Abstract
Lung adenocarcinoma (LUAD) is the most common pathologic subgroup of lung cancer. The role of basic leucine zipper and W2 domains 2 (BZW2) in tumorigenesis has been investigated, while the functions and molecular mechanisms of BZW2 in LUAD remain undetermined. Our study aimed to investigate the effect of BZW2 in LUAD tumorigenesis and prognostic prediction. LUAD patients who underwent complete resection with tumor available for histologic evaluation were collected, and immunohistochemistry (IHC) was performed and scored for intensity of BZW2 expression. Overall survival (OS) and disease free survival (DFS) were estimated and compared between groups. Hazard ratios (HRs) for death were estimated using univariable and multivariable Cox proportional hazards models. BZW2 was considerably raised in the archival tissue samples from LUAD patients relative to those in healthy controls. High BZW2 expression was associated with unfavorable OS and DFS in LUAD patients. Coincidently, the up-regulated BZW2 was related to tumorigenesis, including tumor size, stage, and lymphatic invasion. In addition, we also found a positive correlation between BZW2 and EIF5 expression. BZW2 may be a clinical molecular biomarker for the prognosis of LUAD patients.
Keywords: BZW2, lung adenocarcinoma, immunohistochemistry, biomarker
Introduction
Lung cancer is a globally leading cause of cancer-associated mortality, accounting for millions of cancer deaths in China [1]. In clinical practice, adenocarcinoma is the most frequent histologic subtype [2]. Despite the current standard of care and emerging targeted therapies for LUAD, 5-year overall survival rates still vary from 4-17%, and most LUAD patients are diagnosed at advanced stages with poor prognosis [3]. Identification of potential molecular biomarkers with prognostic and clinicopathological significance is an urgent need for the early diagnosis of LUAD. Up to now, microarray, RNA-seq, and other technologies led to the detection of clinicopathologic markers that drive LUAD genesis [4-6]. Nonetheless, these markers are not adequately specific to enhance the diagnostic accuracy of LUAD, so there is a notable demand for the discovery of novel molecular markers for the prediction of cancer-associated metastasis and recurrence [7].
BZW2 is a member of the basic-region leucine zipper (bZIP) superfamily of transcription factors [8]. There are few studies reported on the effect of bZIP superfamily in cancer [9]. A previous study revealed that BZW2 is up-regulated in hepatocellular carcinoma and its overexpression induces hepatocellular carcinoma cells proliferation and drug resistance by inactivating the PI3K/AKT/mTOR signaling pathway, suggesting BZW2 plays vital roles in the regulation of hepatocellular carcinoma [10], while knockdown of BZW2 gene in bladder cancer cell consistently resulted in the suppression of cell growth, cell arrest at G1-phase, and reduction in viable cell numbers [11,12]. However, the role of BZW2 in LUAD is uncertain.
In this study, we investigated the BZW2 expression by immunohistochemistry in primary LUAD patients, and evaluated its correlation with clinical and pathological features, especially with overall survival (OS) and disease free survival (DFS). Our data showed that BZW2 is a potential prognostic predictor for LUAD.
Materials and methods
Microarray analysis
Public microarray datasets were used to evaluate our hypothesis and results. Survival analysis of large cohort microarray dataset was performed in The Cancer Genome Atlas (TCGA) cohort and Gene Expression Omnibus (GEO) dataset. The correlation between the expression of BZW2 and EIF5 in LUAD was investigated by GSE31210 database [13].
Patients and clinical features
Primary LUAD tissue samples were from 48 patients who underwent radical surgery at the Xiangshui People’s Hospital (Xiangshui, China) from February 2015 to November 2018. None of the LUAD patients received any forms of treatments (immunotherapy, radiation therapy, chemotherapy or other cancer-associated treatments) before radical surgery. The demographic and clinical information, including gender, age, tumor size, smoking history, degree of differentiation, and lymph node metastasis, were provided from pathology and clinical records.
Immunohistochemical analysis and pathologic evaluation
Immunohistochemistry of BZW2 was performed on 3 μm-thick sections of the LUAD tumor, using anti-BZW2 antibody (Bioss, Beijing). In brief, the primary antibody was diluted at 1:200 and incubated with the slices at 4°C overnight. The secondary antibody was applied for 30 min, followed by incubation with the avidin-biotin and streptavidin complex. The images were visualized with microscope (Olympus, Tokyo).
Two pathologists who were blinded to clinical outcome independently performed the IHC scoring. BZW2 expression was initially evaluated according to the percentage and intensity of BZW2-positive cell. In brief, immunostaining intensity of BZW2 was categorized as follows: 0, no staining; 1+, weak staining; 2+, moderate staining; 3+, strong staining. Immunostaining percentage of BZW2 was also scored as 4 levels: 0, 0-25%; 1+, 26-50%; 2+, 51-75%; 3+, 75-100%. The components of the percentage and intensity scores were employed as the ultimate IHC scoring. Low BZW2 expression was defined as a score of 0 and + and high expression as a score of ++ and +++.
Statistical analysis
Various statistical analyses were performed to assess the role of BZW2 expression on clinical features and outcome in LUAD patients. Two-tailed Student’s t-test and one-way analysis of variance were adopted to compare two or multiple experimental groups. Chi-square test was adopted to compare clinical and pathological features between the BZW2 high and BZW2 low groups. Survival curves were made according to the Kaplan-Meier method, and the log-rank test was utilized to analyze significance between these curves. The role of BZW2 on survival was analyzed by univariate and multivariate Cox proportional hazard models. For all analyses, the SPSS v23 and GraphPad Prism 6 were employed, and significance was defined as *P≤0.05.
Results
BZW2 expression in the TCGA database
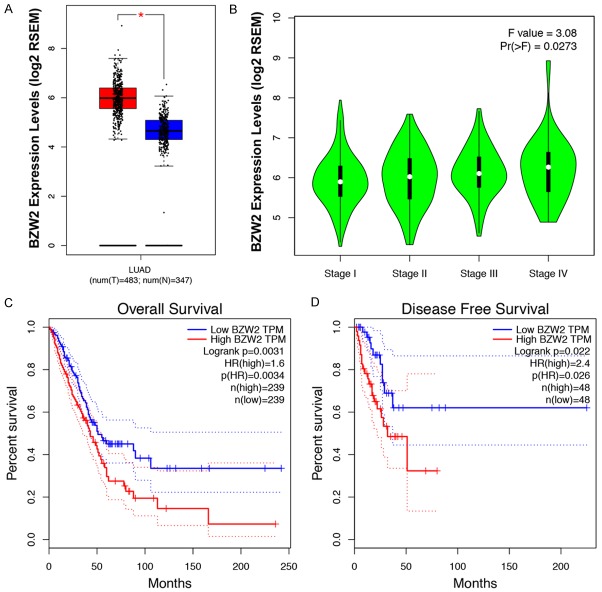
To evaluate the possibility that BZW2 is important for LUAD, we examined BZW2 expression in corresponding noncancerous tissues and LUAD using the Gene Expression Profiling Interactive Analysis (GEPIA) website based on a TCGA database [14]. Notably, BZW2 expression in LUAD samples was significantly higher compared to normal lung samples (P<0.05, Figure 1A). In detail, we further found that BZW2 expression in stage III & IV, the worst sub-group in LUAD patients, was dramatically higher than expression in the other seven groups (P<0.05, Figure 1B). In addition, LUAD patients bearing high BZW2 expression suffered poor clinical outcomes relative to low BZW2 expressing patients in the TCGA cohort. As shown in Figure 1C and 1D, elevated BZW2 expression is linked to a significantly shorter response duration of OS and DFS respectively. Thus we may hypothesize that BZW2 acts as an oncogene in LUAD as well and apply IHC for further investigation.
Figure 1.
Expression level of BZW2 in LUAD. A. BZW2 messenger RNA expression was higher in LUAD tissues than in normal lung tissues. B. BZW2 expression in different LUAD TMN stage cells. C, D. Kaplan-Meier analysis on the overall survival and disease-free survival of LUAD patients in TCGA database based on the BZW2 expression.
Association between BZW2 expression and clinicopathologic characteristics
We tried to characterize BZW2 protein expression in normal samples and in LUAD samples by analyzing immunohistochemical staining images from HPA database [15] and found that normal tissues had no BZW2 staining. Conversely, LUAD had moderate-to-strong BZW2 staining (Figure 2A). To further assess the BZW2 expression in LUAD patients, we performed the staining in 48 cases of LUAD patients and divided these patients into different groups. Twenty-four patients (50%) were classified into the high expression group, depending on the immunostaining intensity (Figure 2B). BZW2 expression levels relative to clinicopathologic characteristics were then investigated (Table 1). More high BZW expression tumors were stage III (66% vs. 29%), and high BZW2 expression tumors were larger (58% vs. 25%). We then explored other histopathologic markers related to tumor invasion, aggressiveness, and metastases. Lymphatic invasion was more common in the high BZW2 expression group (66% vs. 25%); moderate and high histologic architectural tumor grade was more common in the high BZW2 expression group (62% vs. 33%).
Figure 2.
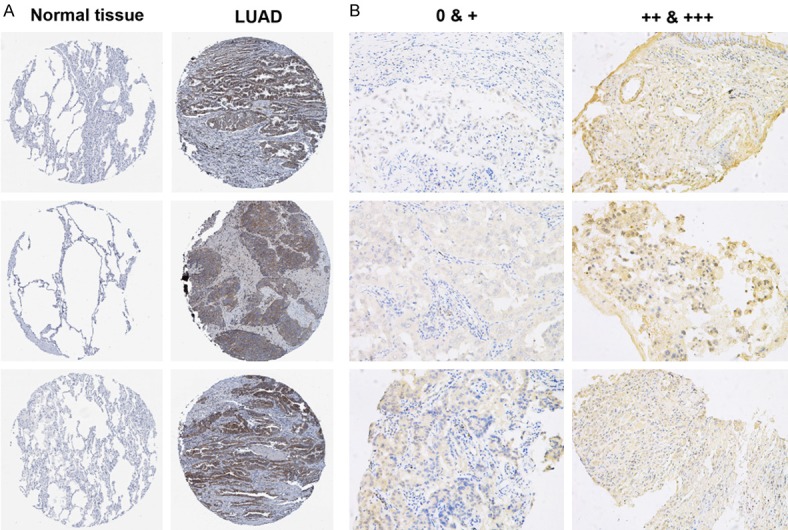
Representative types of BZW2 protein expression in tissue samples. A. BZW2 protein expression was significantly higher in LUAD tissues (right row) in comparison with normal respiratory epithelial tissues (left row). Images were downloaded from the Human Protein Atlas (HPA). B. BZW2 expression in LUAD patients. Representative case with a lack of BZW2 expression (left row); Representative case with stable BZW2 expression (right row).
Table 1.
Relation of the characteristics in 48 LUAD patients
| Characteristic | No. of Patient/Total No. (%) | 0 & + (n=24) | ++ & +++ (n=24) | p value |
|---|---|---|---|---|
| Age (≥65 yr) | 28/48 (58) | 16/24 (66) | 12/24 (50) | 0.241† |
| Male sex | 33/48 (68) | 14/24 (50) | 19/24 (79) | 0.119† |
| Smoking history | 19/48 (39) | 9/24 (37) | 10/24 (41) | 0.767† |
| Degree of differentiation (moderate or high) | 23/48 (47) | 8/24 (33) | 15/24 (62) | 0.043† |
| Tumor size (≥5 cm) | 20/48 (41) | 6/24 (25) | 14/24 (58) | 0.019† |
| Lymph node metastasis (positive) | 22/48 (45) | 6/24 (25) | 16/24 (66) | 0.003† |
| Tumor stage (III) | 23/48 (47) | 7/24 (29) | 16/24 (66) | 0.009† |
The chi-square test was used.
Higher BZW2 expression predicts poor prognosis in LUAD
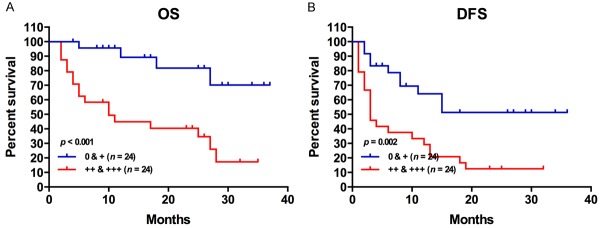
To assess the biological outcomes of elevated BZW2 in MM, we first compered the survival of two groups. In the high BZW2 expression group, 17 (70%) patients died from any cause, with 7 (30%) alive at the end of this study. Comparatively, in the low BZW2 expression group, 4 patients (16%) died from any cause, with 20 (84%) alive at the end of the study. As shown in Figure 3A, LUAD patients with high BZW2 expression had an inferior OS (P<0.001). Furthermore, Table 2 shows the impact of BZW2 expression and clinicopathologic characteristics on outcomes. Univariate analysis showed that BZW2 (HR=2.230, 95% CI: 1.395-3.566, P=0.001), tumor size (HR=2.441, 95% CI: 1.022-5.831, P=0.045), and smoking history (HR=3.135, 95% CI: 1.271-7.736, P=0.013) were significant predictors related to OS in LUAD. Furthermore, in the multivariate Cox analysis, BZW2 expression (HR=3.050, 95% CI: 1.219-7.633, P=0.017) was still an independent prognostic indicator of OS in LUAD.
Figure 3.
High BZW2 expression is associated with reduced overall survival and disease-free survival.
Table 2.
Univariate and multivariate Cox regression analyses for OS in 48 LUAD patients
| Variable | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age ≥65 yr | 0.688 | 0.291-1.625 | 0.394 | |||
| Male sex | 1.895 | 0.631-5.688 | 0.254 | |||
| Smoking history | 3.135 | 1.271-7.736 | 0.013 | 1.923 | 0.783-4.769 | 0.153 |
| Tumor size | 2.441 | 1.022-5.831 | 0.045 | 2.264 | 0.943-5.439 | 0.068 |
| Lymph node | 2.528 | 0.813-7.863 | 0.109 | |||
| Tumor stage | 1.780 | 0.651-4.866 | 0.261 | |||
| Differentiation | 1.689 | 0.674-4.327 | 0.264 | |||
| BZW2 ++ & +++ | 2.230 | 1.395-3.566 | 0.001 | 3.050 | 1.219-7.633 | 0.017 |
In the high BZW2 expression group, 21 (87%) patients died from any cause or distant recurrence, with 3 (13%) alive at the end of this study. Comparatively, in the low BZW2 expression group, 10 patients (41%) had recurrence or died of disease, with 14 (59%) alive without disease at the end of our study. As shown in Figure 3B, LUAD patients with high BZW2 expression had an inferior DFS (P=0.002). Furthermore, Table 3 also shows the impact of BZW2 expression and clinicopathologic characteristics on outcome. Univariate analysis showed that BZW2 (HR=1.685, 95% CI: 1.208-2.350, P=0.002) and smoking history (HR=2.331, 95% CI: 1.115-4.876, P=0.025) were significant predictors related to DFS in LUAD. In the multivariate Cox analysis, BZW2 expression (HR=1.659, 95% CI: 1.194-2.305, P=0.003) was still an independent prognostic indicator of DFS in LUAD.
Table 3.
Univariate and multivariate Cox regression analyses for DFS in 48 LUAD patients
| Variable | Univariate model | Multivariate model | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| HR | 95% CI | P | HR | 95% CI | P | |
| Age ≥65 yr | 0.799 | 0.384-1.661 | 0.548 | |||
| Male sex | 1.159 | 0.528-2.542 | 0.714 | |||
| Smoking history | 2.331 | 1.115-4.876 | 0.025 | 2.556 | 1.220-5.357 | 0.013 |
| Tumor size | 2.011 | 0.965-4.190 | 0.062 | |||
| Lymph node | 2.359 | 0.966-5.756 | 0.059 | |||
| Tumor stage | 2.210 | 0.978-4.990 | 0.056 | |||
| Differentiation | 1.246 | 0.600-2.587 | 0.556 | |||
| BZW2 ++ & +++ | 1.685 | 1.208-2.350 | 0.002 | 1.659 | 1.194-2.305 | 0.003 |
BZW2 positively correlates with the EIF5 in LUAD
BZW2, also known as EIF5-mimic protein, is a paralogous human protein containing a C-terminal HEAT domain that resemble the HEAT domain of EIF5 [16]. Using STRING tools, the protein-protein interaction analysis also showed that BZW2 and EIF5 interact or co-express in the human Homo sapiens protein interaction network (Figure 4A). Given that BZW2 is involved in EIF5 mediated-LUAD progression, we investigated the correlation between BZW2 and EIF5 expression in GSE31210. EIF5 expression was significantly correlated with BZW2 expression, with R value of 0.3262, respectively (Figure 4B). To further investigate whether BZW2 and EIF5 have synergistic or additive effects in patient outcome, the LUAD patients were classified into 2 groups including BZW2 low/EIF5 low and BZW2 high/EIF5 high, and Kaplan-Meier analyses showed clearly that the BZW2 high/EIF5 high group had the worst outcome in OS and DFS (P<0.05, Figure 4C and 4D). These data strongly support findings that BZW2 interacts with EIF5 and promotes its oncogenic function in LUAD.
Figure 4.
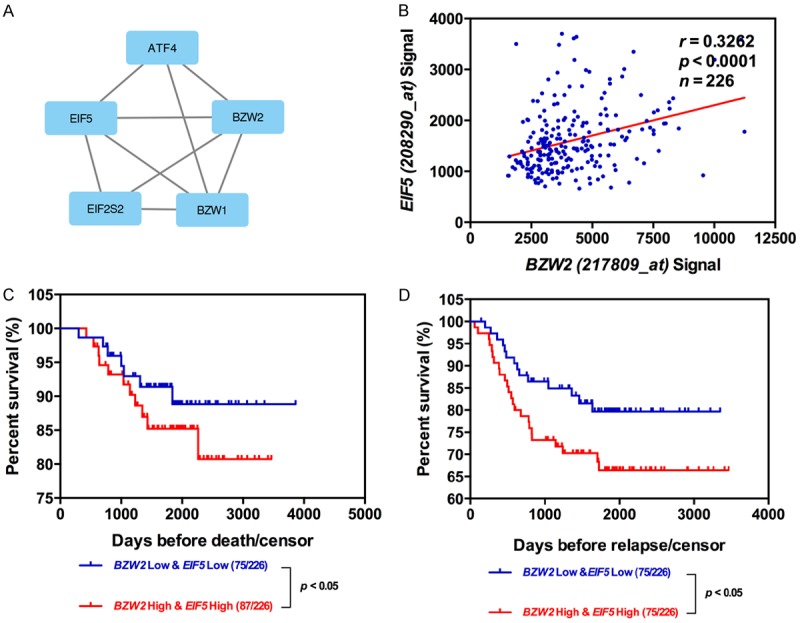
Relationship between the expression of BZW2 and EIF5 in LUAD. A. The Protein network was constructed by online software string. B. Pearson correlation analysis between BZW2 levels and EIF5 levels from GSE31210 microarray dataset. C, D. Kaplan-Meier analyses of OS and DFS among LUAD patients with different expression levels of BZW2 and EIF5.
Discussion
Risk factors to identify LUAD patients who have high risk for prognosis and distant recurrence are still poorly defined [7]. Currently, the most applied risk stratification for LUAD patients is TNM stage [17]. Nevertheless, the long-term survival of LUAD patients remains poor [18,19]. It is important to identify a novel biomarker to improve the outcome of LUAD. BZW2 was initially identified in osteosarcoma, and further research showed BZW2 was overexpressed in multiple tumors. What is more, high expression of BZW2 is associated with tumor aggressiveness [10,11,20]. To our knowledge, there was no study about the expression and clinical significance of BZW2 in LUAD. Therefore, we investigated the BZW2 expression, collected demographic and clinical information, and explored the correlation between the BZW2 expression and clinicopathologic characteristics. Coincidentally, our study showed that the BZW2 was overexpressed in LUAD patients. We further explored the correlations of elevated BZW2 expression and clinicopathologic characteristics in LUAD. As shown in Table 1, the elevated BZW2 expression is related to aggressive features (including lymph node metastasis, poor histopathologic differentiation, and advanced clinical stage).
In survival analysis, BZW2 expression, tumor size, and smoking history were screened as independent factors that were significantly related to OS and DFS of LUAD patients by using univariate modes. Furthermore, multivariate analysis also identified that BZW2 acts as the independently prognostic indicator for LUAD patients. Kaplan-Meier curves also depict that the lifespan of LUAD patients with high BZW2 expression suffered a poor overall survival (OS) and disease free survival (DFS). Currently, this is the first study indicating a correlation between the expression of BZW2 and prognostic significance in LUAD patients. As BZW2 expression was easily evaluated by immunohistochemistry on formalin-fixed and paraffin-embedded sections, immunohistochemistry of BZW2 would identify LUAD with poor prognosis.
It has been established that EIF5 plays essential roles in promoting the ribosome pre-initiation complex assembly and accurate translation initiation [21]. Previous research has shown EIF5A2 was overexpressed of in various human tumors [22-24]. EIF5A2 has been shown to play an important role in malignant cell proliferation, transformation, and metastasis [25]. It can bind to the cap structure located at the 5’ end of mRNA and is fundamental for mRNA translation initiation. It is a major factor in the initial process of protein synthesis. By screening BZW2-interaction proteins reported by STRING database, we found that BZW2 and EIF5 mRNA expression is positively correlated in GSE31210 database. Additionally, the high expression of BZW2 and EIF5 group showed the worst prognosis in several groups of LUAD patients. These clinical results strongly support that BZW2 up-regulates EIF5 expression and promotes its oncogenic function.
To sum up, our study identified that BZW2 was overexpressed in LUAD. The elevated BZW2 expression was related to aggressive clinicopathologic features and poor prognosis, suggesting that BZW2 could be applied as a novel prognostic biomarker in LUAD.
Disclosure of conflict of interest
None.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Zheng YZ, Ma R, Zhou JK, Guo CL, Wang YS, Li ZG, Liu LX, Peng Y. ROR1 is a novel prognostic biomarker in patients with lung adenocarcinoma. Sci Rep. 2016;6:36447. doi: 10.1038/srep36447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hirsch FR, Scagliotti GV, Mulshine JL, Kwon R, Curran WJ Jr, Wu YL, Paz-Ares L. Lung cancer: current therapies and new targeted treatments. Lancet. 2017;389:299–311. doi: 10.1016/S0140-6736(16)30958-8. [DOI] [PubMed] [Google Scholar]
- 4.Bucciarelli PR, Tan KS, Chudgar NP, Brandt W, Montecalvo J, Eguchi T, Liu Y, Aly R, Travis WD, Adusumilli PS, Jones DR. BRMS1 expression in surgically resected lung adenocarcinoma predicts future metastases and is associated with a poor prognosis. J Thorac Oncol. 2018;13:73–84. doi: 10.1016/j.jtho.2017.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cancer Genome Atlas Research Network. Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Fan W, Xu L, Mao Q, Chen Y, Mao Y, Xu L, Wang J. Rab27b is a potential indicator for lymph node metastasis and unfavorable prognosis in lung adenocarcinoma. Dis Markers. 2018;2018:7293962. doi: 10.1155/2018/7293962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koo HK, Jin SM, Lee CH, Lim HJ, Yim JJ, Kim YT, Yang SC, Yoo CG, Han SK, Kim JH, Shim YS, Kim YW. Factors associated with recurrence in patients with curatively resected stage I-II lung cancer. Lung Cancer. 2011;73:222–229. doi: 10.1016/j.lungcan.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Soloway MS. Bladder cancer: lack of progress in bladder cancer--what are the obstacles? Nat Rev Urol. 2013;10:5–6. doi: 10.1038/nrurol.2012.219. [DOI] [PubMed] [Google Scholar]
- 9.Li S, Chai Z, Li Y, Liu D, Bai Z, Li Y, Li Y, Situ Z. BZW1, a novel proliferation regulator that promotes growth of salivary muocepodermoid carcinoma. Cancer Lett. 2009;284:86–94. doi: 10.1016/j.canlet.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 10.Jin X, Liao M, Zhang L, Yang M, Zhao J. Role of the novel gene BZW2 in the development of hepatocellular carcinoma. J Cell Physiol. 2019 doi: 10.1002/jcp.28331. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 11.Cheng DD, Li SJ, Zhu B, Yuan T, Yang QC, Fan CY. Downregulation of BZW2 inhibits osteosarcoma cell growth by inactivating the Akt/mTOR signaling pathway. Oncol Rep. 2017;38:2116–2122. doi: 10.3892/or.2017.5890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao H, Yu G, Zhang X, Yu S, Sun Y, Li Y. BZW2 gene knockdown induces cell growth inhibition, G1 arrest and apoptosis in muscle-invasive bladder cancers: a microarray pathway analysis. J Cell Mol Med. 2019;23:3905–3915. doi: 10.1111/jcmm.14266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Okayama H, Kohno T, Ishii Y, Shimada Y, Shiraishi K, Iwakawa R, Furuta K, Tsuta K, Shibata T, Yamamoto S, Watanabe S, Sakamoto H, Kumamoto K, Takenoshita S, Gotoh N, Mizuno H, Sarai A, Kawano S, Yamaguchi R, Miyano S, Yokota J. Identification of genes upregulated in ALK-positive and EGFR/KRAS/ALK-negative lung adenocarcinomas. Cancer Res. 2012;72:100–111. doi: 10.1158/0008-5472.CAN-11-1403. [DOI] [PubMed] [Google Scholar]
- 14.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45:W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Uhlen M, Zhang C, Lee S, Sjostedt E, Fagerberg L, Bidkhori G, Benfeitas R, Arif M, Liu Z, Edfors F, Sanli K, von Feilitzen K, Oksvold P, Lundberg E, Hober S, Nilsson P, Mattsson J, Schwenk JM, Brunnstrom H, Glimelius B, Sjoblom T, Edqvist PH, Djureinovic D, Micke P, Lindskog C, Mardinoglu A, Ponten F. A pathology atlas of the human cancer transcriptome. Science. 2017;357 doi: 10.1126/science.aan2507. [DOI] [PubMed] [Google Scholar]
- 16.Loughran G, Firth AE, Atkins JF, Ivanov IP. Translational autoregulation of BZW1 and BZW2 expression by modulating the stringency of start codon selection. PLoS One. 2018;13:e0192648. doi: 10.1371/journal.pone.0192648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75. doi: 10.1007/978-3-319-40389-2_3. [DOI] [PubMed] [Google Scholar]
- 18.Kasapoglu US, Arinc S, Gungor S, Irmak I, Guney P, Aksoy F, Bandak D, Hazar A. Prognostic factors affecting survival in non-small cell lung carcinoma patients with malignant pleural effusions. Clin Respir J. 2016;10:791–799. doi: 10.1111/crj.12292. [DOI] [PubMed] [Google Scholar]
- 19.Liang W, Zhang L, Jiang G, Wang Q, Liu L, Liu D, Wang Z, Zhu Z, Deng Q, Xiong X, Shao W, Shi X, He J. Development and validation of a nomogram for predicting survival in patients with resected non-small-cell lung cancer. J. Clin. Oncol. 2015;33:861–869. doi: 10.1200/JCO.2014.56.6661. [DOI] [PubMed] [Google Scholar]
- 20.Sato K, Masuda T, Hu Q, Tobo T, Gillaspie S, Niida A, Thornton M, Kuroda Y, Eguchi H, Nakagawa T, Asano K, Mimori K. Novel oncogene 5MP1 reprograms c-Myc translation initiation to drive malignant phenotypes in colorectal cancer. EBioMedicine. 2019;44:387–402. doi: 10.1016/j.ebiom.2019.05.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hinnebusch AG. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem. 2014;83:779–812. doi: 10.1146/annurev-biochem-060713-035802. [DOI] [PubMed] [Google Scholar]
- 22.Guan XY, Fung JM, Ma NF, Lau SH, Tai LS, Xie D, Zhang Y, Hu L, Wu QL, Fang Y, Sham JS. Oncogenic role of eIF-5A2 in the development of ovarian cancer. Cancer Res. 2004;64:4197–4200. doi: 10.1158/0008-5472.CAN-03-3747. [DOI] [PubMed] [Google Scholar]
- 23.Tang DJ, Dong SS, Ma NF, Xie D, Chen L, Fu L, Lau SH, Li Y, Li Y, Guan XY. Overexpression of eukaryotic initiation factor 5A2 enhances cell motility and promotes tumor metastasis in hepatocellular carcinoma. Hepatology. 2010;51:1255–1263. doi: 10.1002/hep.23451. [DOI] [PubMed] [Google Scholar]
- 24.Zhu W, Cai MY, Tong ZT, Dong SS, Mai SJ, Liao YJ, Bian XW, Lin MC, Kung HF, Zeng YX, Guan XY, Xie D. Overexpression of EIF5A2 promotes colorectal carcinoma cell aggressiveness by upregulating MTA1 through C-myc to induce epithelial-mesenchymaltransition. Gut. 2012;61:562–575. doi: 10.1136/gutjnl-2011-300207. [DOI] [PubMed] [Google Scholar]
- 25.Lee NP, Tsang FH, Shek FH, Mao M, Dai H, Zhang C, Dong S, Guan XY, Poon RT, Luk JM. Prognostic significance and therapeutic potential of eukaryotic translation initiation factor 5A (eIF5A) in hepatocellular carcinoma. Int J Cancer. 2010;127:968–976. doi: 10.1002/ijc.25100. [DOI] [PubMed] [Google Scholar]