Abstract
Objectives: In order to provide further evidence for the origin and differentiation of PEComa, the clinicopathologic and immunophenotype findings were analyzed in 26 cases with literature review. Methods: Immunohistochemistry and special staining were used. Results: Multinucleated giant cells and polymorphism were scattered visibly in 53.8% and 76.9% of the cases and spotty necrosis and hemorrhage were observed in 38% of the cases. Capsular micro-invasion was detected in 46% cases accompanied by hemorrhage and/or necrosis in the tumors with diameters larger than 5 cm. It was also found that 100% of cases diffusely expressed SMA, Melan-A, and vimentin except one negative for HMB-45. The tumor cells partly expressed CD56, CD99, desmin, and S-100 and were negative for CK-pan, TFE3, CD117, CD44, and CD34. Clinical follow-up found that 22 out of 23 patients were alive, with no recurrence or progression, ranging from 42 to 82 months. However, one patient died from leukemia. Conclusions: In this study, the histopathologic features with the co-expressions of SMA and melanin, were the diagnostic basis of PEComas. The interspersed expressions of desmin and S-100 were helpful for the differential diagnosis of leiomyoma and neuroma. The expressions of S-100, CD56, and CD99 supported the origins of the pluripotent cells from the neural crests. Tumors larger than 5 cm in diameter with micro-hemorrhaging/necrosis and micro-capsular invasions should be considered either uncertain or of malignant potential. The spontaneous rupturing of blood vessels may be related to the amyloidosis and desmin negative expression, and broken elastic fibers.
Keywords: PEComa, angiomyolipoma, immunohistochemistry
Introduction
Perivascular Epithelioid Cell (PEComa) tumors are defined by the presence of perivascular epithelioid cells co-expressing both muscle and melanocytic markers and are characterized by similar histologic and immunohistochemical presentations. PEComa include the following: Epithelioid angiomyolipoma (AML); clear cell sugar tumor of the lungs (CCST); lymphangioleiomyomatosis (LAM); hepatic falciform ligament clear cell myomelanocytic tumors (CCMMT); and soft-tissue clear cell myomelanocytic tumors [1], as well as very rare tumors in other locations. PEComas are generally composed of epithelioid or spindle smooth muscle tissue, dysmorphic blood vessels, and mature adipose tissue. PEComas were first described as benign tumors and termed as “angiomyolipoma” in 1951 [2]. In recent years, several hypotheses have been advanced for the purpose of explaining the histogenesis of PEComa. One proposed hypothesis was that the perivascular epithelioid cells had originated from the pluripotent cells derived from the neural crests, which may give rise to smooth muscle cells and melanocytes [3]. In actuality, PEComa tumors have melanosomes by ultrastructure, and are rich in glycogen and cytoplasmic filaments [4]. AMLs were previously classified as hamartomas. However, they are now regarded as belonging to the family of perivascular epithelioid cells.
Materials and methods
Patient data and tissue specimens
In this study, 26 PEComas specimens that had been resected between 2012 and 2016 were obtained from the Department of Pathology at the Affiliated Hospital of Inner Mongolia Medical University. The tumor tissue samples were fixed in 10% neutral buffered formalin and embedded in paraffin. The diagnoses of all the patients who received surgery were confirmed using histologic and immunohistochemical analysis. Then, follow-up surveys were conducted. The clinical data, including age, sex, site, tumor size, and follow-up surveys were available in all of the examined cases, as detailed in Table 1. The histopathologic features were then analyzed, including the cellular type, polymorphism, multinucleated giant cells, hemorrhaging, necrosis, chronic inflammation cell invasions, stromal sclerosis, and capsule invasion. The results are summarized in Tables 2 and 3.
Table 1.
Summary of the clinical information
| Patient ID | Age | Sex | Site | Tumor number (size: cm) | Invasive | Follow-up (month) | Outcome |
|---|---|---|---|---|---|---|---|
| 01 | 55 | F | Renal | 1 (5.0 × 5.0 × 2.0) | Yes | 82 | Alive |
| 02 | 41 | F | Renal | 1 (3.0 × 2.0 × 2.0) | Yes | 81 | Alive |
| 03 | 52 | F | Renal | 1 (1.5 × 1.5 × 1.0) | No | Unavailable | Alive |
| 04 | 53 | F | Renal | 1 (5.0 × 5.0 × 4.0) | No | 78 | Alive |
| 05 | 44 | F | Renal | 1 (4.0 × 3.0 × 3.0) | Yes | 77 | Alive |
| 06 | 49 | F | Renal | 1 (3.0 × 2.0 × 2.0) | Yes | 65 | Alive |
| 07 | 41 | F | Renal | 1 (1.5 × 1.0 × 1.0) | No | 64 | Alive |
| 08 | 58 | F | Renal | 1 (4.0 × 4.0 × 1.0) | No | 64 | Alive |
| 09 | 60 | M | Renal | 2 (from 2.5 to 1.5) | No | 52 | Deceased |
| 10 | 40 | F | Renal | 1 (4.5 × 3.0 × 1.0) | No | 73 | Alive |
| 11 | 55 | F | Renal | 1 (5.0 × .4.0 × 4.0) | No | 73 | Alive |
| 12 | 39 | F | Renal | 3 (from 0.8 to 3) | Yes | 72 | Alive |
| 13 | 29 | F | Renal | 1 (15.0 × 8.0 × 6.0) | Yes | Unavailable | Unknown |
| 14 | 64 | F | Renal | 1 (5.0 × 5.0 × 5.0) | No | Unavailable | Unknown |
| 15 | 59 | F | Renal | 1 (2.0 × 1.5 × 1.0) | No | 68 | Alive |
| 16 | 73 | M | Renal | 1 (2.5 × 2.5 × 2.0) | No | 64 | Alive |
| 17 | 72 | F | Renal | 1 (3.0 × 3.0 × 2.0) | No | 64 | Alive |
| 18 | 51 | F | Renal | 1 (3.0 × 2.0 × 1.0) | No | 63 | Alive |
| 19 | 43 | M | Renal | 1 (5.0 × 5.0 × 4.0) | Yes | 61 | Alive |
| 20 | 73 | F | Renal | 1 (10.0 × 10.0 × 5.0) | Yes | 60 | Alive |
| 21 | 73 | F | Liver | 1 (8.0 × 7.0 × 5.0) | Yes | 51 | Alive |
| 22 | 44 | F | Renal | 1 (10.0 × 4.0 × 4.0) | Yes | 42 | Alive |
| 23 | 62 | F | Renal | 1 (6.0 × 4.0 × 3.0) | Yes | 42 | Alive |
| 24 | 35 | F | Renal | 1 (8.0 × 6.0 × 2.0) | Yes | 42 | Alive |
| 25 | 29 | M | Renal | 1 (5.0 × 4.0 × 2.0) | Yes | 42 | Alive |
| 26 | 44 | F | Retroperitonium | 1 (5.0 × 5.0 × 4.0) | No | 42 | Alive |
Note: The averaged tumor sizes with and without invasive characteristics were 7.42 × 4.75 × 3.17 and 3.83 × 3.21 × 2.43, respectively.
Table 2.
Histopathologic features
| Patient ID | Cell type | Polymorphism | Giant cells | Hemorrhage | Necrosis | LC | Stroma sclerosis | Border invasion |
|---|---|---|---|---|---|---|---|---|
| 01 | Admix | Scattered | Visible | Spotted | No | No | No | Invasive |
| 02 | Admix | Scattered | Visible | No | No | Yes | Yes | Invasive |
| 03 | Epithelioid | Scattered | Invisible | No | No | Yes | No | No |
| 04 | Admix | Scattered | Invisible | No | No | No | No | No |
| 05 | Epithelioid | No | Invisible | Spotted | Scattered | Yes | No | Invasive |
| 06 | Epithelioid | Scattered | Visible | Spotted | Scattered | Yes | Yes | Invasive |
| 07 | Admix | Scattered | Visible | No | Scattered | Yes | Yes | No |
| 08 | Admix | Scattered | Invisible | Spotted | Scattered | No | No | No |
| 09 | Admix | Scattered | Visible | No | No | Yes | No | No |
| 10 | Admix | No | Invisible | No | No | Yes | No | No |
| 11 | Epithelioid | Scattered | Visible | No | No | No | No | No |
| 12 | Admix | Scattered | Visible | No | No | Yes | No | Invasive |
| 13 | Epithelioid | No | Invisible | No | No | Yes | No | Invasive |
| 14 | Epithelioid | Scattered | Visible | Spotted | Scattered | Yes | No | No |
| 15 | Admix | No | Invisible | No | No | No | No | No |
| 16 | Spindle | Scattered | Invisible | No | No | No | No | No |
| 17 | Admix | Scattered | Visible | Spotted | No | Yes | No | No |
| 18 | Admix | No | Invisible | No | No | No | No | No |
| 19 | Admix | Scattered | Visible | Spotted | Scattered | Yes | No | Invasive |
| 20 | Admix | Scattered | Invisible | No | No | No | No | Invasive |
| 21 | Epithelioid | Scattered | Visible | Spotted | Scattered | Yes | Yes | Invasive |
| 22 | Epithelioid | Scattered | Visible | Spotted | Scattered | Yes | No | Invasive |
| 23 | Epithelioid | Scattered | Visible | No | No | Yes | No | No |
| 24 | Spindle | Scattered | Visible | No | Scattered | Yes | Yes | Invasive |
| 25 | Admix | Scattered | Invisible | Spotted | Scattered | Yes | No | Invasive |
| 26 | Spindle | No | Invisible | No | No | No | No | No |
Note: In the table, LC represents lymphocyte infiltration.
Table 3.
Correlations between pathologic characteristics
| Pathological characteristic | Cell types | Giant cells | Blood | Necrosis | Max-diameters | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
|
|||||||
| Ad | S | E | + | - | + | - | + | - | > 5 cm | < 5 cm | |
| Invasive | 6 | 1 | 5* | 8* | 4 | 7* | 5 | 7* | 5 | 8* | 4 |
| Non-invasive | 8 | 2 | 4 | 6 | 8 | 3 | 11 | 3 | 11 | 5 | 9 |
| Necrosis | 4 | 1 | 5# | 7 | 3 | 8 | 2# | ||||
| Non-necrosis | 10 | 2 | 4 | 7 | 9 | 2 | 14 | ||||
Note: In the table;
indicates that the microscopic or macroscopic invasions were much easier to be detected in the epithelioid patterns (5 of 9) than in the spindle or admixed patterns (7 of 17), and correlated with scattered giant cells (8 of 12 versus 6 of 14), both blood and necrosis (7 of 12 versus 3 of 14), and more than 5 cm in maximum diameter (8 of 12 versus 5 of 14);
indicates that the necrosis was more easily to be detected in epithelioid type than in the spindle and admixed types and was correlated with the bleeding (5 of 9 versus 5 of 17).
Immunohistochemistry
The paraffin-embedded sections at 4 micrometers were prepared for IHC staining. This study’s IHC staining procedures were performed using an EnVision two-step immunohistochemical kit. A brief description of the process was as follows: 1. The tissue sections were de-waxed and rehydrated with ethanol, and then quenched with 3% hydrogen peroxide for 10 minutes; 2. The slides were soaked in 0.01 M sodium citrate buffer; 3. A hyper-pressure induced antigen retrieval process was performed for all the primary antibodies, as shown in Table 4; 4. The slides were incubated with primary antibodies overnight at 4°C; 5. The slides were then washed with phosphate buffered saline (PBS) and subsequently incubated with secondary antibodies for 30 minutes; 6. The sections were visualized by incubating then with diaminobenzidine tetrahydrochloride and counterstained with hematoxylin; 7. PBS was used in place of the primary antibody as a negative control.
Table 4.
Primary antibodies
| Antibody | Dilution | Pretreatment | Immunostain | Clone number | Positive control |
|---|---|---|---|---|---|
| SMA | Ready to us | PCA-EDTA | Envision | 1A4 | Artery |
| Calponin | Ready to us | PCA-EDTA | Envision | CALP | Artery |
| HMB45 | Ready to us | PCA-EDTA | Envision | HMB45 | Melanoma |
| MelanA | Ready to us | PCA-EDTA | Envision | A103 | Melanoma |
| Desmin | Ready to us | PCA-EDTA | Envision | D33 | Artery |
| Vimentin | Ready to us | PCA-EDTA | Envision | V9 | Artery |
| Ki67 | Ready to us | PCA-EDTA | Envision | MIB-1 | Breast carcinoma |
| CD56 | Ready to us | PCA-EDTA | Envision | 56C04 | NK/T |
| CD99 | Ready to us | PCA-EDTA | Envision | O13 | PNET |
| S-100 | Ready to us | PCA-EDTA | Envision | 16/f5 | Neurofibroma |
| CD34 | Ready to us | PCA-EDTA | Envision | QBEnd/10 | Vessels |
| CD44v6 | Ready to us | PCA-EDTA | Envision | 2F10 | Breast carcinoma |
| TEF3 | Ready to us | PCA-EDTA | Envision | MRQ-37 | Renal cell carcinoma |
Note: In the table, PCA indicates pressure cooker heating; and EDTA indicates ethylene demine tetra acetic acid buffer (0.01 M; pH 9.0).
Results
Clinical and histopathological features
The study group was composed of 26 patients. The patients’ ages ranged between 29 to 73 years, with a median age at the time of surgery of 51 years. The ratio of females to males was 5.25:1. The anatomic locations of the tumors included the kidneys (24 cases), liver (1 case), and retroperitoneum (1 case), as listed in Table 1. All of the obtained samples were primary tumors. Among the 26 patients in the study group, this study was able to obtain the complete clinical data of 23 of the patients (92%), with a mean follow-up duration of 49.5 (29 to 70) months. However, this study was unable to determine the survival status in three cases due to unavailable phone numbers for those patients (Table 1). Upon completion of the follow-up survey, it was determined that 22 of the 23 patients had healthy prognoses, without tumor recurrence or distant metastases. Meanwhile, one of the patients had died due to another unrelated disease.
PEComa tumors are generally composed of admixtures of a variety of blood vessels, smooth muscle cells, and adipose tissue. The amounts of the aforementioned components tend to differ from case to case. The tumor cells are either epithelioid or spindle arranged in bands, nests, or swirls, and also intersected by different amounts of adipose tissue. In accordance with the predominant (> 90%) cell shapes, PEComa tumors are generally classified into the following three subgroups: Spindle-type; epithelioid-type; and admixed-type. In this study, the spindle-type and epithelioid-type tumors were identified in three and nine cases, respectively. It has been found that the spindle tumor cells are usually arranged arranged in bundles, and appear as eosinophilic cytoplasm (Figure 1A) with different vacuolar degeneration (Figure 1B). In addition, the epithelioid cells appear with bright and finely granulated cytoplasm. The nuclei tend to be relatively small and round-to-oval shaped and are centrally located within the cells (Figure 1C). However, in the case of the liver PEComa, the epithelioid cells displayed larger granular eosinophilic cytoplasm, a round vesicular nucleus, and prominent nucleoli, when compared with those of the kidney PEComa. It was determined that the admixed-type tumors accounted for 53.8% (14/26) of the cases in this study. In addition, the proportions of the epithelioid cells and spindle cells were different for each of the cases. Generally speaking, epithelioid cells are a minor component, arranged in nests, and separated by vascular interstitium (Figure 1D). The tumor cells are arranged around or perpendicular to the blood vessels in the majority of cases (Figure 1E). Moreover, intranuclear inclusion bodies may be occasionally observed. The nuclear membrane tends to be thin, with fine chromatin characterized in part by small eosinophilic and consistent ovals or irregular nuclei, with nuclear grooves frequently visible, as illustrated in Figure 1F.
Figure 1.
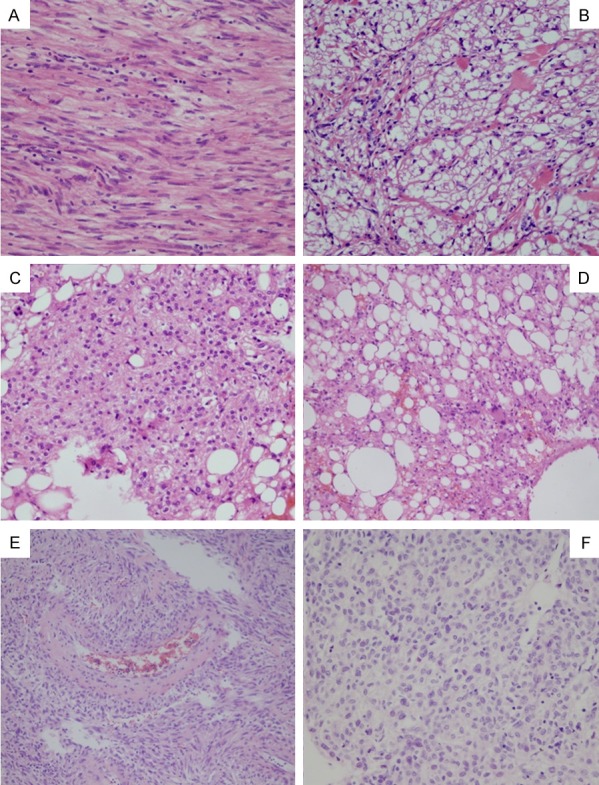
The histologic features of PEComa: Spindle-cell type cells resemble smooth muscle cells with eosinophilic (A) or clear cytoplasm (B). Epithelioid cell type has bright and fine granulated cytoplasm. The nuclei are relatively small and round to oval shaped centrally located in cells (C). Admixed type contains a variety of fatty cells with different amounts of spindle or epithelioid tumor cells (D). Usually tumor cells are around or perpendicular to blood vessels (E). The nucleus is round or rodlike or oval or irregular with nuclear groove depending on the cell type (F). (A-E): 10 × 10; (F): 20 × 10.
In the PEComa cases, changes in the blood vessels were the second prominent characteristic. They were observed to be distributed in clusters and arranged around or radiated by the tumor cells. It was found that the vessel lumen was irregularly dilated, twisted, or dysmorphic in nearly all the study cases (Figure 2A and 2B). However, occasionally it was difficult to observe the vessel lumen due to the mixing together of the spindle tumor cells and the appearance of fissuring (Figure 2C). It was also found that the vessel walls were not consistent in thickness (Figure 2D), with different extents of hyalinization or amyloidosis observed. This study determined that the elastic fibers in both the internal and external lamina of the blood vessels had displayed breakages, particularly in the internal elastic lamina, as shown in the elastic fiber staining results displayed in Figure 2E and 2F. This had caused it to be prone to spontaneous rupturing and hemorrhaging. It was also observed that stromal sclerosis caused by the collagen hyaline degeneration had existed in 65.4% of the cases (Figure 3A) and chronic inflammation cells had accumulated focally in 19% of the cases (Figure 3B). Also, scattered cellular polymorphism was observed in 76.9% (20 of 26) cases and multinucleated giant cells were detected in 53.8% of the cases (14 of 26), as shown in Figure 3C. Focal hemorrhages and spotted necroses are visible in 38.5% of the cases and were found to be positively correlated with each other. The amounts of mature adipose tissue varied from predominant to absent in the examined samples, and displayed a grey-pink or pale coloration with more or less a yellowish appearance and soft texture. Single or small clusters of spindle tumor cells were found to be dispersed in the adipose tissue, with mitosis barely visible. Evidence of capsular invasion was predominantly detected under a microscope and seldom macroscopically in 46% of the cases (12 of 26). Also, as shown in Tables 2 and 3, capsule invasions were more easily detected in the epithelioid type (5 of 9) than the other types (7 of 17), and were mainly accompanied by necrosis, hemorrhages, or both (7 of 12 versus 3 of 14), as well as scattered giant cells (8 of 12 versus 6 of 14) and were larger in size (average size: 7.42 × 4.75 × 3.17 cm) than in the non-invasive cases (8 of 12 versus 5 of 14; average size: 3.83 × 3.21 × 2.43 cm).
Figure 2.
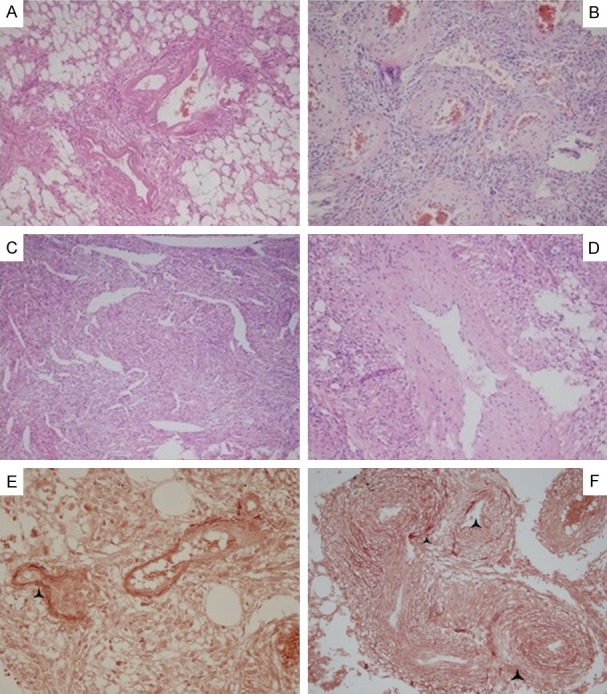
Features of blood vessels: Blood vessels are distributed in clusters and tumor cells are arranged around or radiated with blood vessels. Vessel lumen is irregularly dilated or twisted or dysmorphic with hyaline change (A) and (B), or is not easily observable because of mixing together with spindle tumor cells and resembling fissuring (C) or is not consistent in thickness (D). Elastic fiber staining showed the broken internal and external elastic lamina as a black triangle showed in (E) and (F). (A-F): 10 × 10.
Figure 3.
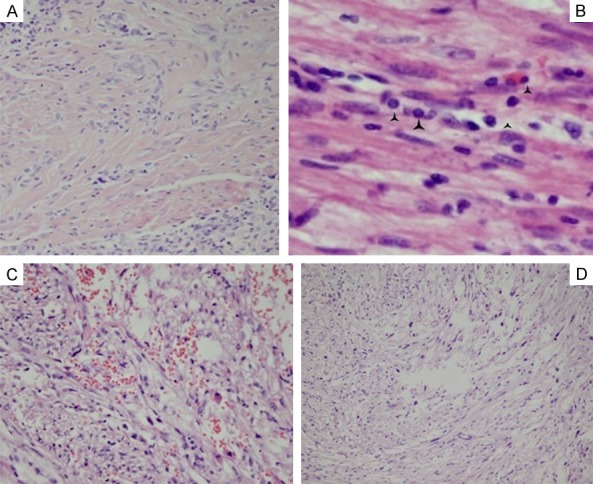
The changes of interstitial stroma: (A) The hyaline change of stroma. The black angles in (B) showed the inflammatory cell infiltrate; (C) Scattered hemorrhage and edema; (D) Focal polymorphism with multinuclear giant cells. (A, D): 4 × 10; (C): 10 × 10; (B): 40 × 10.
Immunohistochemical features
Both the Melan A and HMB-45 were positive in nearly all of the cases (Figure 4A and 4B). Melan A staining revealed results of 81% strong and 19% moderate, which were somewhat higher in intensity and sensitivity than the HMB-45 staining results of 50% diffuse and 46% scattered or focal. It was also found that both the SMA and Calponin staining reactivities (Figure 4C) were diffusely and strongly positive in the tumor cells of all the examined cases. In addition, the tumor cells were weakly to moderately reactive to CD99 in 89% of the cases (Figure 4D), displayed strong to diffuse positive results in 19% of the cases (6 of 26) and moderate to focally positive results in 23% of the cases (7 of 26) for CD56. This study’s desmin staining of the tumor cells also revealed either focal, scattered, or weak to moderate results, but was completely negative within the thickened vessel walls (Figure 4E). The staining results for S-100 were focal or scattered in either the spindle or epithelioid cell nests in only 26.92% of the cases (7 of 26). However, there were single or small clusters of tumor cells interspersed within the fatty tissue which had displayed strong S-100 positive results in 53.8% of the cases (14 of 26) (Figure 4F) and HMB-45 or SMA positive results in only two cases (2 of 26). These findings implied a potential differentiation toward adipose tissue. Furthermore, all of the cases displayed negative results for CD34, CD44, Transcriptional Enhancer Factor 3 (TEF3), and CKpan. The cell proliferative index revealed by the Ki-67 was less than 2%, even in the hot points listed in Table 5. The walls of blood vessels were irregularly thickened and showed different extents of amyloidosis. The smooth muscle cells of blood vessels were positive for SMA (Figure 5A), but negative for Melan A (Figure 5B), desmin (Figure 5C) and HMB-45 (Figure 5D). In addition, they appeared to be brick-red under normal light or have a yellowish-green luster under polarized light by Congo red staining (Figure 5E and 5F).
Figure 4.
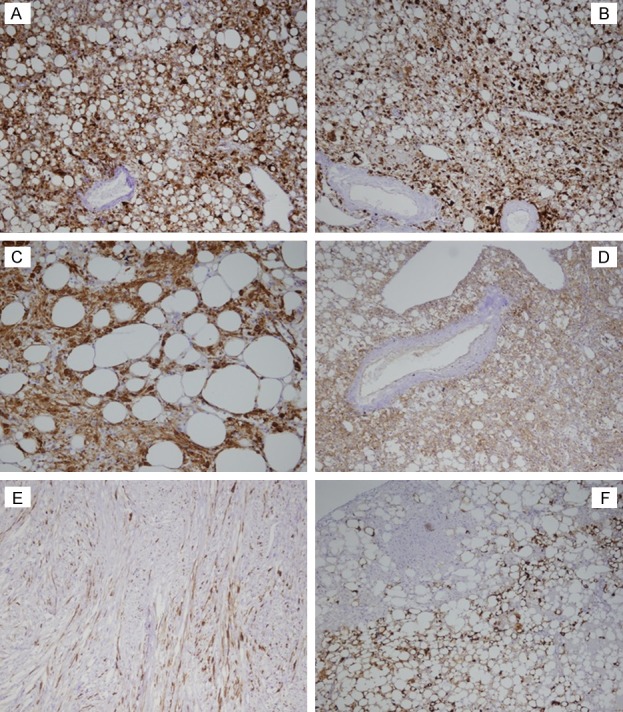
The immunohistochemical phynotypes: (A-C) represent diffused positive to Melan A, HMB-45 and Calponin in all tumor cells respectively. (D) showed both tumor cell and endothelium positive for CD99. Spindle tumor cells showed occasionaly scatter positive for Desmin (E). S-100 is negative for patch of tumor cells, but positive for both fatty cells and tumor cells interspersed in adipose tissue (F). 10 × 10.
Table 5.
Immunohistochemical staining for all antibodies
| Total (n) | SMA/Calp/Vim | HMB-45 | Melan A | Desmin | CD56 | CD99 | S-100 | CD34/CD44/TEF3/CK |
|---|---|---|---|---|---|---|---|---|
| 26 cases | 100% (+++) | 50% (++) | 81% (++) | 0% (++) | 19% (++) | 58% (++) | 0% (++) | 0% (+) |
| 46% (+) | 19% (+) | 85% (+) | 23% (+) | 31% (+) | 26.92% (+) | |||
| 4% (-) | 0% (-) | 15% (-) | 58% (-) | 11% (-) | 73.08% (-) | 100% (-) |
Note: In the table, (++) represents strong or diffuse positive results; (+) represents weak to moderate, focal, or scattered positive results; and (-) represents negative results; Ki67: < 2% in all cases.
Figure 5.
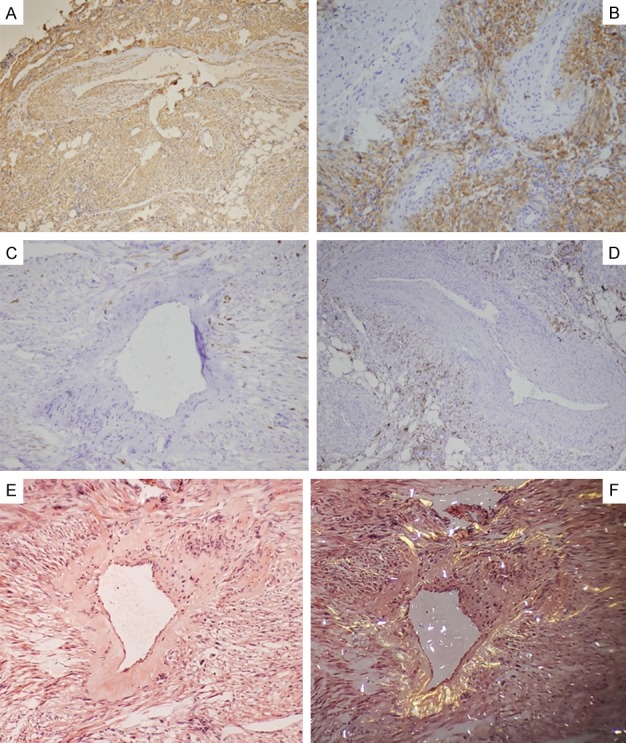
The differences of the spindle tumor cells and smooth muscle cells of blood vessels: SMA was positive for both spindle tumor cells and smooth muscle cells of blood vessels (A). Melan A (B), HMB-45 (D) and occasionally desmin (C) were positive for tumor cells, but completely negative for smooth muscle cells of blood vessels. The wall of blood vessels were negative for amyloidosis and Congo red stain appeared brick-red under normal light microscope (E) or yellow-green lustre under polarizing microscope (F). 10 × 10.
Discussion
PEComa tumors are considered to be heterogeneous. The World Health Organization (WHO) defined PEComas as mesenchymal tumors for the first time in 2002. PEComa tumors have three hypotheses of origin. One is that the tumor cells are derived from neural crest cells, which give rise to smooth muscle cells and melanocytes. Another hypothesis is that the tumor cells are myoblastic in nature. At the present time, angiomyolipomas are thought to be derived from perivascular epithelioid cells, namely pericytes, a type of cell for which no normal counterpart is known. They are essentially considered to be progenitors of fat and muscle cells. The co-expression of melanoma cells and muscle cell markers is one of the main characteristics. However, the intensities and sensitivities of expressions of those two types of markers may differ from case to case. In the present study, all of the cases showed diffusely positive SMA, calponin, and Melan-A results. Furthermore, almost all of the cases showed diffusely or partly positive HMB-45 results. Therefore, Melan A appeared to be more sensitive than HMB-45. The phenotype supported its features with melanosomes by ultrastructure, and richness of the glycogen and cytoplasmic filaments [4]. Also, this study detected positive expressions of S-100, CD56, and CD99, respectively, with different intensities and extents observed between the various examined cases. These findings further provided evidence for the speculation that the origins of the cells were the neural crest cells. As a result, the findings of this study may provide a suitable explanation for the novel concept in which PEComas are considered to be a neoplasm of stem cells which may have acquired defects during differentiation [3].
PEComa tumors are known generally to occur throughout the body in a variety of tissues and organs. The most common sites of PEComa are the kidneys, liver, lungs, and uterus [5], as well as a few cases that have been reported in bladder, prostate, ovaries, pancreas [6,7], and soft tissues [7]. Recent studies have also revealed cases where PEComas have developed in cutaneous [8], stomach [9], and gastrointestinal tracts [10], as well as orbital [11], omentum [12], scrotum [13], mediastinal [14], and even adrenal glands [15]. The available clinical data have shown that these types of tumors predominantly affect women. In the present study, the ratio of female to male patients was 5.25:1. The most common site was the kidneys, which accounted for 24 of the 26 cases in this study. It has been found that PEComa tumors usually develop sporadically. However, up to 20% of the cases may be associated with genetic disorders, such as Tuberous Sclerosis Complex (TSC). TSC is an autosomal dominant disease characterized with deleterious mutations in the tumor suppressors TSC1 (9q34) or TSC2 (16p13). Also, TSC may be characterized by benign neoplasms involving multiple systems. TSC1 and TSC2 gene products typically act to inhibit mammalian Target of Rapamycin (mTOR). There have been several documented cases of malignant angiomyolipoma which exhibit transient responses to mTOR inhibitors and form the basis of the current practice guidelines for malignant PEComa [16], with one exception [17]. TFE3 rearrangements have been found in subsets of PEComas, which include a lack of TSC2 inactivating mutations [18,19], as well as strongly exhibited diffuse positive expressions of HMB-45 and TFE3, weakly positive expressions of SMA, and negative expressions of Melan A [19], with no evident responses to mTOR inhibitors [18].
Based on the results obtained by Dr. Folpe, PEComa tumors have been conferred to a three-tier classification system which includes benign, uncertain malignant potential, and malignant tumor categories. This classification system was used to identify five high-risk features. These high-risk indicators include: 1. Tumor sizes > 5 cm; 2. Infiltrative growth patterns with high nuclear grades and cellularity; 3. Mitotic rate > 1/50 HPF; 4. Necrosis; and 5. Vascular invasion. Any tumors that meet at least two of the aforementioned features should be considered as malignant PEComas. It has been found that the PEComas of uncertain malignant potential can harbor either nuclear pleomorphism and multinucleated giant cells, or cells with diameter sizes greater than 5 cm. Benign PEComas are considered devoid of all high-risk features. However, it is often difficult to confirm the accuracy of Folpe’s criteria when distinguishing malignant from benign PEComa tumors due to the rarity of PEComa cases [6].
In the present study, cellular polymorphism, multinucleated giant cells, and hemorrhage and coagulative necrosis were observed to be scattered or focal in the examined tissues, but not extensive. Although those features were found to be positively correlated and were quite common in the tumors with diameters larger than 5 cm, the low proliferation index and rare mitosis, along with the lack of high nuclear grade and cellularity and no obvious vascular invasions, had indicated uncertain malignant potential, but not malignancy. PEComas undergoing metastasis, recurrence, and aggressive clinical courses are thought to be malignant. The lungs are a common metastatic site of such PEComa tumors. In a previous study, the results of 40 cases of renal AML revealed that approximately 50% of the cases had demonstrated disease progression [20]. Recently, cases of pulmonary metastasis have been reported from uncertain malignant potential or aggressive renal angiomyolipoma [21,22] and malignant perivasular angiomyolipoma cases have even been found to have developed in femur [23]. Additionally, the continuous increases in tumor sizes can potentially result in spontaneous ruptures which may lead to acute and even lethal bleeding in patients.
Acknowledgements
We would like to thank all the patients for providing their details about health status and all authors for their contributions in this study. This work was supported by the subsequent funding of National Natural Science foundation from Inner Mongolia Medical University (YKD2018HXKY001).
Disclosure of conflict of interest
None.
References
- 1.Tan Y, Zhang H, Xiao EH. Perivascular epithelioid cell tumour: dynamic CT, MRI and clinicopathological characteristics analysis of 32 cases and review of the literature. Clin Radiol. 2013;68:555–61. doi: 10.1016/j.crad.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 2.Morgan GS, Straumfjord JV, Hall EJ. Angiomyolipoma of the kidney. J Urol. 1951;65:525–527. doi: 10.1016/S0022-5347(17)68515-X. [DOI] [PubMed] [Google Scholar]
- 3.Lim SD, Stallcup W, Lefkove B, Govindarajan B, Au KS, Northrup H, Lang D, Fisher DE, Patel A, Amin MB. Expression of the neural stem cell markers NG2 and L1 in human angiomyolipoma: are angiomyolipomas neoplasms of stem cells? Mol Med. 2007;13:160–165. doi: 10.2119/2006-00070.Lim. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hirabayashi K, Nakamura N, Kajiwara H, Hori S, Kawaguchi Y, Yamashita T, Dowaki S, Imaizumi T, Osamura RY. Perivascular epithelioid cell tumor (PEComa) of the pancreas: immunoelectron microscopy and review of the literature. Pathol Int. 2009;59:650–655. doi: 10.1111/j.1440-1827.2009.02421.x. [DOI] [PubMed] [Google Scholar]
- 5.Kwon BS, Suh DS, Lee NK, Song YJ, Choi KU, Kim KH. Two cases of perivascular epithelioid cell tumor of the uterus: clinical, radiological and diagnostic challenge. Eur J Med Res. 2017;22:7. doi: 10.1186/s40001-017-0248-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zemet R, Mazeh H, Neuman T, Freund HR, Eid A. Asymptomatic pancreatic perivascular epithelial cell tumor (PEComa) in a male patient: report and literature review. J Oncol Pract. 2011;12:55–58. [PubMed] [Google Scholar]
- 7.Folpe AL, Mentzel T, Lehr HA, Fisher C, Balzer BL, Weiss SW. Perivascular epithelioid cell neoplasms of soft tissue and gynecologic origin: a clinicopathologic study of 26 cases and review of the literature. Am J Surg Pathol. 2005;29:1558–1575. doi: 10.1097/01.pas.0000173232.22117.37. [DOI] [PubMed] [Google Scholar]
- 8.Stuart LN, Tipton RG, DeWall MR, Parker DC, Stelton CD, Morrison AO, Coleman LW, Fosko SW, Vidal CI, Yadira Hurley M, Deeken AH, Gardner JM. Primary cutaneous perivascular epithelioid cell tumor (PEComa): five new cases and review of the literature. J Cutan Pathol. 2017;44:713–721. doi: 10.1111/cup.12972. [DOI] [PubMed] [Google Scholar]
- 9.Shin SA, Choi J, Moon KC, Kim WH. Perivascular epithelioid cell tumor in the stomach. J Pathol Transl Med. 2017;51:428–432. doi: 10.4132/jptm.2016.09.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Z, Han S, Wu J, Xiong M, Huang Y, Chen J, Yuan Y, Peng J, Song W. A systematic review: perivascular epithelioid cell tumor of gastrointestinal tract. Medicine (Baltimore) 2016;95:e3890. doi: 10.1097/MD.0000000000003890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varan A, Bayhan T, Kiratli H, Özoğul E, Kösemehmetoğlu K, Bulut E, Akyüz C. Orbital perivascular epithelioid cell tumor in a 7-year-old boy: case report and review of the literature. J AAPOS. 2017;21:325–328. doi: 10.1016/j.jaapos.2017.05.022. [DOI] [PubMed] [Google Scholar]
- 12.Okamoto K, Okada Y, Ohno K, Yagi T, sukamoto M, Akahane T, Shimada R, Hayama T, Tsuchiya T, Nozawa K, Matsuda K, Ishida T, Kondo F, Hashiguchi Y. A rare case of perivascular epithelioid cell tumor (PEComa) of the greater omentum. World J Surg Oncol. 2018;16:113. doi: 10.1186/s12957-018-1407-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sultan G, Masood B, Qureshi H, Mubarak M. Angiomyolipoma of the scrotum: report of a rarely seen case and review of the literature. Turk J Urol. 2017;43:223–226. doi: 10.5152/tud.2017.26779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim YD, Jeong SC, Jeon HW, Song SW, Shin OR, Choi SY. Successful thoracoscopic resection of a large mediastinal angiomyolipoma. J Thorac Dis. 2017;9:E427–E431. doi: 10.21037/jtd.2017.04.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwazneski Ii D, Merrill M, Young J, Sell H Jr. Angiomyolipoma and malignant PEComa: discussion of two rare adrenal tumors. Case Rep Oncol Med. 2016;2016:5204092. doi: 10.1155/2016/5204092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budde K, Gaedeke J. Tuberous sclerosis complex-associated angiomyolipomas: focus on mTOR inhibition. Am J Kidney Dis. 2012;59:276–283. doi: 10.1053/j.ajkd.2011.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Machado I, Cruz J, Lavernia J, Rayon JM, Poveda A, Llombart-Bosch A. Malignant PEComa with metastatic disease at diagnosis and resistance to several chemotherapy regimens and targeted therapy (m-TOR inhibitor) Int J Surg Pathol. 2017;25:543–549. doi: 10.1177/1066896917701245. [DOI] [PubMed] [Google Scholar]
- 18.Schoolmeester JK, Dao LN, Sukov WR, Wang L, Park KJ, Murali R, Hameed MR, Soslow RA. TFE3 translocation associated perivascular epithelioid cell neoplasm (PEComa) of the gynecologic tract: morphology, immunophenotype, differential diagnosis. Am J Surg Pathol. 2015;39:394–404. doi: 10.1097/PAS.0000000000000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim H, Kim J, Lee SK, Cho EY, Cho SY. TFE3-expressing perivascular epithelioid cell tumor of the breast. J Pathol Transl Med. 2019;53:62–65. doi: 10.4132/jptm.2018.08.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brimo F, Robinson B, Guo C, Zhou M, Latour M, Epstein JI. Renal epithelioid angiomyolipoma with atypia: a series of 40 cases with emphasis on clinicopathologic prognostic indicators of malignancy. Am J Surg Pathol. 2010;34:715–722. doi: 10.1097/PAS.0b013e3181d90370. [DOI] [PubMed] [Google Scholar]
- 21.Okamoto S, Komura M, Terao Y, Kurisaki-Arakawa A, Hayashi T, Saito T, Togo S, Shiokawa A, Mitani K, Kobayashi E, Kumasaka T, Takahashi K, Seyama K. Pneumothorax caused by cystic and nodular lung metastases from a malignant uterineperivascular epithelioid cell tumor (PEComa) Respir Med Case Rep. 2017;22:77–82. doi: 10.1016/j.rmcr.2017.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cooper A, Baugh L, Kelley S, Huang H, Guileyardo J. Pulmonary lymphangioleiomyomatosis associated with aggressive renal angiomyolipoma. Proc Bayl Univ Med Cent. 2018;31:81–83. doi: 10.1080/08998280.2017.1391038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sadigh S, Shah P, Weber K, Sebro R, Zhang PJ. Primary malignant perivascular epithelioid cell neoplasm (PEComa) of the bone mimicking granular cell tumor in core biopsy: a case report and literature review. Oncol Lett. 2018;15:2946–2952. doi: 10.3892/ol.2017.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]


