Abstract
C-X3-C motif ligand 1 (CX3CL1) mediates migration, survival, and adhesion of natural killer (NK) cells, monocytes, and T-cells to endothelial/epithelial cells. Aberrant numbers and/or activation of these decidual immune cells elicit preeclampsia development. Decidual macrophages and NK cells are critical for implantation, while macrophage-derived tumor necrosis factor-α (TNF-α), interleukin-1 β (IL-1β), and NK cell-derived interferon-γ (IFN-γ) are associated with preeclampsia development. Thus, serum and decidual levels of CX3CL1 from first-trimester pregnancy and preeclampsia-complicated term pregnancy were examined by enzyme-linked immunosorbent assay (ELISA) and immunohistochemistry, respectively. The effects of incubating primary human first-trimester decidual cells (FTDCs) with estradiol + medroxyprogesterone acetate + either IL-1β or TNF-α and/or IFN-γ on CX3CL1 expression were also assessed by quantitative reverse transcription-polymerase chain reaction and ELISA. The inhibition of each signaling pathway with each kinase and nuclear factor κB (NFκB) inhibitors was evaluated by ELISA. Chemotaxis of CD56brightCD16− NK cells by various concentrations of CX3CL1 was evaluated. C-X3-C motif ligand 1 is expressed by both cytotrophoblasts and decidual cells in first-trimester decidua. C-X3-C motif ligand 1 expression is increased in term decidua but unchanged in first-trimester and term serum of patients with preeclampsia. Interferon-gamma and either IL-1β or TNF-α synergistically upregulated CX3CL1 expression in FTDCs. Coincubation with IL-1β or TNF-α or IFN-γ, mitogen-activated protein kinase kinase 1 and 2 (MEK1/2), c-JUN N-terminal kinase (JNK), and NFκB inhibitors suppressed CX3CL1 production. C-X3-C motif ligand 1 elicited concentration-dependent enhancement of CD56brightCD16− NK cell migration. In conclusion, the current study suggests that decidual cell-secreted CX3CL1 is involved in the later development of preeclampsia, whereas circulating CX3CL1 levels do not predict preeclampsia. Mitogen-activated protein kinase kinase 1 and 2, JNK, and NFκB signaling mediate IL-1β-, TNF-α-, and IFN-γ-induced CX3CL1 production by FTDCs.
Keywords: CX3CL1, preeclampsia, dNK cell, decidual cell
Introduction
Implanting human blastocyst-derived extravillous cytotrophoblasts (EVTs) invade an underlying decidua comprised primarily of resident decidual cells (50%) and a diverse immune cell population (40%) dominated by decidual natural killer (dNK) cells (70%), macrophages (20%), and T-lymphocytes (10%) with small percentages of dendritic cells and B-lymphocytes.1 As EVTs traverse the decidua and inner third of the myometrium, they detach from anchoring placental villous columns and breech spiral arteries and arterioles. This invasive process can occur either from within the lumen mediated by endovascular EVTs or from the surrounding stroma mediated by interstitial EVTs.2 By both routes, the EVT epithelial cell adhesion molecule phenotype of EVTs is converted to an endothelial cell-like adhesion molecule phenotype with the spiral arteries and arterioles transformed to low-resistance, high-capacity conduits that provide increased uteroplacental blood flow to the developing fetal–placental unit.3,4 In contrast, shallow EVT invasion is accompanied by decreased uteroplacental blood flow leading to fetal growth restriction as well as placental production of antiangiogenic and proinflammatory factors that precede the later occurrence of hypertension and proteinuria, that is, the maternal syndrome of preeclampsia.5
Our previous results showed that coincubation with interferon-gamma (IFN-γ), a primary NK cell product,6 and either of the primary macrophage-derived cytokines tumor necrosis factor alpha (TNF-α) or interleukin-1 beta (IL-1β)7 synergistically enhanced expression of 2 neutrophil, monocyte, and NK cell-recruiting chemokines, interferon-gamma inducible protein 10 (IP-10) (CXCL10) and interferon-gamma inducible T-cell alpha chemoattractant (I-TAC) (CXCL11), in human leukocyte-free first-trimester decidual cells.8 Complementing these in vitro observations, immunostaining revealed increased expression of both IP-10 and I-TAC in preeclampsia-derived decidua. Moreover, significantly elevated IP-10 levels in first-trimester sera from women eventually developing preeclampsia identified IP-10 as an early predictor of the later development of preeclampsia.8
The current study extends these in vitro and in situ observations to include C-X3-C motif ligand 1 (CX3CL1; fractalkine), the sole CX3C chemokine family member.9 Among the 48 chemokines identified to date,10 CX3CL1 alone is expressed as a membrane-bound molecule that features a chemokine domain attached to the cell surface by a mucin-like stalk.9 Cleavage at the base of this stalk is mediated by 2 enzymes, a disintegrin and metalloproteinase domain-containing protein 10 and 17 (ADAM10 and ADAM17), which function, respectively, under homeostatic and inflammatory conditions.11-13 Acting as the singular ligand for its sole receptor, CX3C chemokine receptor 1 (CX3CR1), membrane-bound CX3CL1 enhances flow-resistant adhesion of leukocytes to endothelial or epithelial cells, whereas soluble CX3CL1 mediates migration, adhesion, and survival of NK cells, monocytes, and T-cells.14 In addition, CX3CL1 specifically promotes the invasion of CX3CR1-expressing endovascular EVTs, while CX3CR1 was not detected in interstitial EVTs.15
Intimate roles in human pregnancy are documented for specific “activated” immune cell subtypes in early human decidua in promoting (1) vascular remodeling of spiral arteries and arterioles to increase uterine blood flow and angiogenesis required by the developing fetal–placental unit demonstrated for a specific CD56brightCD16− dNK cell subtype16; (2) aspects of implantation and placentation by macrophages; and (3) immune tolerance of the foreign semi-allogeneic fetal transplant by subsets of T-lymphocytes.17
Preeclampsia is a leading cause of maternal and fetal morbidity and mortality that is generally diagnosed in the second or third trimester of pregnancy.18,19 However, its pathogenesis is frequently initiated early because of shallow EVT invasion accompanied by incomplete remodeling of the spiral arteries and arterioles resulting in inadequate blood flow to the developing fetal–placental unit.20 Accumulating evidence indicates that aberrant numbers and/or activation of decidual immune cells, that is, dNK cells, macrophages, and T-cells are involved in the later development of preeclampsia.21-23 The pleiotropic actions displayed by CX3CL1 in both recruiting and activating these immune cell types24 stimulated the current study to examine the potential association between decidual cell-expressed CX3CL1 and the later development of preeclampsia by utilizing a 2-tiered approach that integrated in vitro and in situ observations. Given the facts that macrophage and NK cell are 2 major immune cell types in the decidua of early pregnancy, while their products TNF-α, IL-1β (macrophage), and IFN-γ (NK cell) were demonstrated to be associated with the development of preeclampsia.25-27 Furthermore, IFN-γ is known to synergistically induce inflammatory genes with other proinflammatory molecules, such as lipopolysaccharide and TNF-α.28,29
Thus, the current study examined the expression of CX3CL1 in (1) first-trimester decidua; (2) first-trimester serum from patients with either normal pregnancy or eventually developed preeclampsia; and (3) serum and decidua from term pregnancy complicated with or without preeclampsia. The effects of incubating primary human first-trimester decidual cells (FTDCs) with either TNF-α or IL-1β and/or IFN-γ on CX3CL1 messenger RNA (mRNA) and protein expression as well as signaling pathways mediating CX3CL1 production were assessed.
Materials and Methods
Immunofluorescent Staining
Decidua basalis specimens were collected from patients with first-trimester elective termination without pregnancy complications or term pregnancy with preeclampsia and from gestational age-matched controls under institutional review board (IRB) approval at Mackay Memorial Hospital, Taipei, Taiwan. Preeclampsia was diagnosed as the existence of elevated blood pressure (≥140/90 mm Hg) and proteinuria (>1+ on a urine dip stick) found twice, 6 hours apart after 20 weeks of gestation without signs and symptoms of chorioamnionitis or chronic villitis. Both preeclampsia and gestational age-matched controls (37–40 weeks) were obtained by cesarean delivery without labor. Serial sections (8 μm) of optimum cutting temperature-embedded decidua were incubated with rabbit antihuman CX3CL1 in Tris-buffered saline (1:500; Sigma-Aldrich, St. Louis, Missouri) for 1 hour at 25°C. Immunostaining specificity was confirmed by substituting a rabbit IgG isotype (DAKO, Santa Clara, California). The sections were then treated with fluorescein isothiocyanate–conjugated donkey antirabbit antibody for 1 hour. After washing, the sections were incubated with goat antihuman vimentin or mouse antihuman cytokeratin 7 (CK-7) antibody for 1 hour at 25°C followed by incubation with rhodamine-conjugated rabbit antigoat antibody (1:400; Sigma-Aldrich) or donkey antimouse antibody (1:500; Millipore; Temecula, California) for 1 hour. Finally, the sections were stained with 4′,6′-diamidino-2-phenylindole (DAPI; Sigma-Aldrich) and mounted in a nonfade mounting medium (DAKO). Immunofluorescence was examined under a Zeiss microscope (Müchen-Hallbergmoos, Germany) equipped with a cooled charge-coupled device camera (Axiocam HRm, Zeiss).
Cell Isolation and Culture
First trimester decidual cells were isolated and cultured as described previously.30 Briefly, the decidua from elective terminations between 6 and 12 weeks of gestation was obtained under IRB approval at E-Da Hospital. Tissues were minced and digested with 0.1% collagenase type IV, 0.01% DNase in Ham’s F-10. The digestate was subjected to 60/50/40% Ficoll-Paque Plus (GE Healthcare, Piscataway, New Jersey) purification. Decidual cells were retrieved from the 40% layer followed by culturing in basal medium (phenol red-free 1:1 vol/vol mix of Dulbecco's Modified Eagle's Medium and Ham’s F-12; Sigma-Aldrich) supplemented with 100 U/mL penicillin, 100 µg/mL streptomycin, 0.25 ng/mL fungizone (Invitrogen, Carlsbad, California), and 10% charcoal-stripped calf serum (Germini Bio-products, West Sacramento, California). The cells were passaged until free of immune cell contamination as determined by flow cytometric analysis of anti-CD14 and anti-CD45 (BD Pharmingen, San Diego, California). Decidualization was carried out by treating confluent decidual cells with estradiol (E) (10−8 mol/L) + medroxyprogesterone acetate (MPA) (10−7 mol/L) for 7 days and confirmed by enhanced expression of prolactin and plasminogen activator inhibitor-1 and inhibited expression of interstitial collagenase and stromelysin-1 (data not shown). These cells were then incubated in serum-free defined medium ± 1 ng/mL IL-1β or TNF-α (R&D Systems, Minneapolis, Minnesota) for 24 hours. Conditioned medium supernatants and whole cell lysates or total RNA were collected and stored at −80°C for future use.
One hundred milliliter of peripheral blood from healthy reproductive-age female donors was obtained under IRB approval at The Ohio State University. Peripheral blood mononuclear cells were isolated from the interphase after Ficoll–Paque (GE Healthcare) centrifugation of CD56brightCD16− NK cells were purified with anti-CD56 paramagnetic beads (Miltenyi Biotec, Auburn, California) according to the manufacturer’s instruction. The purity of NK cells was determined by flow cytometric analysis, confirming that at least 95% purity was achieved.
Signaling Pathway(s) Mediation of CX3CL1 Production in FTDCs
First-trimester decidual cells were preincubated with either a mitogen-activated protein kinase kinase (MAPKK) 1 and 2 (MEK1/2) inhibitor (PD184325, 20 μmol/L) or a c-JUN N-terminal kinase (JNK) inhibitor (SP600125, 20 μmol/L) or a p38 MAPK inhibitor (SB203580, 1 μmol/L; Sigma-Aldrich) or a nuclear factor κB (NFκB) inhibitor (BAY11-7082, 5 μM; Invitrogen) or a PI3 kinase (PI3K) inhibitor (wortmannin, 5 μmol/L) for 1.5 hours before incubation with 10 ng/mL IL-1β or TNF-α or IFN-γ. Conditioned medium was collected after 24 hours for CX3CL1 measurements using a commercial enzyme-linked immunosorbent assay (ELISA) kit from R&D Systems according to the manufacturer’s instructions. Total cell protein levels were measured by a modified Lowry assay (Bio-Rad Labs, Inc, Hercules, California).
Enzyme-Linked Immunosorbent Assay
Frozen tissues were homogenized in RIPA lysis buffer (150 mmol/L NaCl, 1% IGEPAL CA-630, 0.1% sodium dodecyl sulfate, 0.5% sodium deoxycholate, 50 mmol/L Tris, pH 8.0) with protease inhibitor cocktail (Sigma-Aldrich). Total protein levels were measured using protein assay dye reagent (Pierce BCA Protein Assay Kit; Thermo Fisher Scientific, Waltham, Massachusetts). C-X3-C motif ligand 1 levels were measured using an ELISA kit by following the manufacturer’s instruction (R&D Systems). The sensitivity range was 6.0 to 7.2 pg/mL. The intra- and interassay coefficients of variation were 2.1% to 3.2% and 5.4% to 8.9%, respectively.
Natural Killer Cell Chemotaxis Assay
Peripheral blood mononuclear cells were isolated from healthy reproductive-age female donors by gradient Ficoll-Hypaque (GE Healthcare) centrifugation under IRB approval at The Ohio State University. The CD56bright CD16− NK cells were purified with CD56 paramagnetic beads from Miltenyi Biotec according to the manufacturer’s instruction. The chemotactic activity for CD56brightCD16− NK cells by various concentrations of CX3CL1 (0, 0.1, 1, 10, or 50 ng/mL) was performed as described.8 Briefly, CX3CL1 was added into each well in a 24-well culture plate. CD56brightCD16− NK cells (5 × 105 cells/mL) were added to each cell culture insert with P.E.T. membrane (BD Biosciences, San Jose, California) in a well. After incubation for 16 to 18 hours at 37°C in 5% CO2, cell migration was determined by counting the cell number in the wells for 1 minute using flow cytometer.
Quantitative Reverse Transcription-Polymerase Chain Reaction
Total RNA was extracted from macrophages using a total RNA purification plus kit (Norgen Bioteck, Ontario, Canada). Reverse transcription used SuperScript II First-Strand Synthesis System from Invitrogen (Thermo Scientific, Waltham, Massachusetts). Specific primer sets for human CX3CL1 (Taqman Cat # Hs0017086_m1) or β-actin (Thermo Scientific) were used for quantitative-polymerase chain reaction (qPCR). Quantitation of unknowns was determined and adjusted to quantitative expression of β-actin in specific samples. Melting curve analysis determined the specificity of the amplified products and the absence of primer-dimer formation.
Statistics
The variance and normality of data from tissue and cell CX3CL1 ELISAs, quantitative reverse transcription-PCR as well as NK cell migration assay were first examined. Then, the statistical significance of results with equal variance was examined by t test assuming equal variance. The results with unequal variance that either passed or failed normality test were then evaluated by t test assuming unequal variance or the Mann-Whitney rank sum test, respectively. A P < .05 was considered significant.
Results
C-X3-C Motif Ligand 1 Is Expressed by Both Cytotrophoblasts and Decidual Cells
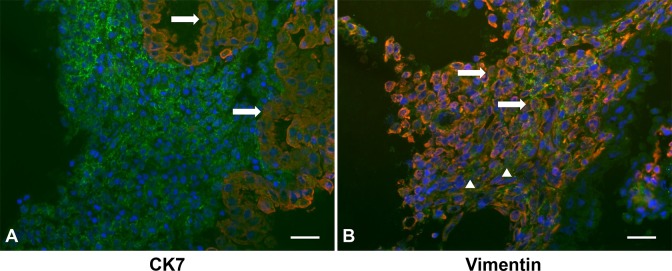
Cryosections of first trimester decidua were immunostained for CX3CL1 (Figure 1; green fluorescence) and then with either vimentin as a decidual cell marker (Figure 1A) or CK7 as a cytotrophoblast marker (Figure 1B; red fluorescence). Nuclei were identified by DAPI staining (blue fluorescence). Overlap between red and green immunostaining appears as yellow to orange staining. Inspection of Figure 1 indicates that CX3CL1 is found on the membrane of cytotrophoblasts in anchoring villi and FTDCs as well as in the extracellular space.
Figure 1.
C-X3-C motif ligand 1 (CX3CL1) is expressed in both first trimester decidual cells (FTDCs) and cytotrophoblasts. Representative photomicrographs of immunofluorescent staining of CX3CL1 (green) in first trimester decidua from elective terminations. These tissues were costained with either (A) anti-cytokeratin 7 or (B) anti-vimentin antibody (red). Arrows indicate either cytotrophoblasts or decidual cells expressing membranous CX3CL1 (yellow or orange). Arrowheads indicate the CX3CL1 in extracellular space. Magnification: 400×; Scale bar: 50 μm.
C-X3-C Motif Ligand 1 Expression Is Increased in Term Decidua and Unchanged in Both First-Trimester and Term Serum of Patients With Preeclampsia
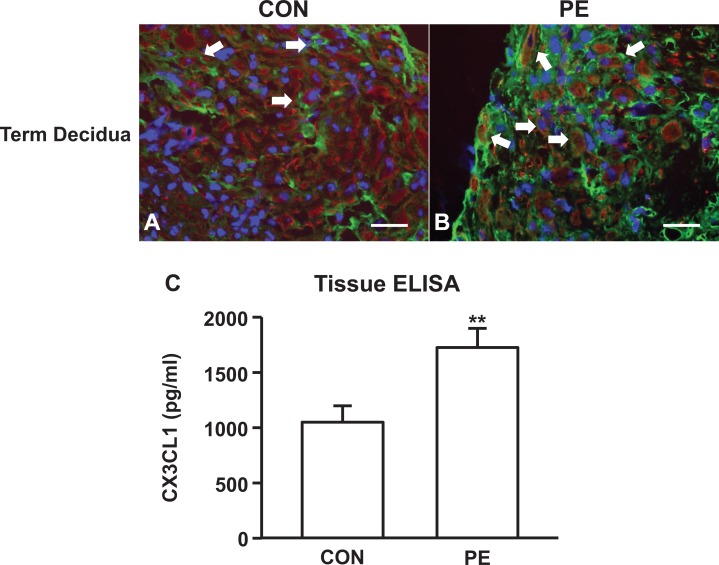
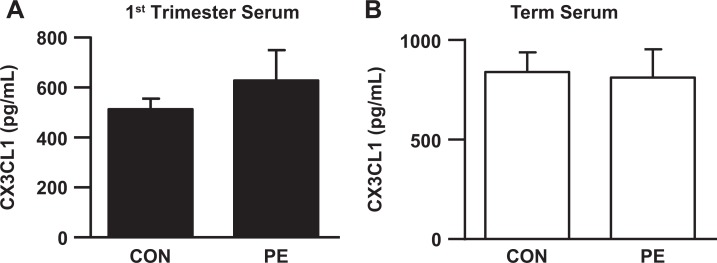
In Figure 2A and B, a statistically significant increase in CX3CL1 expression in term decidua cells is associated with the development of preeclampsia. Tissue ELISA analysis for decidual extracts from patients with preeclampsia finds significantly higher levels of CX3CL1 (Figure 2C) than age-matched controls. In contrast, no such correlation was observed for the first-trimester and term serum levels of CX3CL1 (Figure 3A and B).
Figure 2.
C-X3-C motif ligand 1 (CX3CL1) expression in term decidua from patients with preeclampsia is increased without changes in maternal serum. Representative photomicrographs of immunofluorescent staining of CX3CL1 (green) in decidua from (A) gestational age-matched pregnancies and (B) patients with preeclampsia. These tissues were costained with anti-vimentin antibody (red). C-X3-C motif ligand 1 protein expression in (C) decidua. Arrows indicate decidual cells expressing membranous CX3CL1 (yellow or orange). The results are reported as mean ± standard error of the mean. n = 11; **P < .01.
Figure 3.
C-X3-C motif ligand 1 (CX3CL1) serum levels in patients with preeclampsia remain unchanged. (A) first trimester (B) term maternal serum was measured by ELISA. The results are reported as mean ± standard error of the mean. First trimester: n = 90 for control, n = 30 for preeclampsia; term: n = 40. ELISA indicates enzyme-linked immunosorbent assay
C-X3-C Motif Ligand 1 Expression in FTDCs Is Synergistically Upregulated by IFN-γ and Either IL-1β or TNF-α
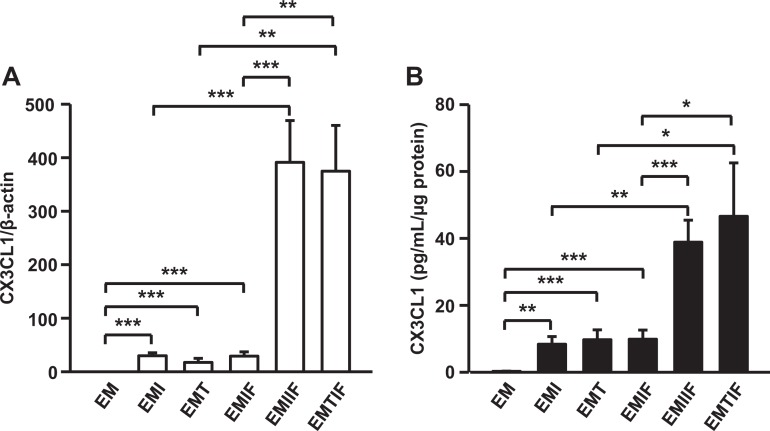
The effects of preeclampsia responsive proinflammatory cytokines, IL-1β and TNF-α, as well as decidual cell- and NK cell-secreted IFN-γ31 were assessed on the production of NK-recruiting CX3CL1 in leukocyte-free FTDCs cultured with E2 + MPA to mimic the hormonal milieu of early pregnancy. The expression levels of CX3CL1 mRNA are increased in FTDCs treated with 1 ng/mL IL-1β, TNF-α or IFN-γ by 178.34 ± 60.01-, 80.01 ± 21.67-, and 119.97 ± 24.99-fold, respectively (Figure 4A). The combination of IFN-γ with either IL-1β, TNF-α increases CX3CL1 mRNA by 2067.12 ± 456.86- and 1502.17 ± 165.91-fold, respectively, that are 7- to 8-fold greater than the sum of each cytokine used alone (Figure 3A). Consistently, IL-1β induces 3.95 ± 1.62 pg/mL/μg protein, an increase of 27.04 ± 9.12-fold over basal output (Figure 4B). Tumor necrosis factor-alpha induces 4.55 ± 2.97 pg/mL/mg protein, an increase of 23.54 ± 9.38-fold over basal output. Interferon-gamma stimulation induces 5.04 ± 1.27 pg/mL/μg protein, an increase of 36.36 ± 8.00-fold over basal output (0.15 ± 0.03 pg/mL/μg protein). The combination of IL-1β with IFN-γ (38.86 ± 6.56 pg/mL/μg protein) produced an increase over basal output of 290.83 ± 52.66-fold, whereas treating with TNF-α and IFN-γ led to a 349.37 ± 121.99-fold increase (46.56 ± 16.02 pg/mL/μg protein). The induction of CX3CL1 protein secretion by combined incubation of FTDCs with IFN-γ plus either IL-1β or TNF-α is 4- to 5-fold greater than the sum of treatment with each cytokine alone.
Figure 4.
Messenger RNA (mRNA) and protein expression in cultured FTDCs is upregulated by TNF-α, IL-1β, and IFN-γ with synergy between IFN-γ and either TNF-α or IL-1β. Leukocyte-free FTDCs were primed in E2 + MPA (EM) for 7 days. Cultures were then switched to defined medium with the steroids and either IL-1β (EMI) or TNF-α (EMT) or IFN-γ (EMIF) or IL-1β+ IFN-γ (EMIIF) or TNF-α + IFN-γ (EMTIF) for 6 hours for mRNA or 24 hours for protein analysis. A, The mRNA levels for CX3CL1 were measured by real-time qRT-PCR and normalized to β-actin. B, Protein levels of CX3CL1were measured by ELISA in conditioned medium supernatants and normalized to total cell protein. The results are reported as mean ± standard error of the mean. n = 6-11; *P < .05; **P < .01; ***P < .005. E indicates estradiol; ELISA, enzyme-linked immunosorbent assay; FTDCs, first trimester decidual cells; IFN-γ, interferon-gamma; IL-1β, interleukin-1 beta; MPA, medroxyprogesterone acetate; mRNA, messenger RNA; qRT-PCR, quantitative reverse transcription-polymerase chain reaction; TNF-α, tumor necrosis factor-alpha.
Signaling Pathways Mediating CX3CL1 Production Induced by Pro-Inflammatory Cytokines in FTDCs
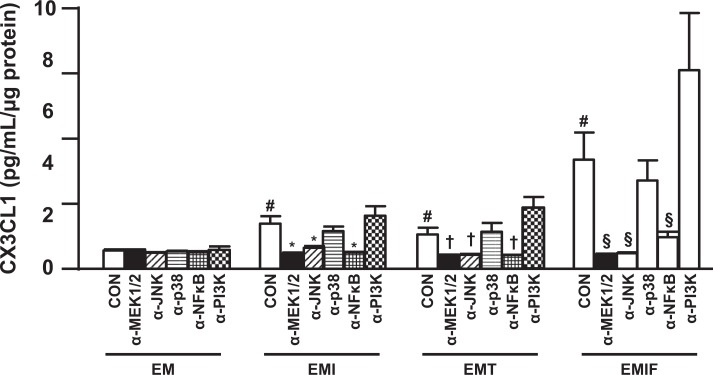
The signaling pathways mediating IL-1β-, TNF-α-, and IFN-γ-induced CX3CL1 production were examined by treating the cultures with various kinase inhibitors. C-X3-C motif ligand 1 production (Figure 5) induced by IL-1β was significantly suppressed by the inhibitors of MEK1/2 (PD184352), JNK (SP600125), and NFκB (BAY11-7082) by 2.57 ± 0.36, 1.93 ± 0.21, 2.74 ± 0.45 folds, respectively. Tumor necrosis factor-alpha-induced CX3CL1 production was correspondingly downregulated by the inhibitors of MEK1/2, JNK, and NFκB by 2.27 ± 0.42, 2.09 ± 0.34, 2.12 ± 0.36 folds (Figure 5). C-X3-C motif ligand 1 production induced by IFN-γ (Figure 4) was suppressed by MEK1/2 inhibitor, JNK inhibitor, and NFκB inhibitor by 7.45 ± 2.12, 7.04 ± 1.59, 3.52 ± 0.85-fold, respectively. In contrast, the inhibitor of p38 MAPK (SB203580) showed no effect on CX3CL1 production induced by IL-1β, TNF-α, or IF N-γ. Although the PI3K inhibitor (Wortmannin) exhibited no effect on IL-1β-induced CX3CL1 expression, the production of CX3CL1 induced by either TNF-α or IFN-γ was enhanced by 2.12 ± 0.31- and 1.73 ± 0.33-fold, respectively (Figure 5).
Figure 5.
C-X3-C motif ligand 1 (CX3CL1) induction by TNF-α, IL-1β, and IFN-γ is mediated by MEK1/2, JNK, and NFκB signaling pathways. A, interferon-gamma inducible protein 10 (IP-10) and (B) leukocyte-free FTDCs were primed in E2 + MPA (EM) for 7 days. Cultures were then switched to defined medium and preincubated with either an MEK1/2 inhibitor (α-MEK1/2, PD184325) or a JNK inhibitor (α-JNK, SP600125) or a p38 MAPK inhibitor (α-p38, SB203580) or a NFκB inhibitor (α-NFκB, BAY11-7082) or a PI3K inhibitor (α-PI3K, wortmannin) for 1.5 hours before incubation with the steroids and either IL-1β (EMI) or TNF-α (EMT) or IFN-γ (EMIF). The results are reported as mean ± standard error of the mean (SEM). n = 4; mean ± SEM; # P < .05 versus CON of EM treatment, *P < .05 versus CON of EMI treatment, † P < .05 versus CON of EMT treatment, § P < .05 versus CON of EMIF treatment. E indicates estradiol; FTDCs, first trimester decidual cells; JNK, c-JUN N-terminal kinase; MAPK, mitogen-activated protein kinase; MEK1/2, mitogen-activated protein kinase kinase 1 and 2; MPA, medroxyprogesterone acetate; NFκB, nuclear factor kappa B; TNF-α, tumor necrosis factor-alpha; IFN-γ, interferon-gamma; IL-1β, interleukin-1 beta; PI3K, PI3 kinase.
CD56brightCD16− NK Cells Migration Is Enhanced by CX3CL1 in a Concentration-Dependent Manner
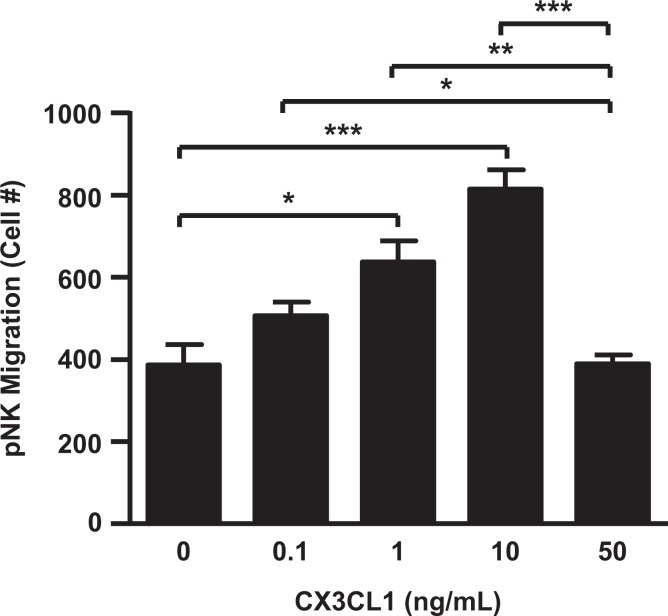
The chemotactic effects of CX3CL1 were also evaluated using CD56brightCD16− NK cells isolated from peripheral blood of healthy reproductive age female donors. Figure 6 demonstrated that CX3CL1 enhanced CD56brightCD16− NK cell migration in a concentration-dependent manner with this NK cell-recruiting effect lost at 50 ng/mL of CX3CL1.
Figure 6.
CD56brightCD16− NK cells are recruited by C-X3-C motif ligand 1 (CX3CL1) in a concentration-dependent manner. CD56brightCD16− NK cells isolated from peripheral blood of healthy reproductive-age female donors. Chemotaxis assays were carried out in the presence of 0, 0.1, 1, 10, or 50 ng/mL of CX3CL1 as a chemoattractant. The results are reported as mean ± standard error of the mean (SEM). n = 3; *P < .05; **P < .01; ***P < .005.
Discussion
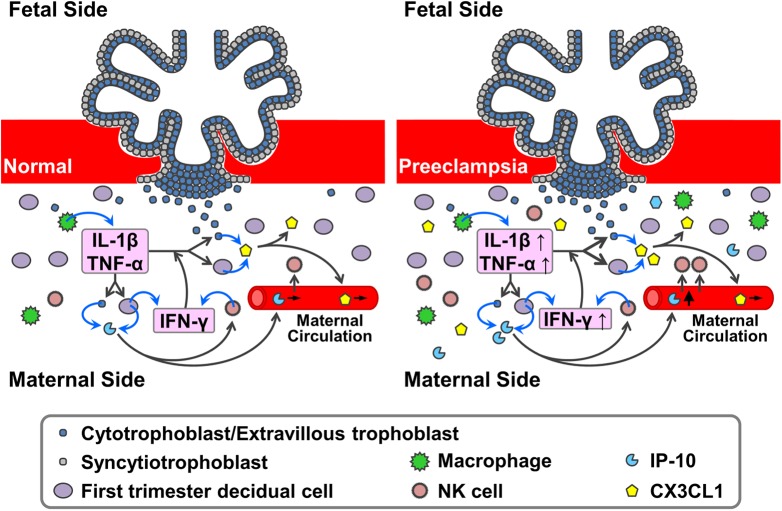
Despite intense investigation, key steps that regulate the pathogenesis of preeclampsia have not been elucidated. Maintenance of the maternal immune balance in response to the semiallogeneic embryo plays a critical role in placentation, which is characterized by trophoblast invasion and decidual vascular remodeling.2 Our previous results demonstrated the importance of an array of human FTDC-secreted NK cell- and monocyte-recruiting and activating chemokines during the development of preeclampsia.8,30 Seeking to complement these previously identified chemokines, the current study scrutinized the role of NK cell-recruiting/activating CX3CL1 in the development of preeclampsia. Thus, the positive immunostaining observed in both the extracellular space and the cell membranes of FTDCs and EVTs indicates the presence of CX3CL1 in the first-trimester decidua and villi in both membranous and secreted forms (Figure 7). Although FTDCs and EVTs have been suggested as the main sources of CX3CL1 in the implantation site, expression of CX3CL1 in other cell types, such as endothelial cells and immune cells, is both expected and deserves investigation.32-34 Complementing a previous report demonstrating increased CX3CL1 expression in syncytiotrophoblasts in villi from patients with severe early-onset preeclampsia,35 immunofluorescent staining of decidua in the current study reveals that CX3CL1 levels on FTDCs and secreted CX3CL1 levels are also increased in patients with preeclampsia, compared to gestational age-matched controls (Figure 7). These results were confirmed by tissue ELISA. However, no significant changes in term serum CX3CL1 levels indicate that the elevation of CX3CL1 expression is a change limited in the decidua during the development of preeclampsia. Moreover, CX3CL1 levels in first-trimester sera did not differ between patients who subsequently developed preeclampsia and normal controls, indicating that unlike elevated first-trimester serum IP-10 levels (Figure 7),8 changes in early serum CX3CL1 levels do not serve as an early predictor for preeclampsia.
Figure 7.
The roles of NK cell-recruiting chemokines in preeclampsia. During early pregnancy, the NK-cell-recruiting and activating chemokines, CX3CL1 and interferon-gamma inducible protein 10 (IP-10), are secreted by first trimester decidual cells and extravillous trophoblasts into decidua and maternal circulation in response to mild inflammation induced by implanting semiallogeneic embryo. In the presence of excessive pro-inflammatory stimuli, the production of CX3CL1 and IP-10 is increased. Thus, the tissue levels of these chemokines are presumably elevated in the early pregnancy in women eventually developing preeclampsia. However, only the levels of IP-10, but CX3CL1, are found to be significantly elevated in the first trimester maternal circulation of women eventually developing preeclampsia. These results indicate that only IP-10 can be used as a potential predictor of later development of preeclampsia. CX3CL1 indicates C-X3-C motif ligand 1; NK, natural killer.
Although various cell types in early human decidua express CX3CL1, FTDCs account for the dominant proportion of total CX3CL1 production. Moreover, CX3CL1 is documented to be both a potent chemoattractant for and activator of NK cells,14 which are a main source of IFN-γ. Elevated expression of IFN-γ together with primary macrophage-derived TNF-α and IL-1β7 have all been implicated in promoting the pathogenesis of preeclampsia.25-27 Therefore, the current study examined the regulation of CX3CL1 expression in response to either TNF-α or IL-1β and/or IFN-γ in primary leukocyte-free FTDCs. The results showed for the first time that TNF-α, IL-1β, and IFN-γ signal through MEK1/2, JNK, and NFκB pathways to induce CX3CL1 production in FTDCs. The current results also provide the first evidence that PI3K inhibits the production of CX3CL1 induced by either IL-1β or TNF-α or IFN-γ. Previously, our laboratory demonstrated that IL-1β and TNF-α each signal through MEK, JNK, p38 kinase, and NFκB pathways in different combinations to upregulate CCL2, CCL4, CCL5, CCL7, CCL20, and CXCL8 in FTDCs. Among these pathways, only NFκB signaling consistently mediates the production of all chemokines in IL-1β- or TNF-α-stimulated FTDCs.36 Moreover, the signaling pathways utilized by both IL-1β and TNF-α are shown to be cell type-specific.37-39 Although IL-1β and NFκB are each established as important upstream40 and downstream41 effectors of the PI3K pathway, respectively, the current study revealed that PI3K inhibitor cotreatment did not attenuate, but unexpectedly increased IL-1β or TNF-α- or IFN-γ-induced CX3CL1 production. These results indicate that in FTDCs, NFκB activation is independent of PI3K and IL-1β and may signal indirectly through PI3K in regulating CX3CL1 expression.
Furthermore, synergy between TNF-α and IFN-γ in upregulating the expression of CX3CL128 and other cytokines29 in various cell types has been documented. For the first time, the current study demonstrates a profound parallel synergistic augmentation of CX3CL1 transcription and translation by IFN-γ and either TNF-α or IL-1β in FTDCs. These results implicate the existence of crosstalk between 2 major decidual immune cell types, dNK cells, and macrophages via paracrine and/or autocrine effects. Since FTDCs are also a major source of IFN-γ,31 these findings suggest that FTDCs not only mediate but also participate in this crosstalk (Figure 7).
In human decidua, CD56brightCD16− dNK cells can be differentiated by decidual cell-derived transforming growth factor-β from CD56dimCD16+ NK cells recruited from peripheral blood.42,43 Nevertheless, upregulation of L-selectin, which mediates interactions with vascular endothelium, indicates that preferential recruitment of the minority circulating CD56brightCD16− NK cells serves as the major source of the dNK cell population.44 In the current study, the results of an NK cell chemotaxis assay verified that CD56bright CD16− NK cells isolated from peripheral blood are chemoattracted by CX3CL1 in a concentration-dependent manner. The soluble form of CX3CL1 responsible for NK cell recruitment is derived from a membranous form cleaved by ADAM10 and ADAM17. Thus, investigations of the regulation of ADAM10 and ADAM17 production in FTDCs and other adjacent cells, such as EVTs and immune cells, are expected to provide further insight in understanding the mechanisms responsible for increased CX3CL1 expression by FTDCs.
In summary, the current study shows that CX3CL1 is expressed in first-trimester decidual cells and cytotrophoblasts. Its expression in term decidual cells is increased in patients with preeclampsia. Nonetheless, CX3CL1 expression in both first trimester and term sera is unchanged. To mimic the proinflammatory conditions in preeclampsia, IL-1β, TNF-α, and IFN-γ signal through MEK1/2, JNK, and NFκB pathways to upregulate CX3CL1 expression in primary FTDCs with synergy between IFN-γ and either IL-1β or TNF-α. C-X3-C motif ligand 1 induces CD56brightCD16− NK cell recruitment in a concentration-dependent manner. Taken together, these results suggest that CX3CL1 play an important role in recruiting CD56brightCD16− NK cells in response to proinflammatory preeclamptic conditions in decidua and however cannot be used as a serological predictor of preeclampsia (Figure 7).
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by USF Foundation E-Da Hospital Research Fund 250333 as well as grants 5R01HD056123 from NICHD, NIH (S.J.H.), EDAHP106035 (SJH), EDAHP106064 (SJH), and EDAHP106006 (CYH).
References
- 1. Trundley A, Moffett A. Human uterine leukocytes and pregnancy. Tissue Antigens. 2004;63(1):1–12. [DOI] [PubMed] [Google Scholar]
- 2. Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod. 2003;69(1):1–7. [DOI] [PubMed] [Google Scholar]
- 3. Pijnenborg R, Vercruysse L, Brosens I. Deep placentation. Best Pract Res Clin Obstet Gynaecol. 2011;25(3):273–285. [DOI] [PubMed] [Google Scholar]
- 4. Young BC, Levine RJ, Karumanchi SA. Pathogenesis of preeclampsia. Annu Rev Pathol. 2010;5:173–192. [DOI] [PubMed] [Google Scholar]
- 5. Velicky P, Knofler M, Pollheimer J. Function and control of human invasive trophoblast subtypes: intrinsic vs. maternal control. Cell Adh Migr. 2016;10(1-2):154–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paolini R, Bernardini G, Molfetta R, Santoni A. NK cells and interferons. Cytokine Growth Factor Rev. 2015;26(2):113–120. [DOI] [PubMed] [Google Scholar]
- 7. Arango Duque G, Descoteaux A. Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol. 2014;5:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lockwood CJ, Huang SJ, Chen CP, et al. Decidual cell regulation of natural killer cell-recruiting chemokines: implications for the pathogenesis and prediction of preeclampsia. Am J Pathol. 2013;183(3):841–856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bazan JF, Bacon KB, Hardiman G, et al. A new class of membrane-bound chemokine with a CX3C motif. Nature. 1997;385(6617):640–644. [DOI] [PubMed] [Google Scholar]
- 10. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity. 2012;36(5):705–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Garton KJ, Gough PJ, Blobel CP, et al. Tumor necrosis factor-alpha-converting enzyme (ADAM17) mediates the cleavage and shedding of fractalkine (CX3CL1). J Biol Chem. 2001;276(41):37993–8001. [DOI] [PubMed] [Google Scholar]
- 12. Hundhausen C, Misztela D, Berkhout TA, et al. The disintegrin-like metalloproteinase ADAM10 is involved in constitutive cleavage of CX3CL1 (fractalkine) and regulates CX3CL1-mediated cell-cell adhesion. Blood. 2003;102(4):1186–1195. [DOI] [PubMed] [Google Scholar]
- 13. Hundhausen C, Schulte A, Schulz B, et al. Regulated shedding of transmembrane chemokines by the disintegrin and metalloproteinase 10 facilitates detachment of adherent leukocytes. J Immunol. 2007;178(12):8064–8072. [DOI] [PubMed] [Google Scholar]
- 14. Imai T, Hieshima K, Haskell C, et al. Identification and molecular characterization of fractalkine receptor CX3CR1, which mediates both leukocyte migration and adhesion. Cell. 1997;91(4):521–530. [DOI] [PubMed] [Google Scholar]
- 15. Hannan NJ, Jones RL, White CA, Salamonsen LA. The chemokines, CX3CL1, CCL14, and CCL4, promote human trophoblast migration at the feto-maternal interface. Biol Reprod. 2006;74(5):896–904. [DOI] [PubMed] [Google Scholar]
- 16. Ashkar AA, Di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192(2):259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faas MM, Spaans F, De Vos P. Monocytes and macrophages in pregnancy and pre-eclampsia. Front Immunol. 2014;5:298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Henderson JT, Thompson JH, Burda BU, Cantor A. Preeclampsia screening: evidence report and systematic review for the US Preventive Services Task Force. JAMA. 2017;317(16):1668–1683. [DOI] [PubMed] [Google Scholar]
- 19. Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: a revised statement from the ISSHP. Pregnancy Hypertens. 2014;4(2):97–104. [DOI] [PubMed] [Google Scholar]
- 20. Caniggia I, Winter J, Lye SJ, Post M. Oxygen and placental development during the first trimester: implications for the pathophysiology of pre-eclampsia. Placenta. 2000;21(suppl A):S25–S30. [DOI] [PubMed] [Google Scholar]
- 21. de Groot CJ, van der Mast BJ, Visser W, De Kuiper P, Weimar W, Van Besouw NM. Preeclampsia is associated with increased cytotoxic T-cell capacity to paternal antigens. Am J Obstet Gynecol. 2010;203(5):496 e1–6. [DOI] [PubMed] [Google Scholar]
- 22. Wu ZM, Yang H, Li M, et al. Pro-inflammatory cytokine-stimulated first trimester decidual cells enhance macrophage-induced apoptosis of extravillous trophoblasts. Placenta. 2012;33(3):188–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fukui A, Yokota M, Funamizu A, et al. Changes of NK cells in preeclampsia. Am J Reprod Immunol. 2012;67(4):278–286. [DOI] [PubMed] [Google Scholar]
- 24. Kervancioglu Demirci E, Salamonsen LA, Gauster M. The role of CX3CL1 in fetal-maternal interaction during human gestation. Cell Adh Migr. 2016;10(1-2):189–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Banerjee S, Smallwood A, Moorhead J, et al. Placental expression of interferon-gamma (IFN-gamma) and its receptor IFN-gamma R2 fail to switch from early hypoxic to late normotensive development in preeclampsia. J Clin Endocrinol Metab. 2005;90(2):944–952. [DOI] [PubMed] [Google Scholar]
- 26. Amash A, Holcberg G, Sapir O, Huleihel M. Placental secretion of interleukin-1 and interleukin-1 receptor antagonist in preeclampsia: effect of magnesium sulfate. J Interferon Cytokine Res. 2012;32(9):432–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wang Y, Walsh SW. TNF alpha concentrations and mRNA expression are increased in preeclamptic placentas. J Reprod Immunol. 1996;32(2):157–169. [DOI] [PubMed] [Google Scholar]
- 28. Yoshida H, Imaizumi T, Fujimoto K, et al. Synergistic stimulation, by tumor necrosis factor-alpha and interferon-gamma, of fractalkine expression in human astrocytes. Neurosci Lett. 2001;303(2):132–136. [DOI] [PubMed] [Google Scholar]
- 29. Paludan SR. Synergistic action of pro-inflammatory agents: cellular and molecular aspects. J Leukoc Biol. 2000;67(1):18–25. [DOI] [PubMed] [Google Scholar]
- 30. Huang SJ, Schatz F, Masch R, et al. Regulation of chemokine production in response to pro-inflammatory cytokines in first trimester decidual cells. J Repdrod Immunol. 2006;72(1-2):60–73. [DOI] [PubMed] [Google Scholar]
- 31. Chen CP, Piao L, Chen X, et al. Expression of interferon gamma by decidual cells and natural killer cells at the human implantation site: implications for preeclampsia, spontaneous abortion, and intrauterine growth restriction. Reprod Sci. 2015;22(11):1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Muehlhoefer A, Saubermann LJ, Gu X, et al. Fractalkine is an epithelial and endothelial cell-derived chemoattractant for intraepithelial lymphocytes in the small intestinal mucosa. J Immunol. 2000;164(6):3368–3376. [DOI] [PubMed] [Google Scholar]
- 33. Truman LA, Ford CA, Pasikowska M, et al. CX3CL1/fractalkine is released from apoptotic lymphocytes to stimulate macrophage chemotaxis. Blood. 2008;112(13):5026–5036. [DOI] [PubMed] [Google Scholar]
- 34. Papadopoulos EJ, Sassetti C, Saeki H, et al. Fractalkine, a CX3C chemokine, is expressed by dendritic cells and is up-regulated upon dendritic cell maturation. Eur J Immunol. 1999;29(8):2551–2559. [DOI] [PubMed] [Google Scholar]
- 35. Siwetz M, Dieber-Rotheneder M, Cervar-Zivkovic M, et al. Placental fractalkine is up-regulated in severe early-onset preeclampsia. Am J Pathol. 2015;185(5):1334–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Li M, Wu ZM, Yang H, Huang SJ. NFκB and JNK/MAPK activation mediates the production of major macrophage- or dendritic cell-recruiting chemokine in human first trimester decidual cells in response to proinflammatory stimuli. J Clin Endocrinol Metab. 2011;96(8):2502–2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Cahill CM, Rogers JT. Interleukin (IL) 1beta induction of IL-6 is mediated by a novel phosphatidylinositol 3-kinase-dependent AKT/IkappaB kinase alpha pathway targeting activator protein-1. J Biol Chem. 2008;283(38):25900–25912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kim MO, Suh HS, Brosnan CF, Lee SC. Regulation of RANTES/CCL5 expression in human astrocytes by interleukin-1 and interferon-beta. J Neurochem. 2004;90(2):297–308. [DOI] [PubMed] [Google Scholar]
- 39. Renaud SJ, Sullivan R, Graham CH. Tumour necrosis factor alpha stimulates the production of monocyte chemoattractants by extravillous trophoblast cells via differential activation of MAPK pathways. Placenta. 2009;30(4):313–319. [DOI] [PubMed] [Google Scholar]
- 40. Reddy SA, Huang JH, Liao WS. Phosphatidylinositol 3-kinase in interleukin 1 signaling. Physical interaction with the interleukin 1 receptor and requirement in NFkappaB and AP-1 activation. J Biol Chem. 1997;272(46):29167–29173. [DOI] [PubMed] [Google Scholar]
- 41. Sizemore N, Leung S, Stark GR. Activation of phosphatidylinositol 3-kinase in response to interleukin-1 leads to phosphorylation and activation of the NF-kappaB p65/RelA subunit. Mol Cell Biol. 1999;19(7):4798–4805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Allan DS, Rybalov B, Awong G, et al. TGF-beta affects development and differentiation of human natural killer cell subsets. Eur J Immunol. 2010;40(8):2289–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keskin DB, Allan DS, Rybalov B, et al. TGFbeta promotes conversion of CD16+ peripheral blood NK cells into CD16− NK cells with similarities to decidual NK cells. Proc Natl Acad Sci U S A. 2007;104(9):3378–3383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Yamaguchi T, Kitaya K, Daikoku N, Yasuo T, Fushiki S, Honjo H. Potential selectin L ligands involved in selective recruitment of peripheral blood CD16(-) natural killer cells into human endometrium. Biol Reprod. 2006;74(1):35–40. [DOI] [PubMed] [Google Scholar]