Abstract
Primary ovarian insufficiency (POI) is defined as ovarian dysfunction in women younger than 40 years. It affects 1% of the women in this age-group and can occur iatrogenically after chemotherapy. Stem cells have been used in attempt to restore ovarian function in POI. In particular, endometrial mesenchymal stem cells (eMSCs) are easily obtainable in humans and have shown great potential for regenerative medicine. Here, we studied the potential for uterine cell (UC) suspensions containing eMSCs to improve ovarian function in a murine model of chemotherapy-induced POI. Green fluorescent protein (GFP)-labeled UC or phosphate-buffered solution (PBS) was delivered intravenously after chemotherapy. There was a significant increase in oocytes production and serum anti-Müllerian hormone concentrations after 6 weeks, as well as a 19% higher body mass in UC-treated mice. Similarly, we observed an increased number of pups in mice treated with UC than in mice treated with PBS. None of the oocytes or pups incorporated GFP, suggesting that there was no contribution of these stem cells to the oocyte pool. We conclude that treatment with UC indirectly improved ovarian function in mice with chemotherapy-induced POI. Furthermore, our study suggests that endometrial stem cell therapy may be beneficial to young women who undergo ovotoxic chemotherapy.
Keywords: primary ovarian insufficiency (POI), uterine cells, endometrial mesenchymal stem cells (eMSCs), ovary, oocytes, AMH, pregnancy, ovarian function, chemotherapy
Introduction
Chemotherapy has become a very effective treatment option for women with cancers and autoimmune diseases; while the number and life span of survivors has increased, some of the long-term adverse effects of these therapies has also become evident. Primary ovarian insufficiency (POI)—defined as a loss of ovarian function before the age of 40 years—is a problem affecting up to 1% of women in that age range as a result of chemotherapy, as well as autoimmune, congenital, or endocrine disorders.1 The consequences of POI are similar to those of menopause, including osteoporosis, hot flashes, sexual dysfunction, and infertility.2
In order to address the problem of infertility caused by radiation and chemotherapy, several methods have been developed that protect the ovaries or mitigate the damaging effect of radiation or chemotherapy, including oophoropexy, the use of gonadotropin-releasing hormone (GnRH) agonists, as well as cryopreservation of oocytes embryos or ovarian tissue.3,4 The use of GnRH agonists, which initially showed promise in the preservation of ovarian function, has been demonstrated to be much less effective than originally thought, with a systematic review revealing a pregnancy incidence of only 10% after the treatment.5 Furthermore, the evidence to support the use of GnRH agonists in this scenario is of variable quality.5,6 Oophoropexy—the transplantation of the ovary to a different location during therapy—protects the ovary from radiation damage7 but does not shield it from the effects of chemotherapy. Furthermore, the effects of POI stretch beyond the sterility, and a treatment that results in reversal of POI and improvement of overall ovarian function would be extremely beneficial.
The last decade has seen the proliferation of research seeking to elucidate the potential of stem cell therapies for regenerative medicine in a number of diseases ranging from oncologic to autoimmune conditions. Several publications have described the attempts to restore ovarian function in chemotherapy-induced POI with the use of stem cells. Several stem cell sources have been investigated with varying degrees of success; these include bone marrow,8,9 amniotic epithelium,10 amniotic fluid,11 and adipose tissue.
Recently, the endometrium has been identified as an easily accessible source of mesenchymal stem cells (MSCs) for regenerative medicine. The presence of endometrium-derived stem cells was demonstrated and characterized in 2004 and accounted for 0.2% to 48% of epithelial cells and 0.3% to 52% of stromal cells.12 Further studies from our laboratory and others have elucidated the presence of MSCs in the endometrium13–16 as well as their multipotency and ability to differentiate into chondrocytes, insulin-producing cells, neuron-like cells, cardiomyocyte-like cells, and other phenotypes.16–21
This article reports on our efforts to improve ovarian function using uterine cells (UCs) in a murine model of POI. In our experiments, whole UC preparations containing endometrial mesenchymal stem cells (eMSCs) were transfused due to the significant loss during isolation of eMSCs via fluorescence-activated cell sorting. The use of whole endometrial cell preparations is also much more readily applicable to the clinical setting. The outcome measures selected to gauge the effect of the treatment on ovarian function were ovulation, anti-Müllerian hormone (AMH) levels, primordial follicle count, and pregnancy rates.
Materials and Methods
Animals
C57BL/6J female wild-type (8 weeks) and ubiquitin-green fluorescent protein (GFP) mice (8-12 weeks) that are expressing GFP were purchased from Charles River Laboratories (Wilmington, Massachusetts) and The Jackson Laboratory (Bar Harbor, Maine), respectively. Mice were housed and maintained (4-5 per cage) in a room (21°C ± 1°C) with a 12-hour light/dark cycle (7:00 am-7:00 pm) with ad libitum access to food (Purina Chow; Purina Mills, Richmond, Indiana) and water, in the Yale Animal Resources Center at Yale School of Medicine. All animal experiments were conducted in accordance with an approved protocol from Institutional Animal Care and Use Committee (IACUC) of Yale University. The IACUC guidelines were clearly followed for animal care and usage of drugs and chemicals.
Induction of POI in Mice
Intraperitoneal (IP) injection of cyclophosphamide and busulfan is a proven method of POI induction in mice by means of destroying the existing pre- and postmeiotic germ cell pools.10,11,22,23 To validate this method, an ovulation induction trial was conducted in 8-week-old female C57BL/6 mice. Fourteen animals were randomized into a chemotherapy group and a control group (n = 7). The chemotherapy group received a single IP injection of 100 μL of cyclophosphamide monohydrate 120 mg/kg body weight (Sigma, St Louis Missouri) and busulfan 30 mg/kg body weight (Sigma) in dimethylsulfoxide (chemotherapy-control group [CTX]). Mice in the control group received an IP injection of 100 μL of 1% Dulbecco phosphate-buffered solution (PBS). One week after the administration, mice in both groups underwent an ovulation induction treatment with an IP injection of 5 IU of pregnant mare serum gonadotropin (PMSG; Sigma) for follicular hyperstimulation followed by an IP injection of 5 IU of human chorionic gonadotropin (β-hCG, Sigma) 48 hours later to trigger ovulation. After 16 to 18 hours, mice were euthanized using CO2 followed by cervical dislocation. Oviducts were retrieved by necropsy and flushed in a solution of Dulbecco modified Eagle medium with fetal bovine serum in a 9:1 ratio. The oocytes from each mouse were then counted.
Endometrium-Derived Cell Suspension Preparation
C57BL/6 female mice of 8 to 12-week old expressing GFP (Jackson Laboratory) were used as donors. These mice were euthanized following the process described earlier and their uteri were harvested, stripped of all adjacent adipose tissue, and digested in a solution of Hank’s balanced salt solution containing DNAse I (0.085 mg/mL) and collagenase B (0.85 mg/mL). Cells were counted using trypan blue by hemocytometer and then cell pellet was washed twice and suspended in PBS, adjusting the cell concentration to 1 × 106 cells/100 μL.
Chemotherapy and Treatment
Female mice (N = 46) of 8 weeks old were randomized into treatment and control groups (N = 23 per group). All mice received the chemotherapy regimen as described earlier. One week after the chemotherapy, 23 mice received a retro-orbital (intravenous) injection under anesthesia with 100 μL PBS to serve as a CTX. The remaining 23 mice received treatment with a UC suspension (1 × 106 cells in 100 μL) by retro-orbital injection under anesthesia, and served as the treatment group (UC).
Primordial Follicle and Oocyte Counts
One week after the mice had received treatment with PBS or UC, 10 mice in each group were hyperstimulated with PMSG and their ovulation was triggered with β-hCG as described earlier. The mice were euthanized and their oocytes were retrieved and counted as described earlier. Ovaries were harvested from 5 mice in each group, placed in 4% paraformaldehyde, embedded in paraffin, sectioned pole to pole, and mounted on glass slides, and stained with hematoxylin and eosin. The number of primordial ovarian follicles—defined as a compact oocyte surrounded by a layer of flattened granulosa cells—was counted in 1 representative section per every 4 ovarian sections. The tissue was then immunohistochemically stained for GFP and the tissue sections on slides were reanalyzed to identify any areas of GFP enhancement.
Anti-Müllerian hormone Levels, Weight, and Breeding Trial
The remaining 26 mice were caged with proven males of the same strain in a 2:1 female-to-male ratio to conduct a breeding trial. Retro-orbital blood draws were conducted under anesthesia before treatment and subsequently at the 2-week, 6-week, and 6-month posttreatment time points. Serum AMH concentrations were obtained using a murine AMH ELISA kit (Cloud-Clone Corporation, Katy, Texas). Importantly, the interpretation of these results is affected by the limited validity of murine AMH assays, of which only 1 was found to be available. The use of this assay proved to be technically challenging.
During this time, all female mice were weighed weekly and the cages were observed daily for pups to confirm suspected pregnancies. A representative sample of the resulting offspring was observed under ultraviolet (UV) light to evaluate any green fluorescence.
Statistical Analysis
The results of primordial follicle counts and ovulation were analyzed by an unpaired t test. Anti-Müllerian hormone levels in both groups were compared to a 2-way analysis of variance (ANOVA), in order to detect differences between groups at each time point and between time points within each group. A χ2 test was used to determine the statistical significance in pregnancy outcomes. One-way ANOVA was employed to analyze changes in body weight between the 2 groups. A P value of .05 was deemed the threshold of statistical significance. All statistical analyses were performed using GraphPad Prism version 7.0 software.
Results
Murine POI Model Validation
To validate the chemotherapy regimen treatment in mice for induction of POI, the ovulation count in each group was determined and compared. The oocyte counts showed that mice in the CTX group ovulated an average of 5.1 oocytes per mouse, compared to 19.6 oocytes per mouse in the PBS group (P < .0004), as shown in Figure 1. As expected, the number of oocytes decreased significantly in CTX group compared to the PBS group.
Figure 1.
Chemotherapy validation. There was a significant reduction in the number of ovulated oocytes in chemotherapy treatment mice compared to mice without treatment. Error bars represent the mean ± SEM (n = 8 mice per group). P < .0004, using an unpaired t test with Welch correction. SEM indicates standard error of the mean.
Primordial Follicle Count and Ovulation
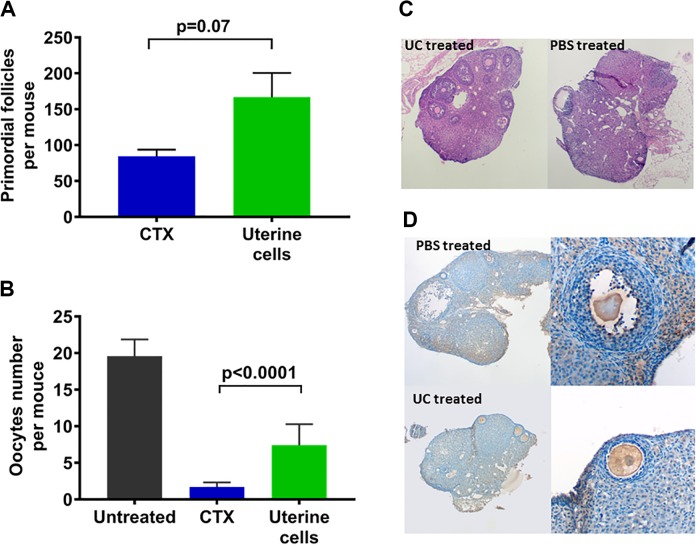
One week after treatment with UC or PBS, primordial follicle number in the ovaries of mice that received UC increased to double that of the mice that received PBS (167 ± 34 vs 84 ± 9; n = 10; P = .07), as shown in Figure 2A, although this difference was not statistically significant. As shown in Figure 2B, mice treated with UC ovulated significantly (4 times) more oocytes than the mice treated with PBS (7.4 ± 0.9 vs 1.7 ± 0.6; n = 20; P < .0001). Better overall morphology and architecture were also noted in the ovaries of UC-treated mice, as shown in Figure 2C, where we found more number of developing follicles and better overall morphology. Staining for GFP revealed no visible presence of GFP-positive cells in the ovaries, as shown in Figure 2D.
Figure 2.
Ovarian function rescued by uterine cell suspension containing endometrial mesenchymal stem cells (eMSCs). A, The number of primordial follicles increased but not significantly in uterine cell suspension-treated mice compared to mice without treatment (P = 7). Error bars represent the mean ± SEM. (n = 10 mice per group). B, The number of oocytes increased significantly in uterine cell suspension-treated mice compared to mice without treatment. Error bars represent the mean ± SEM (n = 10 mice per group). P < .0001, using an unpaired t test with Welch correction. C, Ovary morphology. Ovary of a mouse treated with UC after chemotherapy (left) showing more developing follicles and better overall morphology than that of the mouse treated with PBS (right), which demonstrates less follicles, worse architecture, and areas of vacuolization. D, GFP uptake. No difference in immunohistochemistry slides of GFP uptake in ovaries treated with PBS (top) versus UC (bottom). Some background staining with GFP stain noted on both tissues. GFP indicates green fluorescent protein; PBS, phosphate-buffered saline; SEM, standard error of the mean; UC, uterine cell.
Pregnancy Rates
The 13 mice assigned to the PBS group attained 1 successful pregnancy over 6 months after housed with males of proven fertility. In contrast, the 13 mice assigned to the UC group produced 5 viable litters. This resulted in cumulative pregnancy rates of 7.7% for the PBS group and 38.5% in the UC group, as shown in Figure 3. In those 6 months, the PBS mice delivered a total of 6 pups (0.46 per mouse), whereas the UC mice delivered 26 pups (2 pups per mouse), a 4-fold increase in litter size in the UC group compared to the PBS group P < .029). None of the pups produced by either group displayed green fluorescence under UV light. Notably, all pregnancies took place between 3 and 6 weeks after administering either PBS or UC.
Figure 3.
Determination of pregnancy rates. Pregnancy rate was increased significantly in uterine cell suspension-treated mice compared to mice without treatment. Error bars represent the mean ± SEM (n = 13 mice per group). P < .029, using an unpaired t test with Welch correction. SEM indicates standard error of the mean.
Determination of Body Weight Changes
Although not an anticipated outcome measure, weight was recorded to assess chemotherapy dosing, mice health, and potential gravid status. There was no significant difference in body mass at the start of the trial between the mice that received PBS and those that received UC (19.0 g vs 19.7 g). However, 24 weeks after treatment with either PBS or UC, a weight difference became both grossly apparent (27.8 g vs 34.3 g) and statistically significant (n = 26; P < .0001), as shown in Figure 4A. Necropsy studies showed that mice treated with UC revealed more extensive visceral adipose tissue than their PBS counterparts. Interestingly, in vivo imaging of a biopsy of adipose tissue of mice that had been treated with UC showed GFP fluorescent cells (Figure 4B).
Figure 4.
A, Increased body weight in uterine cell suspension containing eMSCs-treated mice. After 12 weeks, the body weight of the uterine cell suspension-treated mice were increased significantly compared to untreated with uterine cell suspension. No significant changes were observed before 12-week time point. Error bars represent the mean ± SEM (n = 26 mice per group). P < .0001, using an unpaired t test with Welch correction. B, Adipose tissue. GFP fluorescent cell visualized using an in vivo FX PRO system (Carestream, CT) in the adipose tissue of a mouse treated with UC after chemotherapy. eMSCs indicate endometrial mesenchymal stem cells; GFP, green fluorescent protein; SEM, standard error of the mean.
Serum AMH
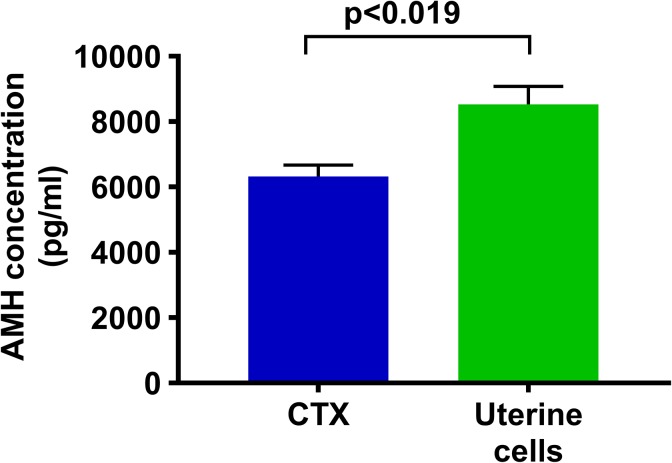
Anti-Müllerian hormone levels were determined from serum samples of mice in which an adequate blood sample was available at all time points (n = 8; 4 mice per group). No difference was observed in AMH concentrations between the 2 groups at 2 weeks posttreatment time point or at 6 months. Only at the 6-week posttreatment blood draw, the concentration of AMH in UC mice was significantly higher than it was in the PBS group (8.530 vs 6.321 pg/mL; n = 8; P < .019), as shown in Figure 5. As previously mentioned, these results are difficult to interpret given the technical challenges and the very limited validation of murine AMH assays and the correlation of murine AMH to mouse reproductive potential.
Figure 5.
Serum AMH levels. The AMH levels were increased significantly in uterine cell suspension-treated mice compared to mice without treatment. Error bars represent the mean ± SEM (n = 10 mice per group, P < .019), using an unpaired t test with Welch correction. AMH indicates anti-Müllerian hormone; SEM, standard error of the mean.
Discussion
Endometrial mesenchymal stem cells have recently been characterized as a potential therapy in regenerative medicine.15,17,21,24,25 Our research demonstrates an improvement in ovarian function in mice receiving chemotherapy and treated with these cells. Mice receiving an eMSC-containing UC suspension after chemotherapy had a 2-fold increase in primordial follicles, a 4-fold increase in oocyte count, and a 19% higher body mass than mice treated with PBS. These findings are suggestive of a potential clinical beneficial role of endometrial cells, easily accessible in humans via endometrial biopsy, in the treatment and prevention of chemotherapy-induced POI. The rest of our findings, including the 5-fold increase in number of pregnancies and the 4-fold difference in total number of pups point to the same conclusion.
Interestingly, the most important time for regeneration after UC therapy appears to be the first 3 to 6 weeks, perhaps owing to a protective effect on the ovary rather than true regenerative effects on the oocyte pool. This theory is supported by the fact that none of the offspring of mice treated with GFP-positive UC displayed fluorescence under UV light, indicating that none of the oocytes were of donor cell origin.
Prior research has shown similar improvements in ovarian function with stem cells from other lineages including tissue sources as diverse as bone marrow,8,9,26–28 amniotic epithelium,10 amniotic fluid,11 human menstrual blood stem cells,29 MSCs,30–32 and adipose tissue.33 The biological mechanism of this improvement has been a topic of discussion for some time. Some studies suggest ovarian and follicular regeneration directly as a result of differentiation of stem cells into ovarian parenchymal tissue.34–36 Another study suggests the promotion of ovarian angiogenesis by stem cell infusion as one of the factors contributing to the observed improvements.26 Mechanical damage to the ovaries, by way of laser drilling or wedge resections, is also thought to promote follicular growth, and Kawamura et al demonstrated a signaling pathway disruption that can be exploited to increase follicular growth.37
Here, the lack of fluorescence in the resulting offspring suggests that the underlying mechanism is more likely related to an enhanced stem cell support of the ovarian stem cell niche rather than to a differentiation into mature oocytes.32
In 2015, Lai et al tested the effect of transplanting human endometrial stem cells into murine models of POI.34 This resulted in an improvement of ovarian function, as evidenced by the reported regularization of estrus cycles and the return of fertility in the treated group. While both studies have important differences in design—Lai et al used double the amount of eMSCs per transplant and 10 times the concentration used in our experiments—the results are overall similar in supporting a beneficial effect of eMSC transplantation in mice with chemotherapy-induced POI. Contrary to our findings, GFP-staining cells were identified histologically in the ovaries of treated mice. However, it is important to mention that the ovaries were harvested 2 months after treatment, as opposed to only 1 week after the cell administration, and that live imaging revealed no GFP activity in the murine pelvis at 1 week. Similar to our observations, the weight of treated mice was significantly higher than that of controls.
The significance of the heavier weight and larger amount of visceral fat in the UC-treated mice lies in that larger weights are positively correlated with the overall health of mice. Indeed, mesenchymal stem cell therapy has been demonstrated to be of use in treating or ameliorating a wide variety of diseases, including coronary artery disease,38 type 1 diabetes mellitus,39 vision loss,40 and arthritis.41 The fact that treated mice gained weight faster after chemotherapy may indicate better overall conditioning, which can in itself affect fertility.42
Additionally, a more specific mechanism of action of the increased visceral adiposity of the treated mice can be postulated. Several studies have pointed to the implication of visfatin to polycystic ovarian syndrome, as well as to its decline with advancing female age. Visfatin administration in mice also results in improvement of ovarian function and oocyte quality by stimulating angiogenesis.43
Future research attempting to administer cell therapies before the administration of chemotherapy could further delineate the possibility of a protective effect on the ovary. Similarly, ongoing projects involving other tissue origins as potential cell sources will surely provide much needed knowledge in regard to the mechanism by which these beneficial effects take place.
Our research suggests that stem cell therapy, particularly of endometrial origin, should be studied in young women undergoing ovotoxic chemotherapy. The endometrium is an easily accessible source of stem cells and results in animal models are promising.
Supplemental Material
Supplemental Material, Supplemental_figure_1 for Uterine Cells Improved Ovarian Function in a Murine Model of Ovarian Insufficiency by Andres Reig, Ramanaiah Mamillapalli, Alexis Coolidge, Joshua Johnson and Hugh S. Taylor in Reproductive Sciences
Acknowledgments
We thank Joshua Huttler for proof reading this manuscript.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by NIH U54 HD052668 and R01 HD076422.
Supplemental Material: Supplemental material for this article is available online.
References
- 1. De Vos M, Devroey P, Fauser BC. Primary ovarian insufficiency. Lancet (London, England). 2010;376(9744):911–921. [DOI] [PubMed] [Google Scholar]
- 2. Nelson LM. Clinical practice. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bedoschi G, Navarro PA, Oktay K. Chemotherapy-induced damage to ovary: mechanisms and clinical impact. Future Oncol (London, England). 2016;12(20):2333–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Oktay K, Harvey BE, Partridge AH, et al. Fertility preservation in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2018;36(19):1994–2001. [DOI] [PubMed] [Google Scholar]
- 5. Lambertini M, Moore HCF, Leonard RCF, et al. Gonadotropin-releasing hormone agonists during chemotherapy for preservation of ovarian function and fertility in premenopausal patients with early breast cancer: a systematic review and meta-analysis of individual patient-level data. J Clin Oncol. 2018;36(19):1981–1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Senra JC, Roque M, Talim MCT, Reis FM, Tavares RLC. Gonadotropin-releasing hormone agonists for ovarian protection during cancer chemotherapy: systematic review and meta-analysis. Ultrasound in Obstet Gynecol. 2018;51(1):77–86. [DOI] [PubMed] [Google Scholar]
- 7. Moawad NS, Santamaria E, Rhoton-Vlasak A, Lightsey JL. Laparoscopic ovarian transposition before pelvic cancer treatment: ovarian function and fertility preservation. J Minim Invasive Gynecol. 2017;24(1):28–35. [DOI] [PubMed] [Google Scholar]
- 8. Lee HJ, Selesniemi K, Niikura Y, et al. Bone marrow transplantation generates immature oocytes and rescues long-term fertility in a preclinical mouse model of chemotherapy-induced premature ovarian failure. J Clin Oncol. 2007;25(22):3198–3204. [DOI] [PubMed] [Google Scholar]
- 9. Fu X, He Y, Xie C, Liu W. Bone marrow mesenchymal stem cell transplantation improves ovarian function and structure in rats with chemotherapy-induced ovarian damage. Cytotherapy. 2008;10(4):353–363. [DOI] [PubMed] [Google Scholar]
- 10. Wang F, Wang L, Yao X, Lai D, Guo L. Human amniotic epithelial cells can differentiate into granulosa cells and restore folliculogenesis in a mouse model of chemotherapy-induced premature ovarian failure. Stem cell Res Ther. 2013;4(5):124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lai D, Wang F, Chen Y, Wang L, Wang Y, Cheng W. Human amniotic fluid stem cells have a potential to recover ovarian function in mice with chemotherapy-induced sterility. BMC Develop Biol. 2013;13:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85. [DOI] [PubMed] [Google Scholar]
- 13. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086. [DOI] [PubMed] [Google Scholar]
- 14. Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16(2):126–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Du H, Naqvi H, Taylor HS. Ischemia/reperfusion injury promotes and granulocyte-colony stimulating factor inhibits migration of bone marrow-derived stem cells to endometrium. Stem cells and Develop. 2012;21(18):3324–3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wolff EF, Wolff AB, Hongling D, Taylor HS. Demonstration of multipotent stem cells in the adult human endometrium by in vitro chondrogenesis. Reprod Sci. 2007;14(6):524–533. [DOI] [PubMed] [Google Scholar]
- 17. Simoni M, Taylor HS. Therapeutic strategies involving uterine stem cells in reproductive medicine. Curr Opin Obstet & Gynecol. 2018;30(3):209–216. [DOI] [PubMed] [Google Scholar]
- 18. Wolff EF, Mutlu L, Massasa EE, Elsworth JD, Eugene Redmond D, Jr, Taylor HS. Endometrial stem cell transplantation in MPTP-exposed primates: an alternative cell source for treatment of Parkinson’s disease. J Cell and Mol Med. 2015;19(1):249–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Santamaria X, Massasa EE, Feng Y, Wolff E, Taylor HS. Derivation of insulin producing cells from human endometrial stromal stem cells and use in the treatment of murine diabetes. Mol Ther. 2011;19(11):2065–2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wolff EF, Gao XB, Yao KV, et al. Endometrial stem cell transplantation restores dopamine production in a Parkinson’s disease model. J Cell and Mol Med. 2011;15(4):747–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Mutlu L, Hufnagel D, Taylor HS. The endometrium as a source of mesenchymal stem cells for regenerative medicine. Biol Reprod. 2015;92(6):138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou K, Yuan Z, Yang Z, et al. Production of offspring from a germline stem cell line derived from neonatal ovaries. Nat Cell Biol. 2009;11(5):631–636. [DOI] [PubMed] [Google Scholar]
- 23. Johnson J, Canning J, Kaneko T, Pru JK, Tilly JL. Germline stem cells and follicular renewal in the postnatal mammalian ovary. Nature. 2004;428(6979):145–150. [DOI] [PubMed] [Google Scholar]
- 24. Massasa EE, Taylor HS. Use of endometrial stem cells in regenerative medicine. Regen Med. 2012;7(2):133–135. [DOI] [PubMed] [Google Scholar]
- 25. Sahin Ersoy G, Zolbin MM, Cosar E, Moridi I, Mamillapalli R, Taylor HS. CXCL12 promotes stem cell recruitment and uterine repair after injury in Asherman’s syndrome. Mol Ther Meth Clin Dev. 2017;4:169–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Herraiz S, Buigues A, Diaz-Garcia C, et al. Fertility rescue and ovarian follicle growth promotion by bone marrow stem cell infusion. Fertil Steril. 2018;109(5):908–918.e902. [DOI] [PubMed] [Google Scholar]
- 27. Liu J, Zhang H, Zhang Y, et al. Homing and restorative effects of bone marrow-derived mesenchymal stem cells on cisplatin injured ovaries in rats. Mol Cells. 2014;37(12):865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kilic S, Pinarli F, Ozogul C, Tasdemir N, Naz Sarac G, Delibasi T. Protection from cyclophosphamide-induced ovarian damage with bone marrow-derived mesenchymal stem cells during puberty. Gynecol Endocrinol. 2014;30(2):135–140. [DOI] [PubMed] [Google Scholar]
- 29. Liu T, Huang Y, Zhang J, et al. Transplantation of human menstrual blood stem cells to treat premature ovarian failure in mouse model. Stem cells Dev. 2014;23(13):1548–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Grady ST, Watts AE, Thompson JA, Penedo MCT, Konganti K, Hinrichs K. Effect of intra-ovarian injection of mesenchymal stem cells in aged mares. J Assist Reprod Genet. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fazeli Z, Abedindo A, Omrani MD, Ghaderian SMH. Mesenchymal stem cells (MSCs) therapy for recovery of fertility: a systematic review. Stem Cell Rev. 2018;14(1):1–12. [DOI] [PubMed] [Google Scholar]
- 32. Mohamed SA, Shalaby SM, Abdelaziz M, et al. Human mesenchymal stem cells partially reverse infertility in chemotherapy-induced ovarian failure. Reprod Sci. 2018;25(1):51–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Sun M, Wang S, Li Y, et al. Adipose-derived stem cells improved mouse ovary function after chemotherapy-induced ovary failure. Stem Cell Res Ther. 2013;4(4):80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lai D, Wang F, Yao X, Zhang Q, Wu X, Xiang C. Human endometrial mesenchymal stem cells restore ovarian function through improving the renewal of germline stem cells in a mouse model of premature ovarian failure. J Trans Med. 2015;13:155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tilly JL, Telfer EE. Purification of germline stem cells from adult mammalian ovaries: a step closer towards control of the female biological clock? Mol Hum Reprod. 2009;15(7):393–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. White YA, Woods DC, Takai Y, Ishihara O, Seki H, Tilly JL. Oocyte formation by mitotically active germ cells purified from ovaries of reproductive-age women. Nat Med. 2012;18(3):413–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kawamura K, Cheng Y, Suzuki N, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci. USA. 2013;110(43):17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Schachinger V, Erbs S, Elsasser A, et al. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355(12):1210–1221. [DOI] [PubMed] [Google Scholar]
- 39. Vanikar AV, Trivedi HL, Thakkar UG. . Stem cell therapy emerging as the key player in treating type 1 diabetes mellitus. Cytotherapy. 2016;18(9):1077–1086. [DOI] [PubMed] [Google Scholar]
- 40. Higuchi A, Kumar SS, Benelli G, et al. Stem cell therapies for reversing vision loss. Trends Biotechnol. 2017;35(11):1102–1117. [DOI] [PubMed] [Google Scholar]
- 41. Freitag J, Wickham J, Shah K, Tenen A. Effect of autologous adipose-derived mesenchymal stem cell therapy in the treatment of acromioclavicular joint osteoarthritis. BMJ Case Rep. 2019;12(2):pii: e227865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Optimizing natural fertility: A committee opinion. Fertil and Steril. 2017;107(1):52–58. [DOI] [PubMed] [Google Scholar]
- 43. Choi KH, Joo BS, Sun ST, et al. Administration of visfatin during superovulation improves developmental competency of oocytes and fertility potential in aged female mice. Fertil Steril. 2012;97(5):1234–1241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material, Supplemental_figure_1 for Uterine Cells Improved Ovarian Function in a Murine Model of Ovarian Insufficiency by Andres Reig, Ramanaiah Mamillapalli, Alexis Coolidge, Joshua Johnson and Hugh S. Taylor in Reproductive Sciences