Abstract
Primary hepatic pregnancy is an extremely rare event which is difficult to diagnose due to its unusual location; moreover the potential mortality rate is five to seven times higher than the rate found in other ectopic pregnancies. We report a case of primary hepatic pregnancy in a 23-year-old woman, who presented with a history of one cesarean section and had taken oral contraceptives within half a year prior to her presentation. Interestingly this patient has no history of amenorrhoea, and no clinical symptoms of chills, fever, nausea, vomiting and diarrhea. Imaging findings showed an abnormal mass on the lower part of the right lobe. The patient was misdiagnosed with a liver tumor prior to operation. Histopathologic examination found that chorionic villi with trophoblasts infiltrated the hepatic tissue. A few trophoblasts were detected in some hepatic veins. HCG immunostaining showed positive reactivity in the trophoblasts. We believe that some risk factors of primary hepatic pregnancy such as the history of cesarean section and oral contraceptive should be taken into serious consideration and raise the index of suspicion, especially in the women of reproductive age, with or without a history of amenorrhoea. Timely diagnosis should be made in order to avoid mortality from rupture of the gestational sac.
Keywords: Primary hepatic pregnancy, cesarean section, oral contraceptive, etiology
Introduction
Abdominal ectopic pregnancy has been defined as an ectopic pregnancy implanted in the peritoneal cavity, but excludes the tube, ovary, or intraligamentous. It accounts for approximately 1% of all ectopic pregnancies [1]. Ordinarily, most abdominal ectopic pregnancy implants in the pelvic cavity, but very rarely it may be implanted on the upper abdominal regions, such as liver, spleen, omentum, or mesentery [2]. Because of high vascular supply, the liver is an exceptional implantation site and a life-threatening condition may result from fatal hemorrhage after rupture of an ectopic pregnancy [3]. To the best of our knowledge, less than 40 cases have been recorded during the past 60 years. The etiology of hepatic ectopic pregnancy is very complicated and the pathogenesis is not clear. We herein present a primary hepatic pregnancy case with a history of cesarean section and oral contraceptives within half a year prior to her presentation, but there was no history of amenorrhoea or the clinical symptoms of chills, fever, nausea, vomiting and diarrhea. We believe that cesarean section and oral contraceptives are risk factors which should be responsible for the primary hepatic pregnancy. We hope that the case we reported herein can make a contribution to the possible etiology of primary hepatic pregnancy.
Case presentation
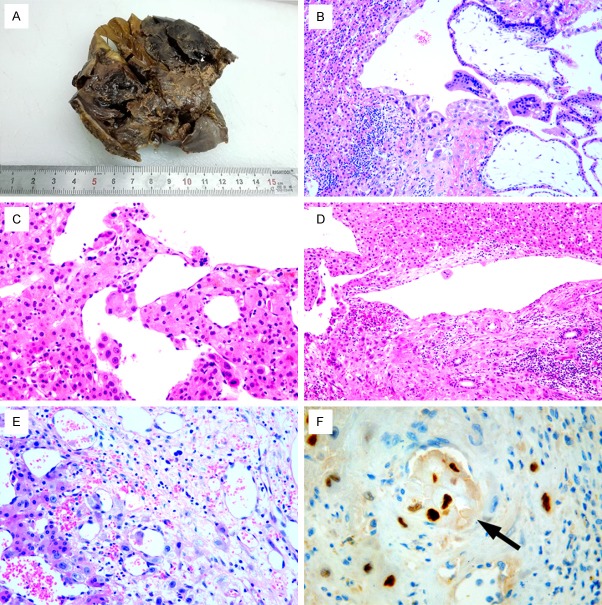
A 23-year-old woman was admitted to hospital for persistent dullness and upper right abdominal pain with a 10-day history. Dullness was relieved by the patient. The patient lacked clinical symptoms of chills, fever, nausea, vomiting and diarrhea. She did not have a history of inducing abortion or a history of cesarean section 6 months prior to her presentation. Moreover, the patient had taken oral contraceptives two times after cesarean section. There was no history of amenorrhoea, but there was a history of vaginal bleeding with black blood clots half a month after the last menstruation and continuing to her presentation. Laboratory tests showed total white blood cells was 9.30×109/L, the number of red cells was 3.97×1012/L and PLT 365×109/L and her pulse, blood pressure were within normal limits. She was in good condition on clinical examination except for right upper quadrant tenderness. The patient’s serum human chorionic gonadotropin (HCG) level dropped to 899 mIU/ml after surgery and became normal 1 week later. We do not have an HCG level because it was misdiagnosed as liver adenoma before the operation. Ultrasound examination of the abdomen revealed no obvious gestational sac and an intrauterine contraceptive device in the uterine cavity, but detected an 11 cm×8.9 cm mixed cystic and solid hyperechoic mass attached to the right liver lobe with unclear boundaries. The uterus, ovaries and fallopian tubes were normal in size and shape. A magnetic resonance imaging (MRI) plain scan of TI-weighted imaging (T1W1) showed an abnormal round-like high signal mass in the lower part of the right lobe (Figure 1A). The focus on T2-weighted imaging (T2W1) appeared to be an evidently high-signal with indistinct margin (Figure 1B). Gallbladder, pancreas, spleen and kidneys were otherwise normal. Grossly pathologic examination found that external surface of the liver was focal bulging and broken. A 5×5×4 cm reddish mass was detected within the hepatic tissues on the cut surface (Figure 2A). Histologically, the chorionic villi with trophoblasts eroded into the hepatic tissues (Figure 2B). A few trophoblasts could be detected in some hepatic veins (Figure 2C-E) and HCG immunostaining showed positive reactivity in the trophoblasts (Figure 2F).
Figure 1.
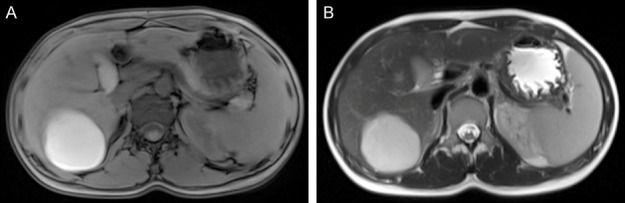
A. MRI T1W1 showed an abnormal round-like high-signal mass in the lower part of the right lobe. B. MRI T2W1 appeared an evidently high signal with unclear margin inside.
Figure 2.
A. Gross pathologic examination found a 5×5×4 cm reddish mass detected within the hepatic tissues on the cut surface. B. Histologically, the chorionic villi with trophoblasts eroded into the hepatic tissues (×100). C-E. A few trophoblasts can be detected in some hepatic veins (×200). F. HCG immnostaining showed positive reactivity in the trophoblasts in some small veins (×400, black arrow).
Discussion
Abdominal pregnancy is a rare event and accounts for approximately 1.0% of all ectopic pregnancies [1]. Abdominal pregnancy can be regarded as either primary or secondary, with the latter most commonly associated with early tubal rupture or uterine rupture and subsequent nidation in the peritoneal cavity [4]. The gestational sac of an abdominal pregnancy usually implants in highly vascular areas such as the liver, spleen, and mesentery [2]. Primary hepatic ectopic pregnancy not only is an exceptionally rare event in abdominal pregnancy but also has high potential mortality [3]. The diagnosis of primary hepatic ectopic pregnancy must satisfy the criteria established by Studdiford [5]. That is, (1) normal tubes and ovaries without evidence of injury, (2) no evidence of utero-peritoneal fistula, (3) pregnancy related exclusively to the peritoneal surface; and (4) pregnancy at an early enough stage of gestation to exclude the possibility of a secondary implantation following an initial eliminated primary tubal nidation. The present case fulfilled all these criteria, and the implantation occurred on the right liver hilum (parenchyma).
Over the past 60 years, less than 40 cases have been reported in the English language medical literature on the basis of a MEDLINE database search. The median age was 29 years (range, 18-46). In most cases, the attachment site of the placenta was on the lower surface of the right lobe of the liver which is enriched in vascular supply. A few reports described an implantation location of the placenta on the upper surface of the right liver lobe or on the caudate lobe [6-9]. Only two cases reported the attachment site on the inferior surface of the left lobe [10,11]. Clinically, most of the patients were admitted to hospital for severe abdominal pain with the history of amenorrhoea and vaginal bleeding [8,10,12-20]. Some cases presented with acute peritonitis and hypovolemic shock resulting from ruptured ectopic pregnancy in the first trimester which occurs due to lack of Arias-Stella reaction and inadequate decidualization in the location of the liver [6,10,12,16,20-25]. Our patient presented with minimal clinical symptoms and abdominal signs because of a slow hemorrhage. Most cases of hepatic pregnancy were characterized by an elevated serum HCG level. We lacked a serum HCG level before surgery, but the serum HCG level was also higher (889 IU/L) than normal. In previously reported cases, it was difficult to discover of the pregnancy through imaging examination including US, CT and MRI preoperatively, similar to the current case. Our case was misdiagnosed as liver adenoma since the patient did not have a history of amenorrhoea and acute intraperitoneal hemorrhage, and also didn’t have the characteristic findings by imaging examination.
The etiology of hepatic ectopic pregnancy is very complicated and the pathogenesis is unknown. It is reported that there are some related factors which can help to understand the pathogenesis of the primary hepatic ectopic pregnancy. First, contraception including oral contraceptives and intrauterine contraceptive devices were reblamed for the hepatic ectopic location. As of now, there are three reported cases with a history of using intrauterine devices [14,19,20]. Second, inflammation of fallopian tubes and pelvis is responsible for the rising incidence of hepatic ectopic pregnancies. Third, the liver is the largest solid organ of the abdominal cavity. It is rich in blood supply and more suitable for embryo growth. Among all of the reported cases, there were only two cases with a history of cesarean section, but the onset time was one year or longer after cesarean section [7,14]. Our case is characterized by a history of having undergone cesarean section 6 months prior to her presentation. We think that the placental villi may enter the injured blood vessels of the uterus and reach the liver parenchyma through blood flow during the operation. The liver is a favorable site of growth for villi because of its rich vascularization. Also in our case the decidual reaction did not take place. Focal and discrete trophoblastic hyperplasia could be detected and eroded into the large unprotected vessels in the hepatic parenchyma, producing hemorrhage. A few trophoblasts could be detected in some hepatic veins, and HCG immunostaining showed positive reactivity in the trophoblasts. It is reported that patients who had been taking oral contraceptives for 5 years are prone to primary hepatic ectopic pregnancy within one year of quitting the drug [26]. There was not one case with a history of oral contraceptives among all of the reported cases. The patient we reported herein had a hepatic pregnancy after taking oral contraceptives twice within 3 months prior to her presentation. We assume that taking oral contraceptives many times in a short period can be another critical risk factor leading to primary hepatic ectopic pregnancy, but the pathogenesis is unknown.
The possibility of primary hepatic pregnancy should be kept in mind in the differential diagnosis of acute abdomen. Most cases are located on the inferior margin of the right hepatic lobe, and get misdiagnosed as the tumors of liver, gastroduodenitis, or diseases of the biliary system. This case was misdiagnosed as liver adenoma in a preoperative phase because of the lack of history of amenorrhoea and the HCG level. Also, the imaging findings showed no definite finding, so the exact diagnosis was made postoperatively. Risk factors such as a history of cesarean section and oral contraceptives should be considered and raise the index of suspicion, especially in women of reproductive age with or without a history of amenorrhoea. The timely diagnosis of primary hepatic pregnancy should be made combing with the clinical manifestations, imaging findings and the HCG level, which can avoid mortality from rupture of the gestational sac.
Acknowledgements
This study was supported by the Scientific and Technological Development Projects of Shandong Province Medical and Health Science. (No. 2015WS0244). Written informed consent was obtained from the patient for publication of this case report.
Disclosure of conflict of interest
None.
References
- 1.Atrash HK, Friede A, Hogue CJ. Abdominal pregnancy in the United States: frequency and maternal mortality. Obstet Gynecol. 1987;69:333–337. [PubMed] [Google Scholar]
- 2.Molinaro TA, Barnhart KT. Ectopic pregnancies in unusual locations. Semin Reprod Med. 2007;25:123–130. doi: 10.1055/s-2007-970051. [DOI] [PubMed] [Google Scholar]
- 3.Tshivhula F, Hall DR. Expectant management of an advanced abdominal pregnancy. J Obstet Gynaecol. 2005;25:298. doi: 10.1080/01443610500106819. [DOI] [PubMed] [Google Scholar]
- 4.Pinto V, Marinaccio M, Causio F, Putignano G. Abdominal pregnancy. An ever present challenge. Minerva Ginecol. 1996;48:321–326. [PubMed] [Google Scholar]
- 5.Studdiford WE. Primary peritoneal pregnancy. Am J Obstet Gynecol. 1942;44:485–487. [Google Scholar]
- 6.Veress B, Wallmander T. Primary hepatic pregnancy. Acta Obstet Gynecol Scand. 1987;66:563–564. doi: 10.3109/00016348709015736. [DOI] [PubMed] [Google Scholar]
- 7.Kuai XP, Wang SY, Qiu JM. Ectopic pregnancy implanted in the liver under the diaphragm. Taiwan J Obstet Gynecol. 2013;52:586–587. doi: 10.1016/j.tjog.2013.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Chui AK, Lo KW, Choi PC, Sung MC, Lau JW. Primary hepatic pregnancy. ANZ J Surg. 2001;71:260–261. doi: 10.1046/j.1440-1622.2001.02085.x. [DOI] [PubMed] [Google Scholar]
- 9.Nichols C, Koong D, Faulkner K, Thompson G. A hepatic ectopic pregnancy treated with direct methotrexate injection. Aust N Z J Obstet Gynaecol. 1995;35:221–223. doi: 10.1111/j.1479-828x.1995.tb01880.x. [DOI] [PubMed] [Google Scholar]
- 10.Wang C, Cheng L, Zhang Z, Yuan Z. Imaging diagnosis of hepatic ectopic pregnancy: a report of one case. Intractable Rare Dis Res. 2012;1:40–44. doi: 10.5582/irdr.2012.v1.1.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao RF, Huang SR, Xu LL, Liu NP, Liang N. Successful management of a live 14-week primary hepatic ectopic pregnancy combined with a residual horn of the uterus using laparoscopy. Chin Med J (Engl) 2017;130:3013–3014. doi: 10.4103/0366-6999.220298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Barbosa Júnior Ade A, de Freitas LA, Mota MA. Abdominal pregnancy. An ever present challenge. Pathol Res Pract. 1991;187:329–331. doi: 10.1016/s0344-0338(11)80792-2. [DOI] [PubMed] [Google Scholar]
- 13.Kato K. [Primary hepatic pregnancy] . Ryoikibetsu Shokogun Shirizu. 1995:523–525. [PubMed] [Google Scholar]
- 14.Cai YY, Xiao EH, Shang QL, Xiao LZ. Ectopic pregnancy in the liver incidentally diagnosed by imaging: a case report. Exp Ther Med. 2017;14:373–376. doi: 10.3892/etm.2017.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu S, Song Q, Chen K, Chen Y. Contrast-enhanced multiphasic CT and MRI of primary hepatic pregnancy: a case report and literature review. Abdom Imaging. 2014;39:731–735. doi: 10.1007/s00261-014-0101-5. [DOI] [PubMed] [Google Scholar]
- 16.Ma J, Zhou C, Duan Z, Jiang Y. Successful management of primary hepatic pregnancy with selective hepatic artery embolization and intra-arterial methotrexate infusion. Int J Gynaecol Obstet. 2013;122:78–79. doi: 10.1016/j.ijgo.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Sibetcheu Tchatou A, Tchounzou R, Mbuagbaw L, Mboudou ET. Successful medical treatment of a hepatic pregnancy: a case report. J Med Case Rep. 2017;11:70. doi: 10.1186/s13256-017-1227-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tu J, Wang E, Shen J. Primary hepatic ectopic pregnancy: a case report. J Reprod Med. 2016;61:175–178. [PubMed] [Google Scholar]
- 19.Yin H, Liu Y, Cao Y, Zhang M, Wang T, Wang Z, Wu J, Yu T, Lu W. Primary hepatic pregnancy. QJM. 2018;111:411–413. doi: 10.1093/qjmed/hcy063. [DOI] [PubMed] [Google Scholar]
- 20.Borlum KG, Blom R. Primary hepatic pregnancy. Int J Gynaecol Obstet. 1988;27:427–429. doi: 10.1016/0020-7292(88)90125-7. [DOI] [PubMed] [Google Scholar]
- 21.Jane Z, Farkasdi J, Jager L, Kneffel P, Koppany C, Markus B, Puskas T. A rare localisation of ectopic pregnancy--a case of hepatic pregnancy. Magy Seb. 2008;61:270–272. doi: 10.1556/MaSeb.61.2008.5.3. [DOI] [PubMed] [Google Scholar]
- 22.Murley AH. Liver pregnancy. Lancet. 1956;270:994–995. doi: 10.1016/s0140-6736(56)91802-5. [DOI] [PubMed] [Google Scholar]
- 23.Delabrousse E, Site O, Le Mouel A, Riethmuller D, Kastler B. Intrahepatic pregnancy: sonography and CT findings. AJR Am J Roentgenol. 1999;173:1377–1378. doi: 10.2214/ajr.173.5.10541123. [DOI] [PubMed] [Google Scholar]
- 24.Mitchell RW, Teare AJ. Primary hepatic pregnancy. a case report and review. S Afr Med J. 1984;65:220. [PubMed] [Google Scholar]
- 25.Paulino-Netto A, Roselli A. Hepatic ectopic pregnancy: successful surgical treatment of a patient with hepatic pregnancy and acute hemorrhage. Mt Sinai J Med. 1986;53:514–517. [PubMed] [Google Scholar]
- 26.Shippey SH, Bhoola SM, Royek AB, Long ME. Diagnosis and management of hepatic ectopic pregnancy. Obstet Gynecol. 2007;109:544–546. doi: 10.1097/01.AOG.0000247293.32523.c3. [DOI] [PubMed] [Google Scholar]