of abdominal aortic aneurysms. Nat. Rev. Cardiol. 2011;8:92–102. doi: 10.1038/nrcardio.2010.180. [DOI] [PubMed] [Google Scholar]
- 48.Galis Z.S., Khatri J.J. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ. Res. 2002;90:251–262. [PubMed] [Google Scholar]
- 49.Zhang L., Cheng H., Yue Y. H19 knockdown suppresses proliferation and induces apoptosis by regulating miR-148b/WNT/β-catenin in ox-LDL -stimulated vascular smooth muscle cells. J. Biomed. Sci. 2018;25:11. doi: 10.1186/s12929-018-0418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lv J., Wang L., Zhang J. Long noncoding RNA H19-derived miR-675 aggravates restenosis by targeting PTEN. Biochem. Biophys. Res. Commun. 2018;497:1154–1161. doi: 10.1016/j.bbrc.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 51.Li D.Y., Busch A., Jin H. H19 induces abdominal aortic aneurysm development and progression. Circulation. 2018;138:1551–1568. doi: 10.1161/CIRCULATIONAHA.117.032184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun W., Lv J., Duan L. Long noncoding RNA H19 promotes vascular remodeling by sponging let-7a to upregulate the expression of cyclin D1. Biochem. Biophys. Res. Commun. 2019;508:1038–1042. doi: 10.1016/j.bbrc.2018.11.185. [DOI] [PubMed] [Google Scholar]
- 53.Sun Y., Zhong L., He X. LncRNA H19 promotes vascular inflammation and abdominal aortic aneurysm formation by functioning as a competing endogenous RNA. J. Mol. Cell. Cardiol. 2019;131:66–81. doi: 10.1016/j.yjmcc.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 54.Hofmann P., Sommer J., Theodorou K. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc. Res. 2018;115:230–242. doi: 10.1093/cvr/cvy206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang Z., Zou G., Chen X. Knockdown of lncRNA PVT1 inhibits vascular smooth muscle cell apoptosis and extracellular matrix disruption in a murine abdominal aortic aneurysm model. Mol. Cells. 2019;42:218–227. doi: 10.14348/molcells.2018.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang Y.N., Shan K., Yao M.D. Long noncoding RNA-GAS5: a novel regulator of hypertension-induced vascular remodeling. Hypertension. 2016;68:736–748. doi: 10.1161/HYPERTENSIONAHA.116.07259. [DOI] [PubMed] [Google Scholar]
- 57.Tang R., Zhang G., Wang Y.C. The long non-coding RNA GAS5 regulates transforming growth factor beta (TGF-beta)-induced smooth muscle cell differentiation via RNA Smad-binding elements. J. Biol. Chem. 2017;292:14270–14278. doi: 10.1074/jbc.M117.790030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yao J., Shi Z., Ma X. lncRNA GAS5/miR-223/NAMPT axis modulates the cell proliferation and senescence of endothelial progenitor cells through PI3K/AKT signaling. J. Cell. Biochem. 2019 Sep;120(9):14518–14530. doi: 10.1002/jcb.28713. [DOI] [PubMed] [Google Scholar]
- 59.Bell R.D., Long X., Lin M. Identification and initial functional characterization of a human vascular cell-enriched long noncoding RNA. Arterioscler. Thromb. Vasc. Biol. 2014;34:1249–1259. doi: 10.1161/ATVBAHA.114.303240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Boulberdaa M., Scott E., Ballantyne M. A role for the long noncoding RNA SENCR in commitment and function of endothelial cells. Mol. Ther. 2016;24:978–990. doi: 10.1038/mt.2016.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lyu Q., Xu S., Lyu Y. SENCR stabilizes vascular endothelial cell adherens junctions through interaction with CKAP4. Proc. Natl. Acad. Sci. U.S.A. 2019;116:546–555. doi: 10.1073/pnas.1810729116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao J., Zhang W., Lin M. MYOSLID is a novel serum response factor-dependent long noncoding RNA that amplifies the vascular smooth muscle differentiation program. Arterioscler. Thromb. Vasc. Biol. 2016;36:2088–2099. doi: 10.1161/ATVBAHA.116.307879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ballantyne M.D., Pinel K., Dakin R. Smooth muscle enriched long noncoding RNA (SMILR) regulates cell proliferation. Circulation. 2016;133:2050–2065. doi: 10.1161/CIRCULATIONAHA.115.021019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ahmed A.S.I., Dong K., Liu J. Long noncoding RNA NEAT1 (nuclear paraspeckle assembly transcript 1) is critical for phenotypic switching of vascular smooth muscle cells. Proc. Natl. Acad. Sci. U.S.A. 2018;115:E8660–e8667. doi: 10.1073/pnas.1803725115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Leung A., Trac C., Jin W. Novel long noncoding RNAs are regulated by angiotensin II in vascular smooth muscle cells. Circ. Res. 2013;113:266–278. doi: 10.1161/CIRCRESAHA.112.300849. [DOI] [PMC free article] [PubMed] [Google Scholar]