Abstract
Pancreatic cancer (PC) has one of the worst survival rates of all cancers. Anoctamin (ANO) inlcudes a family of Ca2+-activated Cl- channels (CaCCs) that participate in tumorigenesis and progression. However, the exact role of ANO5 in PC has not yet been clarified. Our previous study showed that ANO5 is highly expressed in the epithelial cells of the digestive tract, but there have been no reports of ANO5 expression in normal pancreatic tissue and in PC tissue. In the present study, we investigated the expression of ANO5 in normal pancreatic tissues and PC tissues using immunohistochemistry. The results indicated that ANO5 expression was significantly elevated in PC tissues compared with normal pancreatic tissues. Next, we investigated the expression of ANO5 in pancreatic ductal epithelial cells and PC cells using western blotting and quantitative real-time reverse transcription polymerase chain reaction (RT-qPCR). The results indicated that ANO5 expression was significantly elevated in PC cells compared with pancreatic ductal epithelial cells. The impact of siRNA-mediated ANO5 knockdown on PC cell proliferation and migration was detected using CCK-8 assays and scratch experiments. The results indicated that ANO5 siRNA reduced the proliferation and migration of PC cells. Collectively, the results suggest that downregulation of ANO5 inhibits the proliferation and migration of PC cells. Therefore, ANO5 is potentially a novel therapeutic target for PC treatment.
Keywords: Anoctamin 5, pancreatic cancer, proliferation, migration
Introduction
Pancreatic cancer (PC) is an aggressive and highly metastatic malignancy that results in a high mortality rate and is a serious threat to human health worldwide. The incidence and death rates of PC have been increasing in recent years [18]. Recently, investigation of ion channel dysfunction has become an emerging field in cancer research, and the relationship between ion channels and tumors has attracted increasing attention. Differential expression of multiple classes of ion channels has been reported for different cancer tissues. Calcium-activated chloride channels of the ANO (alias TMEM16) protein family have been reported to be abnormally expressed in several cancers and to play a role in cancer development and progression [2,6,7,10,13,20]. One study has shown that exogenous expression of ANO9 in PANC-1 cells significantly increases cell proliferation in cell cultures and in mice [7]. Inhibition of ANO1 expression suppresses growth and invasion in human colorectal cancer cells and in human lung cancer [6,20]. A mutation in ANO5, a member of the anoctamin family, has been reported to be closely related to muscular dystrophy [16,22,23], and some patients with ANO5 mutations suffer from upper gastrointestinal tract syndromes such as dysphagia [25]. In a previous study, we found that ANO5 is widely expressed in epithelial cells of the gastrointestinal tract [19]. Recently, Chang et al. reported that ANO5 regulates cell migration and invasion in thyroid cancer [1]. However, the role of ANO5 in pancreatic cancer remains unknown.
In this study, we found that ANO5 expression was highly upregulated in human pancreatic cancer tissues, and ANO5 expression upregulation was confirmed in two types of human pancreatic cancer cell lines at the mRNA and protein levels. Using siRNA to knock down ANO5 expression, we found that, surprisingly, inhibition of ANO5 could influence the migration and proliferation of PDAC cells.
Materials and methods
Pancreatic tissues and cells
Human pancreatic cancer tissue microarrays were purchased from Xi’an Alena Biotechnology Co., Ltd. of China. In the tissue microarrays, there are pancreatic cancer samples and normal pancreatic tissue samples adjacent to carcinoma tissue, including 36 cancer samples and 4 normal samples. The human PC cell lines HPAC, BxPC-3, and PANC-1 and HEK293 cells were obtained from the American Type Culture Collection (ATCC, USA), and the human pancreatic ductal epithelial cell line HPDE6-C7 was purchased from the cell bank of the Chinese Academy of Sciences (Shanghai, China).
Chemicals and reagents
The ANO5 antibodies or39268 and ab181663 were obtained from Biorbyt Ltd. (Biorbyt, Cambridge, UK) and Abcam (Abcam, Cambridge, UK), respectively. ANO5 siRNA (sc-97047) and negative control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology (Santa Cruz Biotechnology, Dallas, USA), vimentin antibody (ab92547) was obtained from Abcam (Abcam, Cambridge, UK).
Immunohistochemical staining
We used xylene and graded alcohols (ZSGB-BIO, Beijing, China) to dewax and rehydrate the samples. Subsequently, citrate salt buffer (pH 6.0) was used to treat the samples in the microwave for 15 min for antigen retrieval, and then 3% hydrogen peroxide (ZSGB-BIO, Beijing, China) was used to block endogenous peroxidase activity for 15 min. Before the samples were incubated with ANO5 antibody at 4°C overnight, the samples were blocked with 5% donkey blood serum (Jackson, West Grove, USA) in phosphate-buffered saline (PBS) for 1 h at room temperature. Then, the samples were incubated with secondary horseradish peroxidase (HRP)-conjugated antibodies (ZSGB-BIO, Beijing, China) for 1 h at room temperature. Next, diaminobenzidine (DAB) and hematoxylin (ZSGB-BIO, Beijing, China) were used for staining and counterstaining, respectively. Following dehydration with graded alcohols and xylene, the slides were sealed with coverslips and neutral gum.
Western blotting
Total protein was isolated from cells using RIPA (radioimmunoprecipitation) buffer (Beyotime Bio, Shanghai, China) containing a protease inhibitor cocktail and PMSF. The cell suspension was centrifuged at 12,000 × g for 30 min, and the supernatant fractions were collected. Protein concentrations were measured with a PierceTM BCA protein assay kit according to the manufacturer’s instructions (Thermo Scientific, Waltham, USA). Equal amounts of protein were loaded and separated on 8% polyacrylamide gels and then transferred onto nitrocellulose filter (NC) membranes (Millipore, Massachusetts, USA). The membranes were incubated with 5% milk for 1 h at room temperature, and the primary ANO5 antibody (1:1000), vimentin antibody (1:1000) was used to incubate the membranes at 4°C overnight. The membranes were subsequently incubated with secondary goat anti-rabbit IgG HRP-conjugated antibodies (1:1000; LI-COR Biosciences, Nebraska, USA) for 1 h at room temperature. Protein bands were visualized using a chemiluminescence detection system (Odyssey® two-color infrared fluorescence imaging system; LI-COR Biosciences, Nebraska, USA). The protein levels were normalized to GAPDH levels and quantified using ImageJ software (NIH, USA).
RNA isolation and RT-qPCR
Total RNA was isolated from cells using TRIzol reagent (Invitrogen, California, USA). The RNA concentration was measured using a NanoDrop® ND-2000 spectrophotometer (Thermo Scientific, Waltham, USA). cDNA was synthesized with a RevertAid First Strand cDNA Synthesis Kit (Thermo Scientific, Waltham, USA). RT-qPCR primers for ANO5 (forward, 5’-3’: CGAGATGGGATTAGGCAAATTG; reverse, 5’-3’: AAACTCTTTTCTTCTTTCCGCC) were synthesized by Sangon Biotech (Shanghai, China). RT-qPCR was conducted using an Mx3000p RT-PCR detection system and TransStart Top Green qPCR SuperMix (Transgen Biotech, China). The relative gene expression levels were normalized to glyceraldehyde 3-phosphate dehydrogenase (GAPDH) levels.
siRNA-mediated ANO5 knockdown in PANC-1 cells
ANO5 siRNA (sc-97047) and negative control siRNA (sc-37007) were purchased from Santa Cruz Biotechnology. For PANC-1 cell transfection, PANC-1 cells were seeded into 6-well plates and transfected with ANO5 siRNA (50 nM) or negative control siRNA using Lipofectamine 2000 (Invitrogen, Chicago, USA). After 48 h of transfection, mRNA and protein were extracted from the PANC-1 cells. RT-qPCR and western blotting were used to confirm the efficiency of ANO5 knockdown.
Cell proliferation
Before transfection with ANO5 siRNA (50 nM) or negative control siRNA using Lipofectamine 2000, PANC-1 cells (1 × 105) were seeded in a 12-well plate in 1 ml of DMEM containing 10% FBS and incubated at 37°C in 5% CO2 for 24 h. At 24, 48 and 72 h after transfection, cell growth was measured using a CCK-8 cell viability assay (Beyotime Bio, Shanghai, China) according to the manufacturer’s instructions.
Cell migration assay
PANC-1 cells were seeded in a 6-well plate (1 × 105 cells) and incubated at 37°C in 5% CO2 for 24 h. A scratch was made using a 1 ml pipette tip before the cells were transfected with ANO5 siRNA. Images were captured at 0 h, 24 h and 48 h after transfection under an inverted microscope. ImageJ software was used to calculate the area of the scratch. Then, the percentage of wound closure was calculated and compared with that of the negative control group.
Statistical analysis
The data are presented as the mean ± SD. Comparisons between two groups were analyzed with ANOVA test using GraphPad Prism 5 software (GraphPad Software, USA). P < 0.05 was considered significant. All experiments were repeated at least three times.
Results
The expression of ANO5 in human pancreatic cancer and normal human pancreatic tissues
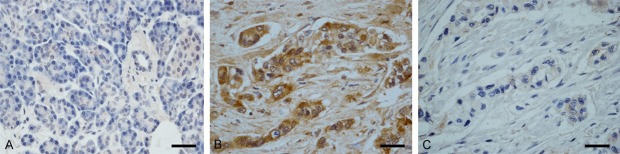
Rabbit polyclonal anti-ANO5 antibodies were used to detect the expression of ANO5 in normal human pancreatic tissues and PC tissues. ANO5 was not expressed in normal pancreatic tissue adjacent to carcinoma tissue, but ANO5 was expressed in PC tissues positively (Figure 1).
Figure 1.
Immunohistochemical staining of ANO5 in normal pancreas and in psancreatic adenocarcinoma (PDAC) tissue. (A) ANO5 in normal pancreatic tissue, (B) ANO5 in PDAC tissue, and (C) Negative control PDAC tissue. Bars, 100 μm.
Approximately 66.7% of the samples were positive for ANO5 (24/36). This result suggests that ANO5 may be involved in the development of tumors.
The expression of ANO5 in human pancreatic cancer cells
Furthermore, we examined the expression of ANO5 in normal human pancreatic duct epithelial cells and pancreatic adenocarcinoma (HPAC) cells. The results of western blotting showed that the expression of ANO5 protein in HPAC cells was significantly increased compared with that in normal human pancreatic duct epithelial cells (HEK293 cells were used as positive controls) (Figure 2). Upon detection of ANO5 protein expression in the three types of pancreatic cancer cells, we found that ANO5 protein expression was different among the three cancer cell types and that the ANO5 protein expression was highest in the PANC-1 cells (Figure 3). The ANO5 mRNA expression results were consistent with those for ANO5 protein expression.
Figure 2.
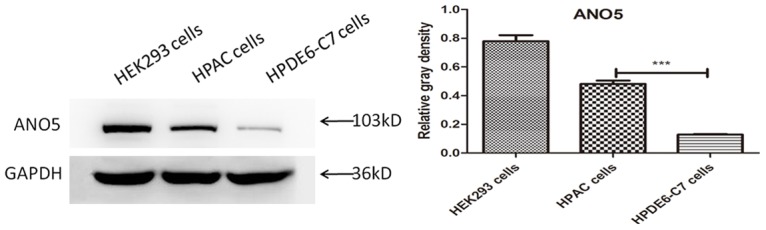
Representative western blotting images showing expression of ANO5 in HPAC cells and HPDE6-C7 cells and densitometry analyses of the blots. ***P < 0.001 compared with HPAC cells.
Figure 3.
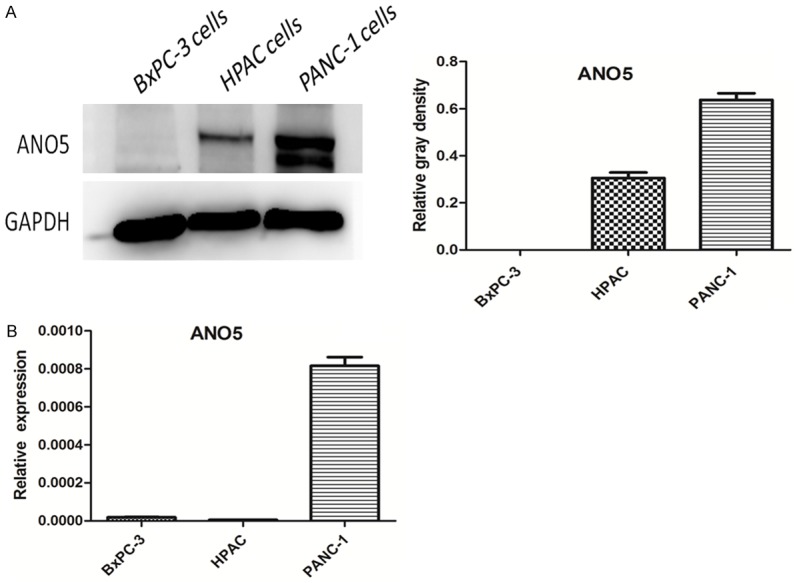
Expression of ANO5 in BxPC-3 cells, HPAC cells, and PANC-1 cells. A. Representative western blot images showing the expression of ANO5 protein in BxPC-3 cells, HPAC cells, and PANC-1 cells and densitometry analyses of the blots. B. RT-qPCR results showing the expression of ANO5 mRNA in BxPC-3 cells, HPAC cells, and PANC-1 cells.
Effect of ANO5 siRNA on the proliferation and migration of PANC-1 cells
To determine whether downregulation of ANO5 changes the biologic properties of PANC-1 cells, ANO5 siRNA (50 nM) was used to knock down ANO5 expression. The results showed that there was a significant downregulation of ANO5 protein expression in PANC-1 cells transfected with ANO5 siRNA, but not in PANC-1 cells transfected with control siRNA (Figure 4). This finding suggests that ANO5 siRNA can effectively silence the expression of the ANO5 gene in PANC-1 cells.
Figure 4.
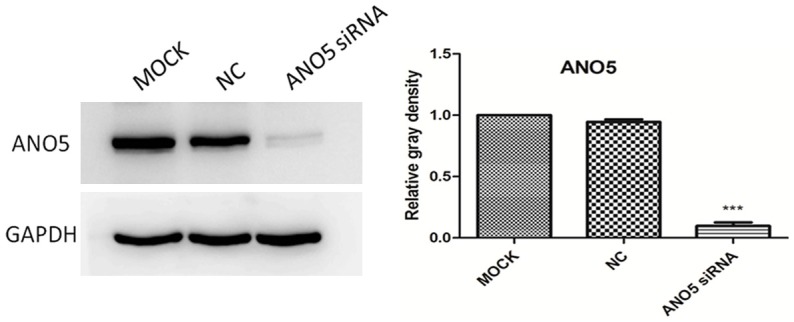
Representative western blotting images of ANO5 protein expression after inhibition with ANO5 siRNA in PANC-1 cells and densitometry analyses of the blots. MOCK: blank control group, NC: negative control group, ANO5 siRNA: ANO5 siRNA group. ***P < 0.001 compared with negative control group.
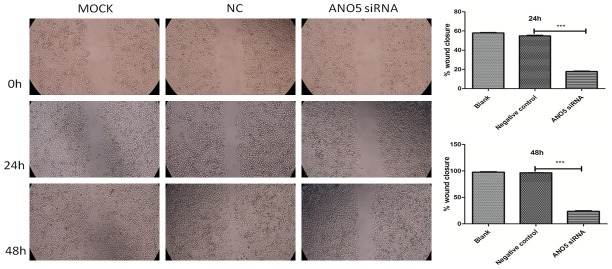
We examined whether ANO5 plays a role in the proliferation and migration of PANC-1 cells. We used a scratch assay (wound healing assay) and a cholecystokinin-8 (CCK-8) assay to measure the effect of ANO5 siRNA on PANC-1 cell migration and proliferation, respectively. The scratch assay showed that, unlike treatment with control siRNA, treatment with ANO5 siRNA (50 nM) inhibited wound closure (as assessed based on the scratch gap) and therefore the migration of PANC-1 cells. As shown in Figure 5, control-siRNA-treated PANC-1 cells migrated into the gap formed by the scratch made in the cell monolayer and covered 54.90% of the gap surface area 24 h after transfection and 96.44% of the gap area 48 h after transfection. In contrast, PANC-1 cells transfected with ANO5 siRNA migrated much more slowly than siRNA-control-treated PANC-1 cells, filling up only 17.74% and 23.86% of the gap after 24 h and 48 h, respectively (comparison with negative control group, P < 0.001).
Figure 5.
Representative microscopic images showing the effect of ANO5 siRNA on the migration of PANC-1 cells and semiquantitative image analysis. MOCK: blank control group, NC: negative control group, ANO5 siRNA: ANO5 siRNA group. ***P < 0.001 compared with negative control group.
Vimentin is an EMT marker. The decreased expression of vimentin indicated that tumor cells recovered the characteristics of epithelial cells, and the ability of tumor cells to migrate and invade was reduced. The result of western blotting showed that there was a significant downregulation of vimentin protein expression in PANC-1 cells transfected with ANO5 siRNA, but not in PANC-1 cells transfected with control siRNA and blank controls (Figure 6B). These results indicate that downregulation of ANO5 severely inhibits the migratory activity of PANC-1 cells.
Figure 6.
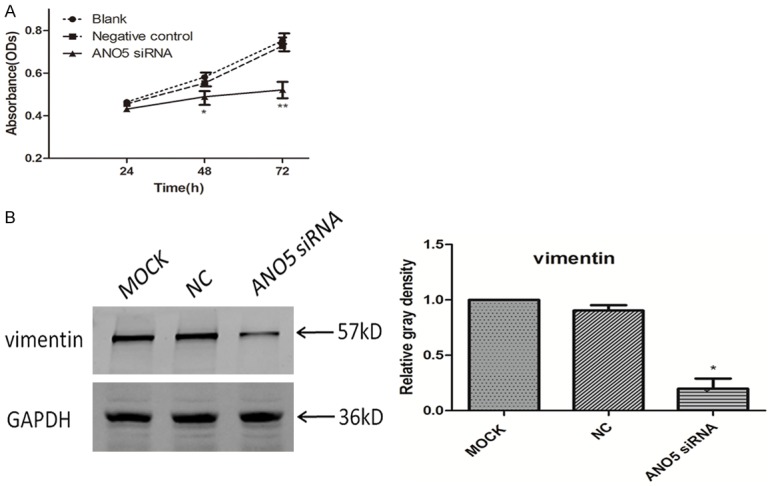
A. Cell growth curve showing that transfection of PANC-1 with ANO5 siRNA significantly reduced PANC-1 cell proliferation compared to that after transfection with negative control siRNA. B. Representative western blotting images of vimentin protein expression after inhibition with ANO5 siRNA in PANC-1 cells and densitometry analyses of the blots. MOCK: blank control group, NC: negative control group, ANO5 siRNA: ANO5 siRNA group. *P < 0.05 compared to negative control group.
We further investigated whether downregulation of ANO5 by siRNA inhibited PANC-1 cell proliferation. ANO5 knockdown significantly inhibited the proliferation of PANC-1 cells (Figure 6A). These data suggest that ANO5 regulates the migration and proliferation of PANC-1 cells.
Discussion
In the present study, we have demonstrated, for the first time, the expression of ANO5 in pancreatic cancer tissue and pancreatic cancer cells. ANO5 was not distributed in normal pancreatic tissue, but ANO5 expression was highly upregulated in human PC tissues and human PC cell lines. Using siRNA to knock down ANO5 expression, we found that, surprisingly, downregulation of ANO5 could influence the migration and proliferation of PC cells.
The genes of the TMEM16 family, also called the ANO family, encode 10 transmembrane proteins with eight hydrophobic helices. The ANO family comprises 10 members, including ANO1-ANO10 [8]. All members of the ANO family have been demonstrated to be Ca2+-activated Cl- channels involved in many physiologic functions [5]. CaCCs play important roles in the cell secretion process in epithelial cells, including pancreatic duct epithelial cells [15]. In a previous study, we found that ANO5 is widely expressed in epithelial cells of the gastrointestinal tract [19]. In this study, we measured ANO5 expression in human pancreatic cancer tissues and normal human pancreatic tissues and found that ANO5 is not expressed in normal pancreatic tissues.
Plasma membrane ion channels are involved in apoptosis, differentiation, proliferation, and cell migration [3,9,12,24]. Chloride intracellular channel 1 (CLIC1) acts as a putative oncogene in pancreatic cancer [11]. CLIC1 can participate the proliferation, migration, and invasion of a variety of tumor cells [21,26]. Inhibition of the CaCC ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer and human prostate carcinoma [6,10]. Downregulation of ANO1 significantly inhibits cancer cell proliferation, migration, and invasion [4,14,17]. ANO9/TMEM16J promotes tumorigenesis by EGFR and is a novel therapeutic target for pancreatic cancer [7]. The results of a previous study have shown that ANO1 channels are pivotal in PDAC cell migration but do not influence cellular proliferation [2]. ANO5 is downregulated in thyroid cancer, and downregulation of ANO5 promotes thyroid cancer cell migration and invasion [1]. The above results show that the physiologic function of ANO family members has been found to vary among different cancer tissues. In the present study, ANO5 was not expressed in normal pancreatic tissue adjacent to carcinoma tissue, but the expression of ANO5 was significantly increased in pancreatic tissues and pancreatic cancer cells. Inhibition of ANO5 by siRNA could inhibit the proliferation and migration of PANC-1 cells. Our results suggest that inhibitors of ANO5 may have therapeutic potential for the treatment of pancreatic cancer or other cancers with high levels of ANO5 expression.
Since ANO5 is less expressed in normal HPDEC-C7 cells, we have not detected the effect of ANO5 on normal HPDEC-C7 cells. We will explore its role in the future.
Acknowledgements
This work was financially supported by the Doctor Scientific Research Foundation of Xinxiang Medical University (XXBSKYZZ201815).
All patients provided written consent for the publication of their data.
Disclosure of conflict of interest
None.
References
- 1.Chang Z, Cai C, Han D, Gao Y, Li Q, Feng L, Zhang W, Zheng J, Jin J, Zhang H, Wei Q. Anoctamin 5 regulates cell migration and invasion in thyroid cancer. Int J Oncol. 2017;51:1311–1319. doi: 10.3892/ijo.2017.4113. [DOI] [PubMed] [Google Scholar]
- 2.Sauter DRP, Novak I, Pedersen SF, Larsen EH, Hoffmann EK. ANO1 (TMEM16A) in pancreatic ductal adenocarcinoma (PDAC) Pflugers Arch. 2015;467:1495–1508. doi: 10.1007/s00424-014-1598-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Du S, Yang L. ClC-3 chloride channel modulates the proliferation and migration of osteosarcoma cells via AKT/GSK3β signaling pathway. Int J Clin Exp Pathol. 2015;8:1622–1630. [PMC free article] [PubMed] [Google Scholar]
- 4.Guan L, Song Y, Gao J, Gao J, Wang K. Inhibition of calcium-activated chloride channel ANO1 suppresses proliferation and induces apoptosis of epithelium originated cancer cells. Oncotarget. 2016;7:78619–78630. doi: 10.18632/oncotarget.12524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hartzell HC, Yu K, Xiao Q, Chien LT, Qu Z. Anoctamin/TMEM16 family members are Ca2+-activated Cl- channels. J Physiol. 2009;587:2127–2139. doi: 10.1113/jphysiol.2008.163709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jia L, Liu W, Guan L, Lu M, Wang K. Inhibition of calcium-activated chloride channel ANO1/TMEM16A suppresses tumor growth and invasion in human lung cancer. PLoS One. 2015;10:e0136584. doi: 10.1371/journal.pone.0136584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jun I, Park HS, Piao H, Han JW, An MJ, Yun BG, Zhang X, Cha YH, Shin YK, Yook JI, Jung J, Gee HY, Park JS, Yoon DS, Jeung HC, Lee MG. ANO9/TMEM16J promotes tumourigenesis via EGFR and is a novel therapeutic target for pancreatic cancer. Br J Cancer. 2017;117:1798–1809. doi: 10.1038/bjc.2017.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Katoh M, Katoh M. GDD1 is identical to TMEM16E, a member of the TMEM16 family. Am J Hum Genet. 2004;75:927–928. doi: 10.1086/425341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kunzelmann K, Nilius B, Owsianik G, Schreiber R, Ousingsawat J, Sirianant L, Wanitchakool P, Bevers EM, Heemskerk JW. Molecular functions of anoctamin 6 (TMEM16F): a chloride channel, cation channel, or phospholipid scramblase. Pflugers Arch. 2014;466:407–414. doi: 10.1007/s00424-013-1305-1. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Lu M, Liu B, Huang Y, Wang K. Inhibition of Ca(2+)-activated Cl(-) channel ANO1/TMEM16A expression suppresses tumor growth and invasiveness in human prostate carcinoma. Cancer Lett. 2012;326:41–51. doi: 10.1016/j.canlet.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Lu J, Dong Q, Zhang B, Wang X, Ye B, Zhang F, Song X, Gao G, Mu J, Wang Z, Ma F, Gu J. Chloride intracellular channel 1 (CLIC1) is activated and functions as an oncogene in pancreatic cancer. Med Oncol. 2015;32:616. doi: 10.1007/s12032-015-0616-9. [DOI] [PubMed] [Google Scholar]
- 12.Martins JR, Faria D, Kongsuphol P, Reisch B, Schreiber R, Kunzelmann K. Anoctamin 6 is an essential component of the outwardly rectifying chloride channel. Proc Natl Acad Sci U S A. 2011;108:18168–18172. doi: 10.1073/pnas.1108094108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matsuba S, Niwa S, Muraki K, Kanatsuka S, Nakazono Y, Hatano N, Fujii M, Zhan P, Suzuki T, Ohya S. Downregulation of Ca2+-activated Cl- channel TMEM16A by the inhibition of histone deacetylase in TMEM16A-expressing cancer cells. J Pharmacol Exp Ther. 2014;351:510–518. doi: 10.1124/jpet.114.217315. [DOI] [PubMed] [Google Scholar]
- 14.Mazzone A, Eisenman ST, Strege PR, Yao Z, Ordog T, Gibbons SJ, Farrugia G. Inhibition of cell proliferation by a selective inhibitor of the Ca(2+)-activated Cl(-) channel, Ano1. Biochem Biophys Res Commun. 2012;427:248–253. doi: 10.1016/j.bbrc.2012.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ouyang H, Lj M, Luk C, Liu N, Karaskova J, Squire J, Tsao MS. Immortal human pancreatic duct epithelial cell lines with near normal genotype and phenotype. Am J Pathol. 2000;157:1623–1631. doi: 10.1016/S0002-9440(10)64800-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schessl J, Kress W, Schoser B. Novel ANO5 mutations causing hyper-CK-emia, limb girdle muscular weakness and Miyoshi type of muscular dystrophy. Muscle Nerve. 2012;45:740–742. doi: 10.1002/mus.23281. [DOI] [PubMed] [Google Scholar]
- 17.Seo Y, Park J, Kim M, Lee HK, Kim JH, Jeong JH, Namkung W. Inhibition of ANO1/TMEM16A chloride channel by idebenone and its cytotoxicity to cancer cell lines. PLoS One. 2015;10:e0133656. doi: 10.1371/journal.pone.0133656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 19.Song HY, Tian YM, Zhang YM, Zhou L, Lian H, Zhu JX. A novel finding of anoctamin 5 expression in the rodent gastrointestinal tract. Biochem Biophys Res Commun. 2014;451:258–262. doi: 10.1016/j.bbrc.2014.07.121. [DOI] [PubMed] [Google Scholar]
- 20.Sui Y, Sun M, Wu F, Yang L, Di W, Zhang G, Zhong L, Ma Z, Zheng J, Fang X, Ma T. Inhibition of TMEM16A expression suppresses growth and invasion in human colorectal cancer cells. PLoS One. 2014;9:e115443. doi: 10.1371/journal.pone.0115443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Y, Guan Y, Jia Y, Meng Q, Yang J. Chloride intracellular channel 1 regulates prostate cancer cell proliferation and migration through the MAPK/ERK pathway. Cancer Biother Radiopharm. 2014;29:339–344. doi: 10.1089/cbr.2014.1666. [DOI] [PubMed] [Google Scholar]
- 22.van der Kooi AJ, Ten DL, Frankhuizen WS, Straathof CS, van Doorn PA, de Visser M, Ginjaar IB. ANO5 mutations in the Dutch limb girdle muscular dystrophy population. Neuromuscul Disord. 2013;23:456–460. doi: 10.1016/j.nmd.2013.03.012. [DOI] [PubMed] [Google Scholar]
- 23.Vihola A, Luque H, Savarese M, Penttilä S, Lindfors M, Leturcq F, Eymard B, Tasca G, Brais B, Conte T, Charton K, Richard I, Udd B. Diagnostic anoctamin-5 protein defect in patients with ANO5-mutated muscular dystrophy. Neuropathol Appl Neurobiol. 2018;44:441–448. doi: 10.1111/nan.12410. [DOI] [PubMed] [Google Scholar]
- 24.Wanitchakool P, Ousingsawat J, Sirianant L, MacAulay N, Schreiber R, Kunzelmann K. Cl- channels in apoptosis. Eur Biophys J. 2016;45:599–610. doi: 10.1007/s00249-016-1140-3. [DOI] [PubMed] [Google Scholar]
- 25.Witting N, Duno M, Petri H, Krag T, Bundgaard H, Kober L, Vissing J. Anoctamin 5 muscular dystrophy in Denmark: prevalence, genotypes, phenotypes, cardiac findings, and muscle protein expression. J Neurol. 2013;260:2084–2093. doi: 10.1007/s00415-013-6934-y. [DOI] [PubMed] [Google Scholar]
- 26.Zhao W, Lu M, Zhang Q. Chloride intracellular channel 1 regulates migration and invasion in gastric cancer by triggering the ROS-mediated p38 MAPK signaling pathway. Mol Med Rep. 2015;12:8041–8047. doi: 10.3892/mmr.2015.4459. [DOI] [PMC free article] [PubMed] [Google Scholar]