Abstract
Background: A randomized, double-blinded controlled trial was performed to evaluate how perioperative goal-directed fluid therapy (GDFT) influences tissue oxygenation in laparoscopic colorectal surgery. Methods: A total of 74 patients undergoing elective laparoscopic colorectal surgery were treated with GDFT (G group) guided by stroke volume variation or conventional fluid therapy (C group). Forearm, crural, and cerebral tissue oxygen saturation (rSO2) were simultaneously measured by near-infrared spectroscopy. Parameters of hemodynamics and rSO2 were obtained at seven time points including before induction of anesthesia (T1), 5 min after trachea cannula (T2), 5, 60, and 120 min after pneumoperitoneum in the Trendeleburg position (T3, T4 and T5, respectively), after desufflation in the Trendeleburg position (T6), and at the end of the operation in a supine position (T7). The postoperative outcomes were recorded. Results: Compared to C group, intraoperatively, patients in the G group received more colloid (P<0.05). The stroke volume variation in G group at T5, T6 and T7 was significantly lower than that in C group (P<0.05). The cardiac index, forearm and crural rSO2 in G group at T4, T5, T6 and T7 were significantly higher than those in C group (P<0.05). No significant differences were observed for the cerebral rSO2 between the two groups (P > 0.05). The postoperative hospital stay and complications also showed no differences between these two groups. Conclusions: Although the implementation of GDFT cannot increase cerebral rSO2, the forearm and crural rSO2 are improved during the laparoscopic colorectal surgery, which is helpful to reduce the risk of regional tissue hypoxia.
Keywords: Goal-directed fluid therapy, tissue oxygen saturation, cardiac index, colorectal surgery
Introduction
Laparoscopic surgery is widely used for colorectal cancer, which can decrease pain, reduce postoperative infection and shorten duration of hospitalization [1]. A steep Trendelenburg position is often required during laparoscopic colorectal surgery, which alters fluid balance and increases oxygen consumption [2]. Meanwhile, it increases intraabdominal pressure, but reduces microcirculatory flow and tissue oxygenation tension [3,4]. The blood may reallocate to the “more vital” organs (such as brain and heart), thus reducing the peripheral tissue perfusion and oxygen delivery [5]. The changes will have significant effects on the body recovery and may result in the development of the dysfunctions [6-8]. Therefore, it is important to keep appropriate capacity and oxygenation in patients, especially in older people [9].
Goal-directed fluid therapy (GDFT) is a method based on standardized fluid-related hemodynamic variables to achieve an optimized stroke volume (SV), cardiac index (CI) and oxygen delivery [10]. Although GDFT is reported to be associated with improved clinical outcomes based on clinical investigations, the utility and advantage of GDFT in clinical practice remain controversial [10-12]. Some researchers suggested that GDFT has few or no benefit to the patients undergoing major abdominal surgery, and pointed out that only the patients with decreased aerobic fitness may benefit from GDFT [2,13]. However, they did not directly test whether GDFT could improve the tissue perfusion and oxygenation in patients who received laparoscopic colorectal surgery.
Various measures such as CI, SV, systemic vascular resistance (SVR), stroke volume variation (SVV), mixed venous oxygen saturation (SvO2) and plethysmography variability index (PVI) have been investigated in GDFT [14-20]. From the viewpoint of physiology, it is speculated that the flow-derived dynamic variable (i.e. SVV) is very meaningful in predicting fluid responsiveness, since blood flow is the primary determinant of oxygen delivery [14]. Moreover, the method to monitor SVV using the FloTrac system is less invasive, because the complications of peripheral arterial catheter placement are less than 1% [15]. SVV is currently considered to be a simple and sensitive predictor of fluid responsiveness and preload status [16,17]. The changes of regional tissue oxygenation (rSO2) reflecting early organ ischemia and/or local metabolic disturbance, have been reported to precede the lactate levels, hemodynamics, and pulse oximetry (SpO2) [18,19]. The cerebral rSO2 is even regarded as a surrogate for central venous oxygen saturation (ScvO2) and SvO2 [20].
In the present study, in order to explore whether GDFT can increase tissue oxygenation and thereby improve clinical outcomes of patients, we monitored the changes of brain oxygenation and peripheral muscular tissue oxygenation following GDFT in laparoscopic colorectal surgery. The results may provide guidance to the clinical application of GDFT in colorectal therapy.
Materials and methods
Patients
The prospective double-blind randomized controlled study was undertaken at the Qianfoshan Hospital, Shandong University, Jinan, China. A total of 74 patients undergoing elective laparoscopic colorectal surgery between September 2017 and December 2018 were enrolled in this study. Inclusion criteria: patients aged between 65 and 80 years old, undergoing general anesthesia during laparoscopic resection for colorectal cancer, and classified as American Society of Anesthesiologists (ASA) physical status I-II. Exclusion criteria: patients with uncompensated cardiac (including arrhythmias) and/or respiratory disease, neurological diseases, peripheral vascular disease, coagulopathy, anemia (Hb<90 g/L), renal impairment (creatinine > upper limit of normal values), and major operation history. Prior written and informed consent was obtained from every patient, and the clinical trial was approved by the Institutional Review Board (Approval No: S056), and registered at http://www.chictr.org.cn (Registration number: ChiCTR-IIR-17012414).
Perioperative care
On the morning of the surgery, the participants were allocated by a random number table generator to either the GDFT group (G group) or the traditional fluid therapy group (C group). The surgeons, patients and investigators were kept blinded. Prophylactic antibiotics were given to all patients in the operating room. Both groups had the arteria radialis puncture and catheterization, and arterial cannula was connected to a FloTrac/Vigileo monitoring system (Edwards Lifesciences, Irvine, CA, USA). All patients were monitored for hemodynamics, SpO2, bispectral index (BIS), temperature, rSO2. Anesthesia was induced with midazolam (0.05 mg/kg), sufentanil (0.5 μg/kg), atracurium (0.8 mg/kg) and etomidate (0.3 mg/kg). After intubation, volume control mechanical ventilation was carried out in a mixture of 50% oxygen and 50% air. End-tidal carbon dioxide (ETCO2) was maintained between 35 and 45 mmHg. Anesthesia was maintained with propofol and remifentanil to keep BIS between 40 and 60. After the surgery, transversus abdominis plane (TAP) block was performed with a portable ultrasound device (Sonosite Inc., Bothell, WA, USA). All patients received 15 mL of 0.375% (w/v) ropivacaine to each side for bilateral block [21,22]. Then patients controlled intravenous analgesia (PCIA) were administered with the mixture of 1 μg/mL sufentanil, 1 mg/mL flurbiprofen axetil and 2.5 μg/mL palonosetron.
Fluid management
All patients took polyethylene glycol electrolyte powder and/or received mechanical bowel preparation on the day before the surgery. A forearm vein was used for the administration of study fluid. Before the induction of anesthesia, 5 mL/kg lactated Ringer’s was infused for the patients. All fluid was pre-warmed before use.
In the GDFT group, SVV was used as a predictor of fluid responsiveness. When the SVV was below 13%, additional boluses of 200 mL colloidal solution were administered in 10 min. Otherwise, the CI was further assessed based on the algorithm given in Figure 1. Lactated Ringer’s solution (2 mL/kg/h) was administered as a background maintenance infusion until the end of the surgery.
Figure 1.
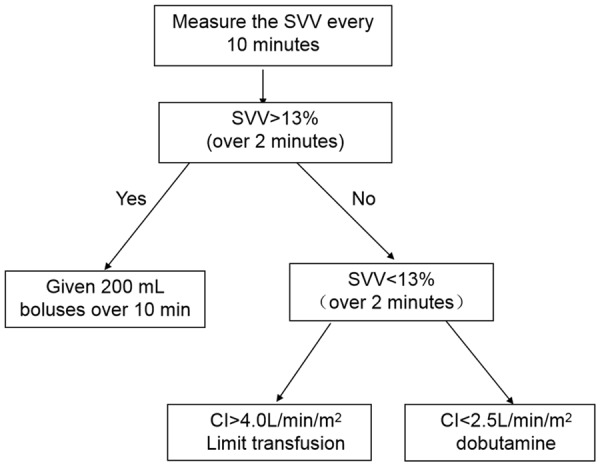
Algorithm developed for intraoperative fluid management in the GDFT group. SVV: stroke volume variation; CI: cardiac index.
In the control group, lactated Ringer’s solution (5-10 mL/kg/h) was administered to avoid the urinary rate less than 0.5 mL/kg/h. In the case of systolic pressure and heart rate variation more than 20%, dobutamine, norepinephrine or nitroglycerin was administered. The starting rates of the three drugs were 2.5 μg/kg.min, 0.03 μg/kg.min and 25 μn/min, respectively. In addition to vasoactive agent, colloid was administered according to clinical requirement. To avoid the bias, the FloTrac/Vigileo monitor screen was concealed from the anesthetist and surgeons.
Measurement of tissue oxygenation
For cerebral rSO2 measurement, Near Infrared Spectroscopy (NIRS) tissue oximeter (FORE-SIGHTTM, CASMED, Branford, CT, USA) was used. Cerebral oximeter probes were placed at least 2 cm above the eyebrow on the left forehead, while BIS motor was placed on the right forehead. For the brachioradialis muscle rSO2 measurement, NIRS tissue oximeter (INVOS® 5100C, Mansfield, MA, USA) was placed on the lateral side of the anterior surface of the left forearm contralateral to the vein and radial artery cannulation site. For the gastrocnemius muscle rSO2 measurement, the NIRS probe was placed on the lateral side of the left calf, proximally 15 cm from the ankle joint. For the three sites, single-depth 25 mm probes were attached to the measurement spots with opaque elastic bandages enfolded. All measurements were performed at the same room temperature of 20°C.
Data collection
Heart rate (HR), mean arterial pressure (MAP), SVV, CI and rSO2 were all measured before induction of anesthesia (T1), 5 min after trachea cannula (T2), 5, 60 and 120 min after pneumoperitoneum in a 20 Trendeleburg position (T3, T4 and T5, respectively), after desufflation in a Trendeleburg position (T6), and at the end of operation in a supine position (T7). Arterial blood samples were collected at T1, T4 and T7, and oxygen delivery index (DOI) was calculated.
Clinical outcomes were collected by a different investigator, blinded to group allocation. The exhaust time, fasting time and postoperative hospital stay were recorded. The presence of postoperative complications, including vomiting, bleeding, infection, acute kidney injury, anastamotic leak and so on were chosen and graded according to predefined criteria [23,24].
Statistical analysis
Shapiro-Wilk analysis was performed to assess the normal distribution of the data. Continuous variables were summarized as the mean ± standard deviation (SD) or median with interquartile range if appropriate. Categorical variables were analyzed using χ2 or Fisher’s exact test as appropriate. Time-dependent data were compared using repeated measures analysis of variance (between periods in each group and between groups). A preplanned subgroup analysis was conducted using unpaired t-test if the data were normally distributed, while the Mann-Whiteney U-test was conducted if they were not normally distributed. All statistical tests were two-sided and a P value <0.05 was considered significant. Data analysis was performed using Statistical Product and Service Solutions (SPSS) version 21.0 (IBM Corp., USA) and Microsoft Excel 2007 (Microsoft Co., USA).
Results
General information
Overall, general information including the demographic (age, sex, body mass index), risk indices (hypertension, diabetes, ASA), operative data (type of surgery, duration of surgery, intraoperative fluids, Hartmann’s procedure, colectomy) and anesthetic characteristics (duration of anaesthesia, proportion and total dose of vasoactive drugs) was compared between the two groups (Table 1). It was found that there were no differences in age, sex, body mass index, hypertension, diabetes, ASA, type of surgery, duration of surgery, duration of anaesthesia, and intraoperative fluids between G group and C group. Furthermore, compared with C group, less crystalloid and more colloids were needed in the G group. There were imbalances in the vasopressors: more dobutamine was needed in the G group, while more norepinephrine was needed in the C group.
Table 1.
Patient characteristics
| Items | G group | C group | P value |
|---|---|---|---|
| Age (yr) | 69.2±4.7 | 70.3±4.8 | 0.320 |
| Sex (M/F) | 23/14 | 23/14 | 1.000 |
| Body mass index (kg/m2) | 22.0±2.3 | 22.6±2.8 | 0.381 |
| Hypertension | 13 | 15 | 0.632 |
| Diabetes | 7 | 6 | 0.760 |
| ASA | 0.744 | ||
| I | 6 | 5 | |
| II | 31 | 32 | |
| Type of surgery | 0.619 | ||
| Abdomino-perineal resection (Miles) | 16 | 12 | |
| Rectal low anterior resection (Dixon) | 12 | 17 | |
| Hartmann’s procedure | 2 | 1 | |
| Colectomy | 7 | 7 | |
| Duration of surgery (min) | 185.1±22.4 | 177.6±22.9 | 0.159 |
| Duration of anaesthesia (min) | 223.2±25.0 | 216.1±27.4 | 0.247 |
| Intraoperative fluids (mL) | 1979.7±170.6 | 2001.4±228.7 | 0.646 |
| Crystalloid (mL) | 904.1±97.5* | 1340.5±265.3 | 0.000 |
| Colloid (mL) | 1075.7±201.9* | 660.8±199.4 | 0.000 |
| Blood loss (mL) | 65.7±17.1 | 62.4±15.9 | 0.401 |
| Urine output (mL) | 547.6±155.3 | 515.4±135.2 | 0.345 |
| Proportion of vasoactive drugs (%) | |||
| Dobutamine | 73.0* | 21.6 | 0.000 |
| Norepinephrine | 16.2* | 67.6 | 0.000 |
| Nitroglycerin | 13.5 | 18.9 | 0.528 |
| Total dose of vasoactive drugs | |||
| Dobutamine (mg) | 14.2* | 2.5 | 0.000 |
| Norepinephrine (μg) | 3.0* | 74.2 | 0.000 |
| Nitroglycerin (μg) | 10.8 | 13.5 | 0.705 |
Note: Values are expressed as mean ± SD or absolute number.
P<0.05 vs. Group C.
ASA, American Society of Anesthesiologists.
Intraoperative haemodynamics and oxygenation
Hemodynamic monitoring was performed to evaluate the changes of the circulation using different fluid therapies. HR (Figure 2A) and MAP (Figure 2B) were significantly lowered compared with the basic value at T1 (P<0.05). However, there were no statistical significances in HR (Figure 2A) and MAP (Figure 2B) between the G group and the C group at any time points (P > 0.05). Compared to T1, the SVV (Figure 2C) and CI (Figure 2D) were significantly reduced at the other time points in both groups (P<0.05). At T5, T6 and T7, the SVV of the G group was significantly lower than that in the C group (P<0.05) (Figure 2C). Patients in the G group had significantly greater CI than the C group at T4, T5, T6 and T7 (Figure 2D).
Figure 2.
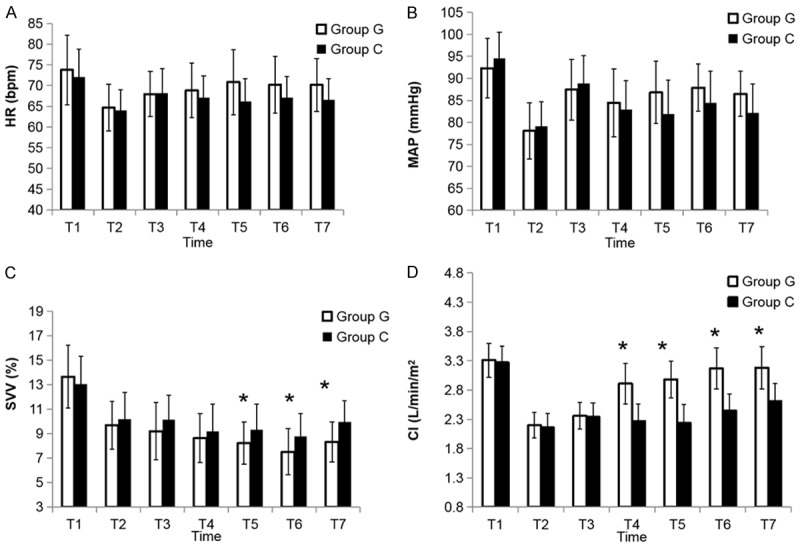
Cardiovascular variables during the study periods. Data are presented as mean ± SD; *P<0.05, vs. C group. A. HR: heart rate; B. MAP: mean arterial pressure; C. SVV: stroke volume variation; D. CI: cardiac index. Measurements were performed at 5 min before induction of anesthesia (T1), 5 min after intubation (T2), 5, 60 and 120 min after pneumoperitoneum in a Trendelenburg and lithotomy position (T3, T4 and T5, respectively), 5 min after desufflation in a Trendelenburg position (T6), and 5 min after operation in a supine position (T7).
To compare the effects of different fluid therapies on regional tissue oxygenation, forearm, crural, and cerebral rSO2 monitoring was simultaneously performed. Compared with the baseline, the cerebral rSO2 at T3 was significantly upregulated (P<0.05), but it was decreased at T5, T6 and T7 (P<0.05) (Figure 3A). The changes over time were similar between the two groups. Forearm rSO2 in both groups was increased throughout the operation, but it was significantly higher in the G group than that in the C group at T4-T7 (P<0.05) (Figure 3B). Compared to the G group, the crural rSO2 was increased in the C group at T4-T7 (P<0.05). The crural rSO2 at T3 was the lowest within both groups (Figure 3C).
Figure 3.
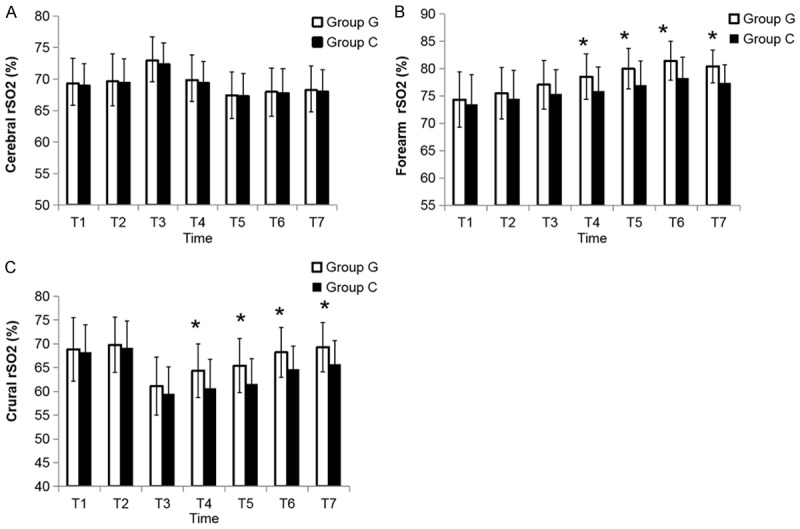
The time courses of cerebral, forearm and crural rSO2. The time points were the same as those described in the figure legend of Figure 2. The values are presented as mean ± SD; *P<0.05, vs. C group. rSO2, regional oxygen saturations.
In addition, the DOI showed no difference between the G group and the C group at T1 (P > 0.05, Table 2). Moreover, the DOI was downregulated in the G group in comparison with the control at T4 and T7 (P<0.05). For Lac and Hb, the differences between the two groups did not show statistical significance (P > 0.05, Table 2). These results indicate that the changes in rSO2 between the two groups are not caused by changes in Hb during surgery.
Table 2.
Data of oxygen delivery index and blood gas
| Items | G group | C group | P value |
|---|---|---|---|
| DOI (mL/min/m2) | |||
| T1 | 523.4±41.5 | 515.8±90.6 | 0.644 |
| T4 | 473.9±40.7* | 402.4±31.4 | 0.000 |
| T7 | 493.1±38.1* | 440.4±40.6 | 0.000 |
| Hb (g/L) | 0.217 | ||
| T1 | 121.9±13.5 | 124.8±13.9 | |
| T4 | 113.2±11.7 | 116.9±12.0 | |
| T7 | 108.1±10.7 | 111.7±12.0 | |
| Lac (mmol/L) | 0.102 | ||
| T1 | 0.85±0.23 | 0.87±0.19 | |
| T4 | 0.92±0.23 | 1.0±0.23 | |
| T7 | 0.96±0.28 | 1.1±0.35 |
Note: Data are presented as mean ± SD.
P<0.05 vs. Group C.
T1, 5 min before induction of anesthesia; T4, 60 min after pneumoperitoneum in a Trendelenburg and lithotomy position; T7, 5 min after operation in a supine position. DOI, oxygen delivery index; Hb, haemoglobin; Lac, lactate.
Postoperative outcomes
To find whether GDFT leads to improved surgical outcomes, postoperative clinical data including exhaust time, fasting time, postoperative hospital stay, nausea and vomiting, abdominal distension, infection, anastomotic leak, bleeding, lower limb venous thrombosis, hypotension, ventricular arrhythmias, acute kidney injury, and wound dehiscence were analyzed. It was found that there were no differences in all measured postoperative clinical data (P > 0.05). Each group had two patients who developed an anastomotic leak and the one in the C group required reoperation. Major and minor complications were not significantly different (Table 3).
Table 3.
Postoperative outcome following different therapies
| Items | G group | C group | P value |
|---|---|---|---|
| Exhaust time (days) | 2.2±0.9 | 2.5±0.9 | 0.123 |
| Fasting time (days) | 3.1±1.1 | 3.5±1.1 | 0.141 |
| Postoperative hospital stay (days) | 10.6±2.3 | 11.4±2.6 | 0.185 |
| Nausea and vomiting | 8 | 11 | 0.425 |
| Abdominal distension | 10 | 13 | 0.451 |
| Infection | 4 | 7 | 0.327 |
| Anastomotic leak | 2 | 2 | 1.000 |
| Bleeding | 4 | 2 | 0.670 |
| Lower limb venous thrombosis | 0 | 1 | 1.000 |
| Hypotension | 1 | 5 | 0.201 |
| Ventricular arrhythmias | 1 | 0 | 1.000 |
| Acute kidney injury | 2 | 0 | 0.2473 |
| Wound dehiscence | 2 | 2 | 1.000 |
Discussion
Laparoscopic colorectal surgery has been widely adopted for the treatment of colorectal neoplasia, with steady increases in use over the past 15 years, and this technique includes multiport laparoscopy, single-incision laparoscopy, and hand-assisted laparoscopy [25,26]. Previous studies have suggested a positive effect of GDFT on clinical outcome, which has long been assumed to be related to the improved tissue perfusion and oxygenation [27,28]. In this study, a randomized, double-blinded controlled trial was designed to evaluate the influences of perioperative GDFT on tissue oxygenation in laparoscopic colorectal surgery.
Measures, such as arterial pressure and oxygen content, cannot reflect tissue-level perfusion or oxygenation, and other indices including central venous oxygen saturation and Lac are relatively nonspecific [18,29]. This study is distinguished from previous works because the change of tissue oxygenation was directly measured in a noninvasive way during the laparoscopic surgery. Cerebral rSO2 and forearm rSO2 were chosen as the parameters representing the upper-body oxygenation, and crural rSO2 was used to represent the lower-body oxygenation status. In the trial, NIRS probe was put on the lateral side of the anterior surface of the forearm brachioradialis muscle rather than the thenar eminence, the most widely used site of measurement. This is because several researchers have suggested that, compared with thenar rSO2, the forearm rSO2 is more appropriate to monitor the change of peripheral microcirculation [30,31]. The gastrocnemius lateralis is the common measurement spot for crural rSO2 [32]. The result of this study showed that the forearm rSO2 is continuously increased during the surgery, suggesting the preoperative peripheral tissue has inadequate perfusion and oxygenation due to the fasting and bowel preparation. The high SVV level also confirms the condition. The DOI is reduced in both groups because of the pneumoperitoneum and anesthetic, while the forearm rSO2 is increased during the operation. One explanation for this phenomenon can be an increase of oxygen extraction. In the present study, forearm rSO2 is maintained higher by GDFT than by the conventional method. The changing trend is in accordance with CI, suggesting that the increase in cardiac output benefits the oxygenation. The reason for a significant difference between the two groups is related to dobutamine administration. A β1-agonist-mediated increase in myocardiac contractility and β2-agonist-mediated increase in peripheral vasodilation may generate an increased rSO2 and CI [33]. This study showed that the dosage and frequency used in the G group are significantly higher than those in the C group. Therefore, the use of GDFT to achieve a higher CI is responsible for the higher rSO2. The elevated values of crural rSO2 in the G group have also been observed. At 5 min after the Trendelenburg and lithotomy positioning, there is an obvious decline of crural rSO2 in both groups, contributing to the great changes of blood redistribution.
In the present study, cerebral rSO2 reaches the highest in this period and followed by a falling at 120 min after pneumoperitoneum in a Trendelenburg position. These results are in agreement with Kumagai’s research [34]. The trend of cerebral rSO2 between the two groups is similar, suggesting that the cerebral blood flow has a relatively strong autoregulation [35]. Likewise, the cerebral rSO2 varies to a lower degree in comparison with the peripheral muscle tissue. Furthermore, although forearm and crural rSO2 are higher in the G group than that in the C group, the clinical outcomes showed no significant differences between two groups. These results indicate that peripheral rSO2 may not be used for predicting the risk of complications. Nevertheless, the forearm and crural rSO2 are more sensitive to hemodynamic changes than the Lac, which suggests that peripheral rSO2 can reflect the early inadequate tissue perfusion, and can be beneficial to the patients with peripheral vascular disease or shock.
The current study showed no obvious clinical benefit in outcome when comparing GDFT with the routine method, which was different from some previous studies [36]. One factor accounting for the finding is that neither the GDFT nor the routine infusion gives rise to fluid overload or hypoxia. At the end of operation, the value of the oxygen delivery index is favorable evidence. Another factor is that good-quality perioperative care, included proper temperature, antibiotics, and adequate postoperative analgesia have offset the advantage of the GDFT [7].
There are some weaknesses in this study. First, cerebral rSO2 and peripheral rSO2 were measured by different machines produced by different manufacturers, using distinct algorithms. However, the applied methodology is not rare. Recently, multisite monitoring of rSO2 using NIRS is increasing [37,38]. In this study, they were performed simultaneously using the same 25 mm probes. Therefore, it is more valuable to follow the trend but not the absolute values in this practice. Second, the observation of the post-operative outcomes is limited to the hospitalization period only. Although the results tend to demonstrate better outcomes in the G group, no significant benefit are found in the pilot study. A further research with larger sample size and longer period is needed. Third, central venous oxygen saturation (ScvO2) was not collected to obtain the oxygen consumption index, because our institutional protocol was not to implement central vein catheterization during the surgery. Finally, during the period from general anaesthetic to the end of surgery, the average CI of the control group is less than 2.5 L/min/m2, but do not cause tissue acidosis and affect postoperative reassignment. Therefore, it is more appropriate to maintain the value of CI after anesthesia, which needs a further investigation.
Conclusion
In summary, a higher level of forearm and crural rSO2, but no statistically significant changes in cerebral rSO2, were observed in GDFT compared to the conventional fluid therapy during laparoscopic colorectal surgery. This indicates that GDFT could dramatically reduce the risk of regional tissue hypoxia and has an important significance in laparoscopic colorectal surgery.
Disclosure of conflict of interest
None.
References
- 1.Devoto L, Celentano V, Cohen R, Khan J, Chand M. Colorectal cancer surgery in the very elderly patient: a systematic review of laparoscopic versus open colorectal resection. Int J Colorectal Dis. 2017;32:1237–42. doi: 10.1007/s00384-017-2848-y. [DOI] [PubMed] [Google Scholar]
- 2.Challand C, Struthers R, Sneyd JR, Erasmus PD, Mellor N, Hosie KB, Minto G. Randomized controlled trial of intraoperative goal-directed fluid therapy in aerobically fit and unfit patients having major colorectal surgery. Br J Anaesth. 2012;108:53–62. doi: 10.1093/bja/aer273. [DOI] [PubMed] [Google Scholar]
- 3.Fleischmann E, Kurz A, Niedermayr M, Schebesta K, Kimberger O, Sessler DI, Kabon B, Prager G. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes Surg. 2005;15:813–9. doi: 10.1381/0960892054222867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambert KG, Wakim JH, Lambert NE. Preoperative fluid bolus and reduction of postoperative nausea and vomiting in patients undergoing laparoscopic gynecologic surgery. AANA J. 2009;77:110–4. [PubMed] [Google Scholar]
- 5.Spruit RJ, Schwarte LA, Hakenberg OW, Scheeren TW. Association of intraoperative tissue oxygenation with suspected risk factors for tissue hypoxia. J Clin Monit Comput. 2013;27:541–50. doi: 10.1007/s10877-013-9460-7. [DOI] [PubMed] [Google Scholar]
- 6.De Blasi RA, Palmisani S, Boezi M, Arcioni R, Collini S, Troisi F, Pinto G. Effects of remifentanil-based general anaesthesia with propofol or sevoflurane on muscle microcirculation as assessed by near-infrared spectroscopy. Br J Anaesth. 2008;101:171–7. doi: 10.1093/bja/aen136. [DOI] [PubMed] [Google Scholar]
- 7.Levy BF, Fawcett WJ, Scott MJ, Rockall TA. Intra-operative oxygen delivery in infusion volume-optimized patients undergoing laparoscopic colorectal surgery within an enhanced recovery programme: the effect of different analgesic modalities. Colorectal Dis. 2012;14:887–92. doi: 10.1111/j.1463-1318.2011.02805.x. [DOI] [PubMed] [Google Scholar]
- 8.Meierhenrich R, Gauss A, Vandenesch P, Georgieff M, Poch B, Schutz W. The effects of intraabdominally insufflated carbon dioxide on hepatic blood flow during laparoscopic surgery assessed by transesophageal echocardiography. Anesth Analg. 2005;100:340–7. doi: 10.1213/01.ANE.0000143566.60213.0A. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson UO, Scott MJ, Schwenk W, Demartines N, Roulin D, Francis N, McNaught CE, Macfie J, Liberman AS, Soop M, Hill A, Kennedy RH, Lobo DN, Fearon K, Ljungqvist O Enhanced Recovery After Surgery (ERAS) Society, for Perioperative Care; European Society for Clinical Nutrition and Metabolism (ESPEN); International Association for Surgical Metabolism and Nutrition (IASMEN) Guidelines for perioperative care in elective colonic surgery: enhanced recovery after surgery (ERAS((R))) society recommendations. World J Surg. 2013;37:259–84. doi: 10.1007/s00268-012-1772-0. [DOI] [PubMed] [Google Scholar]
- 10.Quinn TD, Brovman EY, Urman RD. Analysis of variability in intraoperative fluid administration for colorectal surgery: an argument for goal-directed fluid therapy. J Laparoendosc Adv Surg Tech A. 2017;27:892–7. doi: 10.1089/lap.2017.0336. [DOI] [PubMed] [Google Scholar]
- 11.Brandstrup B, Svendsen PE, Rasmussen M, Belhage B, Rodt SÅ, Hansen B, Møller DR, Lundbech LB, Andersen N, Berg V, Thomassen N, Andersen ST, Simonsen L. Which goal for fluid therapy during colorectal surgery is followed by the best outcome: near-maximal stroke volume or zero fluid balance? Br J Anaesth. 2012;109:191–9. doi: 10.1093/bja/aes163. [DOI] [PubMed] [Google Scholar]
- 12.Gómez-Izquierdo JC, Trainito A, Mirzakandov D, Stein BL, Liberman S, Charlebois P, Pecorelli N, Feldman LS, Carli F, Baldini G. Goal-directed fluid therapy does not reduce primary postoperative ileus after elective laparoscopic colorectal surgery: a randomized controlled trial. Anesthesiology. 2017;127:36–49. doi: 10.1097/ALN.0000000000001663. [DOI] [PubMed] [Google Scholar]
- 13.Lai CW, Starkie T, Creanor S, Struthers RA, Portch D, Erasmus PD, Mellor N, Hosie KB, Sneyd JR, Minto G. Randomized controlled trial of stroke volume optimization during elective major abdominal surgery in patients stratified by aerobic fitness. Br J Anaesth. 2015;115:578–89. doi: 10.1093/bja/aev299. [DOI] [PubMed] [Google Scholar]
- 14.Vos JJ, Kalmar AF, Struys MM, Wietasch JK, Hendriks HG, Scheeren TW. Comparison of arterial pressure and plethysmographic waveform-based dynamic preload variables in assessing fluid responsiveness and dynamic arterial tone in patients undergoing major hepatic resection. Br J Anaesth. 2013;110:940–6. doi: 10.1093/bja/aes508. [DOI] [PubMed] [Google Scholar]
- 15.Kitaguchi K, Gotohda N, Yamamoto H, Kato Y, Takahashi S, Konishi M, Hayashi R. Intraoperative circulatory management using the FloTrac™ system in laparoscopic liver resection. Asian J Endosc Surg. 2015;8:164–70. doi: 10.1111/ases.12158. [DOI] [PubMed] [Google Scholar]
- 16.Choi SS, Kim SH, Kim YK. Fluid management in living donor hepatectomy: recent issues and perspectives. World J Gastroenterol. 2015;21:12757–66. doi: 10.3748/wjg.v21.i45.12757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pala S, Aletti F, Toschi N, Guerrisi M, Coniglione F, Dauri M, Baselli G, Ferrario M. Comparisons of predictors of fluid responsiveness in major surgery. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:3128–30. doi: 10.1109/EMBC.2012.6346627. [DOI] [PubMed] [Google Scholar]
- 18.Benedik PS. Monitoring tissue blood flow and oxygenation: a brief review of emerging techniques. Crit Care Nurs Clin North Am. 2014;26:345–56. doi: 10.1016/j.ccell.2014.04.003. [DOI] [PubMed] [Google Scholar]
- 19.O’Brien-Lambert A, Driver B, Moore JC, Schick A, Miner JR. Using near infrared spectroscopy for tissue oxygenation monitoring during procedural sedation: the occurrence of peripheral tissue oxygenation changes with respiratory depression and supportive airway measures. Acad Emerg Med. 2016;23:98–101. doi: 10.1111/acem.12843. [DOI] [PubMed] [Google Scholar]
- 20.Kim JW, Shin WJ, Park I, Chung IS, Gwak M, Hwang GS. Splanchnic oxygen saturation immediately after weaning from cardiopulmonary bypass can predict early postoperative outcomes in children undergoing congenital heart surgery. Pediatr Cardiol. 2014;35:587–95. doi: 10.1007/s00246-013-0824-z. [DOI] [PubMed] [Google Scholar]
- 21.De Oliveira GS Jr, Fitzgerald PC, Marcus RJ, Ahmad S, Mccarthy RJ. A dose-ranging study of the effect of transversus abdominis block on postoperative quality of recovery and analgesia after outpatient laparoscopy. Anesth Analg. 2011;113:1218–25. doi: 10.1213/ANE.0b013e3182303a1a. [DOI] [PubMed] [Google Scholar]
- 22.Tan TT, Teoh WH, Woo DC, Ocampo CE, Shah MK, Sia AT. A randomised trial of the analgesic efficacy of ultrasound-guided transversus abdominis plane block after caesarean delivery under general anaesthesia. Eur J Anaesthesiol. 2012;29:88–94. doi: 10.1097/EJA.0b013e32834f015f. [DOI] [PubMed] [Google Scholar]
- 23.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jammer I, Wickboldt N, Sander M, Smith A, Schultz MJ, Pelosi P, Leva B, Rhodes A, Hoeft A, Walder B, Chew MS, Pearse RM European Society of Anaesthesiology (ESA) and the European Society of Intensive Care Medicine (ESICM); European Society of Anaesthesiology; European Society of Intensive Care Medicine. Standards for definitions and use of outcome measures for clinical effectiveness research in perioperative medicine: european perioperative clinical outcome (EPCO) definitions: a statement from the ESA-ESICM joint taskforce on perioperative outcome measures. Eur J Anaesthesiol. 2015;32:88–105. doi: 10.1097/EJA.0000000000000118. [DOI] [PubMed] [Google Scholar]
- 25.Liu ZH, Liu JW, Chan FS, Li MK, Fan JK. Intraoperative colonoscopy in laparoscopic colorectal surgery: a review of recent publications. Asian J Endosc Surg. 2019 doi: 10.1111/ases.12704. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 26.Parker JM, Feldmann TF, Cologne KG. Advances in laparoscopic colorectal surgery. Surg Clin North Am. 2017;97:547–60. doi: 10.1016/j.suc.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 27.Giglio MT, Marucci M, Testini M, Brienza N. Goal-directed haemodynamic therapy and gastrointestinal complications in major surgery: a meta-analysis of randomized controlled trials. Br J Anaesth. 2009;103:637–46. doi: 10.1093/bja/aep279. [DOI] [PubMed] [Google Scholar]
- 28.Jhanji S, Vivian-Smith A, Lucena-Amaro S, Watson D, Hinds CJ, Pearse RM. Haemodynamic optimisation improves tissue microvascular flow and oxygenation after major surgery: a randomised controlled trial. Crit Care. 2010;14:R151. doi: 10.1186/cc9220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nardi O, Polito A, Aboab J, Colin G, Maxime V, Clair B, Friedman D, Orlikowski D, Sharshar T, Annane D. StO2 guided early resuscitation in subjects with severe sepsis or septic shock: a pilot randomised trial. J Clin Monit Comput. 2013;27:215–21. doi: 10.1007/s10877-013-9432-y. [DOI] [PubMed] [Google Scholar]
- 30.Bartels SA, Bezemer R, de Vries FJ, Milstein DM, Lima A, Cherpanath TG, van den Meiracker AH, van Bommel J, Heger M, Karemaker JM, Ince C. Multi-site and multi-depth near-infrared spectroscopy in a model of simulated (central) hypovolemia: lower body negative pressure. Intensive Care Med. 2011;37:671–7. doi: 10.1007/s00134-010-2128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bezemer R, Karemaker JM, Klijn E, Martin D, Mitchell K, Grocott M, Heger M, Ince C. Simultaneous multi-depth assessment of tissue oxygen saturation in thenar and forearm using near-infrared spectroscopy during a simple cardiovascular challenge. Crit Care. 2009;13(Suppl 5):S5. doi: 10.1186/cc8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Boezeman RP, Kelder JC, Waanders FG, Moll FL, de Vries JP. In vivo measurements of regional hemoglobin oxygen saturation values and limb-to-arm ratios of near-infrared spectroscopy for tissue oxygenation monitoring of lower extremities in healthy subjects. Med Devices (Auckl) 2015;8:31–6. doi: 10.2147/MDER.S73103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Beest PA, Vos JJ, Poterman M, Kalmar AF, Scheeren TW. Tissue oxygenation as a target for goal-directed therapy in high-risk surgery: a pilot study. BMC Anesthesiol. 2014;14:122. doi: 10.1186/1471-2253-14-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumagai M, Ogawa S, Doe A, Suzuki K. Cerebral oxygenation measured by near-infrared spectroscopy and jugular vein oxygen saturation during robotic-assisted laparoscopic radical prostatectomy under total intravenous anaesthesia. Int J Med Robot. 2015;11:302–7. doi: 10.1002/rcs.1629. [DOI] [PubMed] [Google Scholar]
- 35.Eichhorn L, Erdfelder F, Kessler F, Doerner J, Thudium MO, Meyer R, Ellerkmann RK. Evaluation of near-infrared spectroscopy under apnea-dependent hypoxia in humans. J Clin Monit Comput. 2015;29:749–57. doi: 10.1007/s10877-015-9662-2. [DOI] [PubMed] [Google Scholar]
- 36.Hamilton MA, Cecconi M, Rhodes A. A systematic review and meta-analysis on the use of preemptive hemodynamic intervention to improve postoperative outcomes in moderate and high-risk surgical patients. Anesth Analg. 2011;112:1392–402. doi: 10.1213/ANE.0b013e3181eeaae5. [DOI] [PubMed] [Google Scholar]
- 37.Balaguru D, Bhalala U, Haghighi M, Norton K. Computed tomography scan measurement of abdominal wall thickness for application of near-infrared spectroscopy probes to monitor regional oxygen saturation index of gastrointestinal and renal circulations in children. Pediatr Crit Care Med. 2011;12:e145–8. doi: 10.1097/PCC.0b013e3181e8b430. [DOI] [PubMed] [Google Scholar]
- 38.Ledo A, Aguar M, Núñez-Ramiro A, Saénz P, Vento M. Abdominal near-infrared spectroscopy detects low mesenteric perfusion early in preterm infants with hemodynamic significant ductus arteriosus. Neonatology. 2017;112:238–45. doi: 10.1159/000475933. [DOI] [PubMed] [Google Scholar]


