Abstract
Snakebite envenomation is considered a neglected tropical disease, although it also occurs outside the tropics. In this work, we analyzed the literature on Philodryas species in Chile (Philodryas chamissonis, P. simonsii, and P. tachymenoides) from 1834 to 2019, searching for epidemiological, clinical, and molecular aspects of envenomation. Ninety-one percent of the studies found regarded taxonomy, ecology, and natural history, suggesting that snakebites and venom toxins are a neglected issue in Chile. All snakebite cases reported and toxicological studies concerned the species Philodryas chamissonis. Using 185 distributional records from the literature and museum collections for this species, we show for the first time that the reported snakebite cases correlate with human population density, occurring in the Valparaiso and Metropolitan regions in Central Chile. The reduced number of snakebite cases, which were previously considered as having a low incidence in Chile, may be a consequence of under-reported cases, probably due to the inadequate publication or scarce research on this issue. Absence of information about official pharmacological treatment, post-envenoming sequels, clinical management of particular patient groups (e.g., with non-communicable diseases, pregnant women, and the elderly) was also detected. In conclusion, despite having over 185 years of literature on Chilean snakes, knowledge on the envenomation of Philodryas genus remains scarce, seriously affecting adequate medical handling during an ophidic accident. This review highlights the need to develop deep research in this area and urgent improvements to the management of this disease in Chile.
Keywords: snakebite, opisthoglyphous, Philodryas, toxins, colubrid, therapeutics
1. Introduction
Snakebite envenomation is considered a neglected tropical disease (NTD) that also occurs outside of the tropics [1,2] and requires local attention in each country to understand the disease’s epidemiology in order to improve the effectiveness of treatments and to contribute to reduced deaths and disabilities from a medical perspective [3]. The World Health Organization (WHO) has stated that the control and documentation of snakebite envenoming has long been hampered by poor-quality epidemiological data and poor investment in the study of toxicology beyond the ecological aspects of snakes [4,5].
The genus Philodryas (Wagler 1830) is currently composed of 23 rear-fanged snake species widely distributed in South America [6,7,8,9]. Most species inhabit the lowlands of cis-Andean South America, while only four occur along the trans-Andean zones in Chile, Peru, and Ecuador [6,8].
These species are generally considered harmless for humans; however, abundant data on human snakebites with medical significance [10] and their epidemiological implications are available for the cis-Andean species group, especially for P. baroni [11], P. olfersii [12,13,14,15], P. patagoniensis [13,16,17], and P. viridissima [18,19]. The clinical manifestation of envenoming by some Philodryas species, such as local pain, swelling, erythema, ecchymosis, and regional lymphadenopathy, resemble the local symptoms of Bothrops bites [20,21], and P. olfersii and P. patagoniensis venoms exhibit immunological cross-reactivities to anti-Bothrops sp. serum [21]. Moreover, the components of cis-Andean Philodryas venom secretions have been studied using transcriptomic and biochemical approaches [22], identifying at least five classes of toxins involved in the main clinical effects observed in bitten humans, such as snake venom metalloproteases (SVMPs), snake venom serineproteases (SVSP), cysteine-rich secretory proteins (CRISPs), C-type lectin proteins (CLPs), and natriuretic peptides (NPs) [23,24,25,26,27,28]. All this contrasts with the scarce toxicological information of the species of trans-Andean group P. amaru, P. chamissonis, P. simonsii, and P. tachymenoides [29].
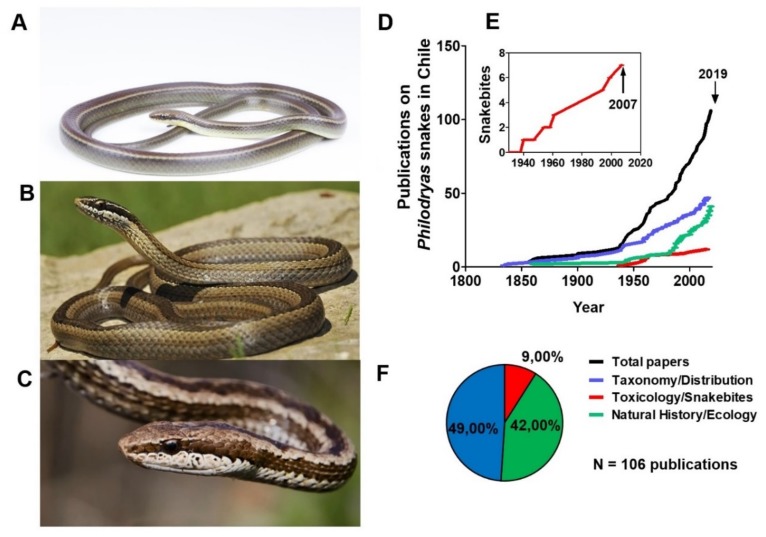
Although evidence concerning snake envenoming caused by Chilean snake species of the genus Philodryas exists from the 1940s, poorly available information on snakebite envenoming situates Chile within countries with insufficient clinical and epidemiological information [30]. Moreover, the apparent low incidence of snakebites and lack of human mortality associated to snake envenoming [31,32] have led to the underestimation of morbidity and non-inclusion in official health programs [33]. Certainly, this knowledge gap highlights the need for identifying the research focus that requires important contributions for new treatments and effective diagnosis as well as molecular and preclinical information. In this work, the literature from 1834 to 2019 on Chilean Philodryas species P. chamissonis, P. simonsii, and P. tachymenoides (Figure 1A–C) [34] was reviewed and analyzed for the first time, revealing that snakebites and venom toxins are neglected issues by researchers in Chile.
Figure 1.
Species of the Philodryas genus recognized for Chile. (A) Philodryas simonsii, referential photography, specimen QCAZR13878, Pontifical Catholic University of Ecuador Collection; (B) Philodryas chamissonis from Maule Region, Chile; (C) Philodryas tachymenoides from Arica and Parinacota Region, Chile. (D) The articles published on snakes in Chile covering taxonomic/geographical distribution (blue line), toxicology/human snakebite reports (red line), and natural history/ecology (green line). Total compiled works: 106. (E) The cumulative total number of reports on ophidism by Philodryas species and the (F) percentage of articles published on Philodryas snakes present in Chile.
2. Results and Discussion
A total of 106 articles about Philodryas species in Chile were reviewed for the period 1834–2019 (185 years, Table S1). As detailed in Figure 1D,E, the period 1938–2007 concentrates all the snakebite case reports on Chilean snakes, showing that the first formal report published on Philodryas snakes was in 1940 [35]. Nine percent of the studies focused on snakebite reports or toxicological aspects of venom; 42% on natural history and ecology; and 49% on the distribution and taxonomy of these species (Figure 1F). The obtained data suggest historic biases in the studies on the Philodryas genus, suggesting snakebites and venom toxins are neglected issues in Chile.
2.1. Epidemiological Aspects
The literature, from 1834 to date, shows that all snakebite cases reported for the Philodryas genus in Chile (seven demonstrable cases in total) belonged to Philodryas chamissonis. For P. simonsii and P. tachymenoides, no reported cases of biting and envenoming in humans were found.
We obtained 185 distributional records from the literature and museum collections for P. chamissonis (Table S2), and we studied if the reported snakebite cases correlated with human population density. As Figure 2 shows, reported snakebite accidents may be related to a greater human contact (high population density) as shown by the greater number of P. chamissonis records, especially in the Valparaíso and Metropolitan regions (Figure 2). We did not rule out the occurrence of unreported snakebite accidents throughout its 25° to 40° south latitude distribution, from Los Ríos to Tarapacá regions in Chile.
Figure 2.
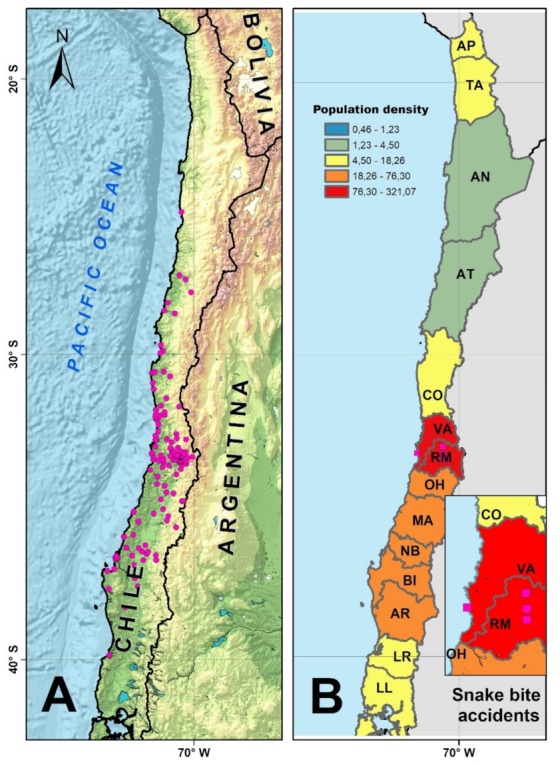
Distribution of Philodryas chamissonis and the location of snakebite reports for Chile. (A) P. chamissonis distribution in Chile obtained from the literature and museum records shown in circles. (B) Human population density, inhabitants per square kilometer, in the different regions of Chile (abbreviated denominations according to ISO 3166-2). Reported cases of snakebite accidents in the Valparaíso and the Metropolitan regions are shown in squares.
All snakebites produced by P. chamissonis were due to the fact of improper animal manipulation or casual snakebites in the upper extremities, especially the fingers. The age of the victims ranged from 11 to 25 years; six cases involved men and one case a woman [31,36,37,38]. All accidents occurred during the summer season, in December and January. Interestingly, similar epidemiological observations have been reported for patients with ophidism by P. patagoniensis and P. olfersii in Brazil [14,17]. Notably, a non-scientific paper mentions a case of human death produced by P. chamissonis envenoming [6]; however, this citation is a wrong translation from an original paper in Spanish published by Gigoux [35], which states: “…una persona mordida”, a bitten person and not a dead person (“…una persona muerta”). Consistent with previous observations [38], our literature analysis did not find evidence of Philodryas envenoming-caused death in Chile.
2.2. Clinical Aspects and Pharmacological Treatment
Front-fanged species of the Crotalus, Bothrops, Lachesis, and Micrurus genera are responsible for the majority of snakebite-related human deaths in South America [32,39,40,41]. Despite this, clinical manifestations of envenoming by Philodryas resemble the symptoms produced by front-fanged species, as Bothrops spp. [21], suggesting an adoption of a more careful evaluation of the victims and its medical treatment [13]. In contrast to other South American countries, there are only rear-fanged snakes of the Pseudalsophis, Tachymenis, and Philodryas genera in Chile. From all they, P. chamissonis snakebite reports with clinical importance have been published describing the evolution of symptoms. At the time of the bite, all patients did not report pain [31,38]. Local lesions were made up of small punctiform wounds produced by snake teeth [42] which, in some cases, remained in the bite site [38]. The reported cases recorded inflammation at the inoculation site in the first 10 to 30 min, accompanied by an intense throbbing pain and heat sensation spreading throughout the affected limb in the following hours [36,38]. Laboratory tests for pediatric and adult patients showed hypoprothrombinemia and prolonged activated partial thromboplastin time (aPTT) with liver, urine, electrocardiogram, plasma electrolyte, and blood glucose tests in normal ranges within the first 24 h after ophidic accident [16,31,36]. The local symptoms produced by cis-Andean Philodryas species, such as P. olfersii, P. patagoniensis, P. viridissima, and P. baroni, are similar to those described for P. chamissonis, i.e., edema, intense pain, bleeding for few minutes, swelling, ecchymosis, and, in some cases, lymphadenopathy; the duration of these effects ranged from 1 to 7 days, with positive clinical results [11,12,14,17,18].
During this time, edema with a stiff appearance induced by P. chamissonis snakebite extended to the shoulder region and chest area with ecchymosis spots and supra-epitrochlear and axillary adenopathies sensitive to palpation [16,31,36]. Some patients exhibited fever, headache, nausea, and a tendency to hypotension. There were no available data about specific or official pharmacological treatment for snakebite patients; instead, empiric treatments consisted of the use of corticosteroids (betamethasone), intravenous antihistamines, and antibiotics (cloxacillin + gentamicin or ampicillin) that reduced edema, headache, nausea and fever, without affecting pain. This treatment caused the regression of the ophidism at 3–4 days. After 7–10 days, ecchymosis and residual inflammation [36] were observed, affecting movement (e.g., writing) with slight pain [38]. Some recommendations of P. chamissonis snakebite have been proposed by Neira et al. [31] such as immobilizing the bite limb, avoiding the use of tourniquets or suction of the wound, washing with warm soapy water immediately, and followed with clinical management for hydration, analgesia, and systemic corticosteroids [31].
2.3. Toxicological Aspects: Pre-Clinical and Molecular Evidence
We found three published reports on experimental toxicity in animals or toxin studies for Philodryas species in Chile, representing the 3% of the analyzed literature which only involved Philodryas chamissonis [29,43,44]. Studies conducted to understanding the effect of the P. tachymenoides (Dromicus tachymenoides) and P. simonsii bites in guinea pigs showed uncertain results [44], because data was not detailed in any publication; therefore, no work in toxicology for both species has been reported (Table 1). The envenoming gland is white with small granulations that connect with the posterior teeth [44]. These are located behind the posterior edge of the eye, are moderately curved, and have a partially closed venom-conducing canal [45]. Extensive details on cranial osteology can be found in Habit et al. [46]. The venom secreted from the envenoming gland has been described as having a milky-white appearance [44]. Although a size-related shift in the dietary habits of neonates and adult P. chamissonis has been reported [47,48], ontogenetic variations in the dental apparatus and venom gland morphology are still unknown.
Table 1.
List of publications that describe clinical and toxicological aspects of venom of Philodryas species in Chile.
Even though the toxicological effects of venom of several Philodryas species have been extensively studied in animal models, such as hematological alterations [4]; edema [49], myotoxicity [50], and neurotoxicity [51], the effects of P. chamissonis venom in vivo have been poorly explored. In Mus musculus mice, the 2.5 mg intraperitoneal inoculation of venom gland extract of P. chamissonis produces dyspnea, decay, ataxia, and hind limb paralysis, producing death at 115 min, but minor doses do not cause death [43,44]. These animals present lesions with hemorrhages at the inoculation site, compromising the abdominal muscles and affecting the small intestine, mesentery, pleural cavity, spleen, and kidneys with hemorrhagic infiltrations [43,44]. Consistent with this, the direct P. chamissonis inoculation in a mouse causes damage in the peritoneum with incipient hemorrhagic sites [29]. In muscle, bites produce hemorrhage with evident edema, accompanied by small coagulopathies in the subcutaneous tissue [29]. This evidence has been related with the presence of toxins with protease, anti-coagulant, and pro-inflammatory action [29].
From studies on venom of cis-Andean Philodryas species, it has been recognized that it presents high proteolytic and hemorrhagic activities but lack of esterase [52], nucleotidase/DNAase [53] and phospholipase A2 activities [21,54] Consistent with this, biochemical characterizations of the venom using extracts from dialyzed and vacuum-dried parotid glands of P. chamissonis suggest a high dose-dependent proteolytic activity in vitro. These extracts complete the gelatin proteolysis in 3 h and lack hemolytic or coagulant effects at 24 h of exposure [43]. In line with this, molecular identification of five toxin-encoding genes [29] which are widely described in snakes of the families Elapidae and Viperidae [22,55,56] have been reported: snake venom metalloprotease type-III (SVMP), snake venom serine protease (SVSP), cysteine-rich secretory protein (CRISP), C-type lectin-like protein (α and β CLP), and natriuretic peptide (NP) [29].
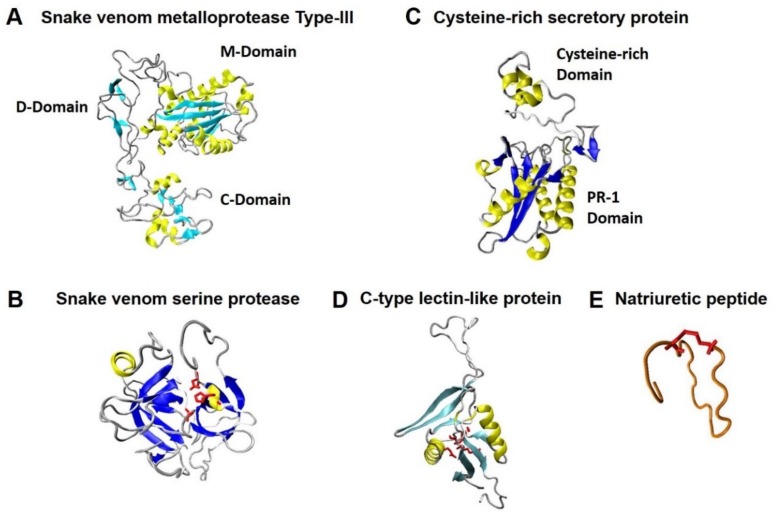
The SVMP-Pch (Figure 3A) is a predicted single polypeptide of 615 amino acids that exhibits a disintegrin-like domain with an aspartic–cysteine–aspartic (DCD) motif and a cysteine-rich domain similar to the sequences reported for P. olfersii [24] which may have α-fibrinogenolytic, hemorrhagic, and pro-inflammatory activities as patagonfibrase, a SVMP isolated from P. patagoniensis venom [26,58]. In addition, SVSP-Pch (Figure 3B) is a putative peptide of 261 amino acids that includes a signal peptide, an activation peptide sequence, and a native serine protease of 226 amino acids with an extension of cysteine residue-containing C-terminal region. In Figure 3B, conserved residues H74, D119, and S213 that compose the catalytic site are shown in red. The SVSP-Pch exhibits three putative N-glycosylation sites (112Asn-Cys-Thr114, 126Asn-Ser-Ser128, and 130Asn-Asn-Ser132) that may contribute to the enzyme stability and its macromolecular selectivity, similar to other SVSPs [59,60]. On the other hand, the non-enzymatic CRISP toxins from front-fanged snakes induces depolarization-induced concentration of arterial smooth muscle of rat tail by blocking cyclic nucleotide-gated channels, voltage-gated Ca2+ channels, voltage-gated K+ channels, and Ca2+-activated K+ channels activities [61,62,63]. The molecular characterization of CRISP-Pch (Figure 3C) reveals that this toxin lacks all the putative binding sites and domains required for the inhibition of ionic channels, suggesting that CRISP-Pch may have different biological effects than those produced by CRISP from front-fanged snakes [29]. Consistent with this observation, the predicted amino acid sequence for CRISP-Pch has matched with fragments of the N-terminal and PR-1 domain of patagonin, a CRISP toxin isolated from P. patagoniensis venom with unusual myotoxic activity [28].
Figure 3.
Predicted 3D structures for P. chamissonis toxins. (A,B) Putative toxins with enzymatic activities and (C–E) putative catalytic activity-lacking toxins with ligand properties. Representations from Urra et al. [29].
The C-type lectin-like proteins are known from front-fanged snake venoms, affecting the blood coagulation and the platelet aggregation [64,65]. C-type lectin-like protein-Pch is a putative α/β heterodimeric CLP lacking calcium-binding sites, of which its biological effect remains unknown [29]. Figure 3D details the amino acidic residues of the β-subunit (Ser66, Leu68, Glu72, and Lys159) that do not form the Ca2+-binding site. Natriuretic peptides are present in the venom of Viperidae and Elapidae species and have potent hypotensive effects [66]. The predicted peptide NP-Pch exhibits the common ring of the vasoactive natriuretic peptides constituted by 17 amino acids (CFGX12GC motif), bridged by an intra-molecular disulfide bond by Cys-265 and Cys-281 [29] (Figure 3E, disulfide bond is shown in red) which is required to interact with membrane-bound receptors with guanylyl cyclase activity [67].
In addition, taking into account that the toxins responsible for causing inflammation and initial hemorrhage, such as SVMP type-III and SVSP described for P. chamissonis [29], are evolutionarily conserved, and being present in other species of the genus, such as P. patagoniensis [26,27,58], P. olfersii [24,51,68], P. baroni [69], it is possible that the venom of the remaining trans-Andean species of Philodryas also have these toxins and the same activities.
2.4. Systemic Symptoms and Dry Bites
In Philodryas snakes, systemic envenoming is infrequent; however, there exists a case of 2 year old child bitten in the finger by P. olfersii that presented the common local effects (local pain, ecchymosis, transient bleeding) with abdominal pain and vomiting [14]. Interestingly, Peichoto et al. [70] reported the local effects of a bite by P. olfersii latirostris as a localized and burning pain and minimal bleeding in the elbow of the arm that rapidly disappeared. A few days after the bite, the victim presented labyrinthine syndrome, including rotator dizziness, nausea, and vomiting. Probably, these effects were related with the snakebite [70]. In addition, de Medeiros et al. [17] reported mild dizziness as a unique systemic manifestation found among 297 studied cases of bite by P. patagoniensis. For Chile, we found the case of a 25 year old man that was bitten in the finger by P. chamissonis and had intense pain, edema, vertigo, headache, fever, and debility [36]. Although dizziness or headache are considered a systemic effect, it may also be manifested in patients with anxiety or hyperventilation.
On the other hand, the presence of lymphadenopathy in the affected limb suggests that venom absorption can occur through the lymphatic system, but blood levels of the venom should be minimal to pose a danger to the victim [71]. Frequently, venom is not inoculated by Philodryas species, because the opistogliph condition gives restricted access to the bite site, an event known as dry bite [72]. In Chile, we did not find formal reports concerning dry bites by Philodryas species.
2.5. Misidentification of Species and Wrong Diagnosis
Identification of offending species and objective description of symptoms are incomplete or confusing in most of the Chilean analyzed literature. Chilean clinical reports generally do not identify the offending species, leaving unclear whether it was involved a Philodryas or Tachymenis specimens (e.g., [73]). For an unidentified snake species involved in a clinical case described by Rayo [73], the specimen was later assigned to Tachymenis peruviana based on its total length (300 mm) by Neira et al. [31]; however, it is currently recognized that the size of Chilean snakes is not a correct criterion for taxonomic identification [48].
2.6. Consequences Due to the Snakebite by Philodryas Species: Sequels and Secondary Infections
Reports on the medical consequences or complications of bites by rear-fanged snakes are poor. Favorable sintomatological evolution in Philodryas species have been described without important consequences for the health of the victim, producing mild, such as for P. viridissima [18], to moderately severe effects such as for P. olfersii and P. patagoniensis [14,17]. For some snakebite cases produced by these two latter species, hands and arms remained with edema, pain, and partial loss of their movements for a prolonged time (15 days) and then recovered their normal appearance [13]. For a snakebite case by P. chamissonis, 10 days after, a discrete increase in the volume of the hand and residual ecchymosis in the arm were observed [37]. We did not find evidence of organic or functional consequences, including allergic reactions, produced for the P. chamisonis envenoming.
In certain cases, the snakebites produced abscesses containing aerobic and anaerobic bacteria and its multiplication can be favored by venom-inducing edema [74,75]. For Philodryas species, secondary infections were confirmed for three of 297 bite cases (1.0%) produced by P. patagoniensis [17] and one case reported about the edema produced by P. olfersii bite progressed to a wound with exudates which was treated with an anti-tetanus vaccine and daily medical control to prevent possible gangrene [13]. Although no infectious complications have been described [31], 43% of Chilean cases were correctly assigned to P. chamissonis snakebites and were prophylactically treated with antibiotics (e.g., cloxaciline, gentamicine, and penicillin) [36,37].
3. Conclusions
Over 104 years, descriptions of Chilean Philodryas snakes have been published without analyzing venom properties, and mention of snake accidents only began to occur in 1938 [73,76] and did not include further details on the species. Our analysis of 185 years of literature on Chilean Philodryas species revealed that studies regarding taxonomy, ecology, and natural history represent 90% of the literature, positioning snakebites and venom toxins as neglected issues in Chile.
Within the trans-Andean group of Philodryas genus, P. chamissonis is the only species widely distributed in high population density areas, such as the central-southern zone of Chile, inhabiting sites where the species may be in contact with human activity, increasing the possibility of snakebites as revealed by our data analysis of 185 locations (Figure 2). The reduced number of snakebite cases recorded in 185 years of literature (equivalent to one report of ophidic accident every 20.5 years) was correlated with a low incidence of snakebite accidents in Chile [31]; however, it may also be a consequence of under-reporting of cases due to the inadequate publication of ophidic accidents or scarce research in this area. Consistent with this idea, it is surprising that all reported cases of ophidic accident by P. chamissonis occurred in the Valparaíso and Metropolitan regions which represent a small portion of the distributional range of this species. A greater effort is required in the other areas of Chile to study this species.
Several aspects of ophidism by Chilean species of the genus Philodryas remain unknown. The toxicological effects of venom and the clinical implications of a potential bite in humans by P. simonsii and P. tachymenoides are entirely unknown. Moreover, although different pharmacological options have been used successfully for treatment of snakebites by P. chamissonis, currently there is no official scheme despite proposed recommendations [31]. All ophidian accidents in Chile occurred in young people, mostly men, and the effects of envenoming and its clinical management in infants, pregnant women, and the elderly are currently unknown. It is also unknown if its presence in patients with non-communicable disease which are of high prevalence in Chile, such as cardiovascular diseases, could contribute to ophidism progression and its treatment. Finally, this review highlights the need to develop deep research in the toxicological aspects of snakebites by Philodryas species and urgent improvements to the management of this neglected disease in Chile.
4. Materials and Methods
4.1. Recollection of Literature
A search of published articles between 1834 and 2019 on Web of Science, Scopus, and Google Scholar for the three species of the genus Philodryas in Chile was conducted using the keywords Chile* AND reptiles OR snakes OR serpientes OR culebra OR ophidism OR snakebite and the scientific names of the studied species (P. chamissonis, P. simonsii, and P. tachymenoides). All works published in peer-reviewed journals were included, and unpublished theses, seminars, and books were excluded. Works not available on digital platforms were obtained in the libraries of the University of Buenos Aires, Faculty of Veterinary Sciences (Buenos Aires, Argentina); Academia Colombiana de Ciencias Exactas, Físicas y Naturales (Bogotá, Colombia), Library of the Faculty of Medicine; University of Chile (Santiago, Chile), Library of Lucas Sierra Foundation (Viña del Mar, Chile); Library of the Medical Society of Santiago (Santiago, Chile), Michigan State University Archives and Historical Collections (East Lansing, MI, USA).
4.2. Data of Geographic Distribution and Location of Snakebite Cases
The locations of P. chamissonis were obtained from the literature [8,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99] and examination of specimens from herpetological collections: Museo Nacional de Historia Natural (MNHN; Santiago, Chile) and the Museo Regional de Concepción (MRC; Concepción, Chile). The locations of snakebites were obtained from the literature. Maps were done using ARCGIS 10.7 software. Population density estimations (inhabitants per km2) were conducted using 2017 census results obtained from the Instituto Nacional de Estadísticas, Chile (INE).
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6651/11/12/697/s1, Table S1: Examined specimens, Table S2: list of literature analyzed.
Author Contributions
F.A.U. designed the study, F.A.U. and A.B.M.-C. collected and analyzed the data from the literature and herpetological collections, F.A.U. and R.A.-M. wrote the manuscript.
Funding
This work was supported by FONDECYT grant #1180069 (RAM), Programa de Investigación Asociativa en Cáncer Gástrico (PIA-CG, RU2107; RAM) and FONDECYT postdoctoral fellowship #3170813 (FAU), CONICYT PCI-Biotechnology #Redbio0027 (FAU, RAM). The authors thank Omar Torres-Carvajal (Ecuador), Baudillo Rebollo-Fernandez (Spain), and Jorge Fuentes (Chile) for the photographs of specimens of P. simonsii, P. chamissonis, and P. tachymenoides, respectively.
Conflicts of Interest
The authors declare no conflict of interest.
Key Contribution
This is the first comprehensive analysis of toxicological aspect of venom and snakebite reports of Philodryas species in Chile.
References
- 1.Chippaux J.P. Snakebite envenomation turns again into a neglected tropical disease! J. Venom Anim. Toxins. Incl. Trop. Dis. 2017;23:38. doi: 10.1186/s40409-017-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Harrison R.A., Hargreaves A., Wagstaff S.C., Faragher B., Lalloo D.G. Snake envenoming: A disease of poverty. PLoS. Negl. Trop. Dis. 2009;3:e569. doi: 10.1371/journal.pntd.0000569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Williams D.J., Faiz M.A., Abela-Ridder B., Ainsworth S., Bulfone T.C., Nickerson A.D., Habib A.G., Junghanss T., Fan H.W., Turner M., et al. Strategy for a globally coordinated response to a priority neglected tropical disease: Snakebite envenoming. PLOS Negl. Trop. Dis. 2019;13:e0007059. doi: 10.1371/journal.pntd.0007059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Minghui R., Malecela M.N., Cooke E., Abela-Ridder B. WHO’s snakebite envenoming strategy for prevention and control. The Lancet Global Health. 2019;7:e837–e838. doi: 10.1016/S2214-109X(19)30225-6. [DOI] [PubMed] [Google Scholar]
- 5.Williams H.F., Layfield H.J., Vallance T., Patel K., Bicknell A.B., Trim S.A., Vaiyapuri S. The Urgent Need to Develop Novel Strategies for the Diagnosis and Treatment of Snakebites. Toxins (Basel) 2019;11:363. doi: 10.3390/toxins11060363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomas R.A. Doctoral Dissertation. Texas A & M University; College Station, TX, USA: 1976. A revision of the South American colubrid snake genus Philodryas Wagler, 1830. [Google Scholar]
- 7.Grazziotin F.G., Zaher H., Murphy R.W., Scrocchi G., Benavides M.A., Zhang Y.-P., Bonatto S.L. Molecular phylogeny of the New World Dipsadidae (Serpentes: Colubroidea): A reappraisal. Cladistics. 2012;28:437–459. doi: 10.1111/j.1096-0031.2012.00393.x. [DOI] [PubMed] [Google Scholar]
- 8.Zaher H., Arredondo J., Valencia J., Arbeláez E., Rodrigues M., Altamirano-Benavides M. A new Andean species of Philodryas (Dipsadidae, Xenodontinae) from Ecuador. Zootaxa. 2014;3785:469–480. doi: 10.11646/zootaxa.3785.3.8. [DOI] [PubMed] [Google Scholar]
- 9.Cacciali P., Cabral H., Ferreira V., Köhler G. Revision of Philodryas mattogrossensis with the revalidation of P. erlandi (Reptilia: Squamata: Dipsadidae) Salamandra. 2016;52:293–305. [Google Scholar]
- 10.Weinstein S.A., White J., Keyler D.E., Warrell D.A. Non-front-fanged colubroid snakes: a current evidence-based analysis of medical significance. Toxicon. 2013;69:103–113. doi: 10.1016/j.toxicon.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 11.Kuch U., Jesberger U. Human envenomation from the bite of the South American colubrid snake species Philodryas baroni Berg, 1895. The Snake. 1993;25:63–65. [Google Scholar]
- 12.Silveria P.V., Nishioka Sde A. Non-venomous snake bite and snake bite without envenoming in a Brazilian teaching hospital. Analysis of 91 cases. Rev. Inst. Med. Trop. Sao Paulo. 1992;34:499–503. doi: 10.1590/S0036-46651992000600002. [DOI] [PubMed] [Google Scholar]
- 13.de Araujo M.E., dos Santos A.C. Cases of human envenoming caused by Philodryas olfersii and Philodryas patagoniensis (Serpentes: Colubridae) Rev. Soc. Bras. Med. Trop. 1997;30:517–519. doi: 10.1590/S0037-86821997000600013. [DOI] [PubMed] [Google Scholar]
- 14.Ribeiro L.A., Puorto G., Jorge M.T. Bites by the colubrid snake Philodryas olfersii: A clinical and epidemiological study of 43 cases. Toxicon. 1999;37:943–948. doi: 10.1016/S0041-0101(98)00191-3. [DOI] [PubMed] [Google Scholar]
- 15.Correia J.M., Santana Neto Pde L., Pinho M.S., Silva J.A., Amorim M.L., Escobar J.A. Poisoning due to Philodryas olfersii (Lichtenstein, 1823) attended at Restauracao Hospital in Recife, State of Pernambuco, Brazil:Case report. Rev. Soc. Bras. Med. Trop. 2010;43:336–338. doi: 10.1590/S0037-86822010000300025. [DOI] [PubMed] [Google Scholar]
- 16.Nishioka S.A., Silveira P.V. Philodryas patagoniensis bite and local envenoming. Rev. Inst. Med. Trop. Sao Paulo. 1994;36:279–281. doi: 10.1590/S0036-46651994000300013. [DOI] [PubMed] [Google Scholar]
- 17.de Medeiros C.R., Hess P.L., Nicoleti A.F., Sueiro L.R., Duarte M.R., de Almeida-Santos S.M., Franca F.O. Bites by the colubrid snake Philodryas patagoniensis: A clinical and epidemiological study of 297 cases. Toxicon. 2010;56:1018–1024. doi: 10.1016/j.toxicon.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 18.Means D.B. Ophidism by the green palmsnake. Wilderness. Environ. Med. 2010;21:46–49. doi: 10.1016/j.wem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- 19.Silva A.M.D., Mendes V., Monteiro W.M., Bernarde P.S. Non-venomous snakebites in the Western Brazilian Amazon. Rev. Soc. Bras. Med. Trop. 2019;52:e20190120. doi: 10.1590/0037-8682-0120-2019. [DOI] [PubMed] [Google Scholar]
- 20.Acosta O., Leiva L.C., Peichoto M.E., Marunak S., Teibler P., Rey L. Hemorrhagic activity of the Duvernoy’s gland secretion of the xenodontine colubrid Philodryas patagoniensis from the north-east region of Argentina. Toxicon. 2003;41:1007–1012. doi: 10.1016/S0041-0101(03)00074-6. [DOI] [PubMed] [Google Scholar]
- 21.Rocha M.M., Paixao-Cavalcante D., Tambourgi D.V., Furtado Mde F. Duvernoy’s gland secretion of Philodryas olfersii and Philodryas patagoniensis (Colubridae): Neutralization of local and systemic effects by commercial bothropic antivenom (Bothrops genus) Toxicon. 2006;47:95–103. doi: 10.1016/j.toxicon.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 22.Junqueira-de-Azevedo I.L., Campos P.F., Ching A.T., Mackessy S.P. Colubrid venom composition: An -omics perspective. Toxins (Basel) 2016;8:230. doi: 10.3390/toxins8080230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Assakura M.T., Reichl A.P., Mandelbaum F.R. Isolation and characterization of five fibrin(ogen)olytic enzymes from the venom of Philodryas olfersii (green snake) Toxicon. 1994;32:819–831. doi: 10.1016/0041-0101(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 24.Ching A.T., Rocha M.M., Paes Leme A.F., Pimenta D.C., de Fatima D.F.M., Serrano S.M., Ho P.L., Junqueira-de-Azevedo I.L. Some aspects of the venom proteome of the Colubridae snake Philodryas olfersii revealed from a Duvernoy’s (venom) gland transcriptome. FEBS Lett. 2006;580:4417–4422. doi: 10.1016/j.febslet.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 25.Fry B.G., Vidal N., Norman J.A., Vonk F.J., Scheib H., Ramjan S.F., Kuruppu S., Fung K., Hedges S.B., Richardson M.K., et al. Early evolution of the venom system in lizards and snakes. Nature. 2006;439:584–588. doi: 10.1038/nature04328. [DOI] [PubMed] [Google Scholar]
- 26.Peichoto M.E., Teibler P., Mackessy S.P., Leiva L., Acosta O., Goncalves L.R., Tanaka-Azevedo A.M., Santoro M.L. Purification and characterization of patagonfibrase, a metalloproteinase showing alpha-fibrinogenolytic and hemorrhagic activities, from Philodryas patagoniensis snake venom. Biochim. Biophys. Acta. 2007;1770:810–819. doi: 10.1016/j.bbagen.2006.12.014. [DOI] [PubMed] [Google Scholar]
- 27.Peichoto M.E., Paes Leme A.F., Pauletti B.A., Batista I.C., Mackessy S.P., Acosta O., Santoro M.L. Autolysis at the disintegrin domain of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Biochim. Biophys. Acta. 2010;1804:1937–1942. doi: 10.1016/j.bbapap.2010.06.005. [DOI] [PubMed] [Google Scholar]
- 28.Peichoto M.E., Mackessy S.P., Teibler P., Tavares F.L., Burckhardt P.L., Breno M.C., Acosta O., Santoro M.L. Purification and characterization of a cysteine-rich secretory protein from Philodryas patagoniensis snake venom. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2009;150:79–84. doi: 10.1016/j.cbpc.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 29.Urra F., Pulgar R., Gutiérrez R., Hodar C., Cambiazo V., Labra A. Identification and molecular characterization of five putative toxins from the venom gland of the snake Philodryas chamissonis (Serpentes: Dipsadidae) Toxicon. 2015;108:19–31. doi: 10.1016/j.toxicon.2015.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Kasturiratne A., Wickremasinghe A.R., de Silva N., Gunawardena N.K., Pathmeswaran A., Premaratna R., Savioli L., Lalloo D.G., de Silva H.J. The global burden of snakebite: a literature analysis and modelling based on regional estimates of envenoming and deaths. PLoS Med. 2008;5:e218. doi: 10.1371/journal.pmed.0050218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Neira O.P., Jofre M.L., Oschilewski L.D., Subercaseaux S.B., Munoz S.N. Snake bite by Philodryas chamissonis. A case presentation and literature review. Rev. Chilena. Infectol. 2007;24:236–241. doi: 10.4067/S0716-10182007000300012. [DOI] [PubMed] [Google Scholar]
- 32.Chippaux J.P. Incidence and mortality due to snakebite in the Americas. PLoS. Negl. Trop. Dis. 2017;11:e0005662. doi: 10.1371/journal.pntd.0005662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Longbottom J., Shearer F.M., Devine M., Alcoba G., Chappuis F., Weiss D.J., Ray S.E., Ray N., Warrell D.A., Ruiz de Castaneda R., et al. Vulnerability to snakebite envenoming: A global mapping of hotspots. Lancet. 2018;392:673–684. doi: 10.1016/S0140-6736(18)31224-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruiz De Gamboa M. Lista actualizada de los reptiles de Chile. Boletín Chileno de Herpetología. 2016;3:7–12. [Google Scholar]
- 35.Gigoux E.R. Los ofidios chilenos. Bol. Museo. Nacional. Hist. Natural. (Chile) 1940;18:5. [Google Scholar]
- 36.Schenone H., Bertín V., Mann G. Un nuevo caso de ofidismo. Bol. Chil. Parasitol. 1954;9:88–89. [PubMed] [Google Scholar]
- 37.Arzola J., Schenone H. Dos casos nuevos de ofidismo en Chile. Bol. Chil. Parasitol. 1994;49:69–70. [PubMed] [Google Scholar]
- 38.Kuch U. Notes on two cases of human envenomation by the South American colubrid snakes Philodryas olfersi latirostris COPE, 1862 and Philodryas chamissonis (WIEGMANN, 1834) Herpetozoa. 1999;12:11–16. [Google Scholar]
- 39.Chippaux J.P., Postigo J.R. Appraisal of snakebite incidence and mortality in Bolivia. Toxicon. 2014;84:28–35. doi: 10.1016/j.toxicon.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Andrade F., Chippaux J.P. Snake bite envenomation in Ecuador. Trans. R. Soc. Trop. Med. Hyg. 2010;104:588–591. doi: 10.1016/j.trstmh.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 41.Otero-Patino R. Epidemiological, clinical and therapeutic aspects of Bothrops asper bites. Toxicon. 2009;54:998–1011. doi: 10.1016/j.toxicon.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 42.Schenone H., Reyes H. Animales ponzoñosos de Chile. Bol. Chil. Parasitol. 1965;20:104–109. [PubMed] [Google Scholar]
- 43.Donoso-Barros R., Cardenas S. Estudio del veneno de Dromicus chamissonis (Wiegmann) Inv. Zool. Chil. 1959;5:93–95. [Google Scholar]
- 44.Donoso-Barros R., Cardenas S. El veneno de las culebras chilenas. Not. Mens. Mus. Hist. Nat. 1962;74:2–4. [Google Scholar]
- 45.Donoso-Barros R. Reptiles de Chile. Univ. Chile; Santiago, Chile: 1966. pp. 458 + cxlvi. [Google Scholar]
- 46.Habit E., Ortiz J., Victoriano P. Osteología craneana de Philodryas chamissonis (Wiegmann, 1834) (Colubridae, Serpentes) Bol. Soc. Biol. Concepción (Chile) 1992;63:83–92. [Google Scholar]
- 47.Cabeza O., Vargas E., Ibarra C., Urra F. Observations on reproduction in captivity of the endemic long-tailed snake Philodryas chamissonis (Wiegmann, 1835) (Reptilia, Squamata, Dipsadidae) from Chile. Herpetozoa. 2019;32 doi: 10.3897/herpetozoa.32.e36705. [DOI] [Google Scholar]
- 48.Greene H., Jaksic F. The feeding behavior and natural history of two Chilean snakes, Philodryas chamissonis and Tachymenis chilensis (Colubridae) Rev. Chil. Historia Nat. 1992;65:485–493. [Google Scholar]
- 49.Lopes P.H., Rocha M.M.T., Kuniyoshi A.K., Portaro F.C.V., Goncalves L.R.C. Edema and nociception induced by Philodryas patagoniensis venom in mice: A pharmacological evaluation with implications for the accident treatment. J. Pharmacol. Exp. Ther. 2017;361:349–354. doi: 10.1124/jpet.116.239640. [DOI] [PubMed] [Google Scholar]
- 50.Peichoto M.E., Acosta O., Leiva L., Teibler P., Marunak S., Ruiz R. Muscle and skin necrotizing and edema-forming activities of Duvernoy’s gland secretion of the xenodontine colubrid snake Philodryas patagoniensis from the north-east of Argentina. Toxicon. 2004;44:589–596. doi: 10.1016/j.toxicon.2004.05.030. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez-Acosta A., Lemoine K., Navarrete L., Giron M.E., Aguilar I. Experimental ophitoxemia produced by the opisthoglyphous lora snake (Philodryas olfersii) venom. Rev. Soc. Bras. Med. Trop. 2006;39:193–197. doi: 10.1590/S0037-86822006000200012. [DOI] [PubMed] [Google Scholar]
- 52.Peichoto M.E., Tavares F.L., Santoro M.L., Mackessy S.P. Venom proteomes of South and North American opisthoglyphous (Colubridae and Dipsadidae) snake species: A preliminary approach to understanding their biological roles. Comp. Biochem. Physiol. Part. D Genom. Proteom. 2012;7:361–369. doi: 10.1016/j.cbd.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 53.Sales P.B., Santoro M.L. Nucleotidase and DNase activities in Brazilian snake venoms. Comp. Biochem. Physiol. C. Toxicol. Pharmacol. 2008;147:85–95. doi: 10.1016/j.cbpc.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 54.Zelanis A., Teixeira da Rocha M.M., de Fatima Domingues Furtado M. Preliminary biochemical characterization of the venoms of five Colubridae species from Brazil. Toxicon. 2010;55:666–669. doi: 10.1016/j.toxicon.2009.09.015. [DOI] [PubMed] [Google Scholar]
- 55.Fry B.G., Vidal N., van der Weerd L., Kochva E., Renjifo C. Evolution and diversification of the Toxicofera reptile venom system. J. Proteomics. 2009;72:127–136. doi: 10.1016/j.jprot.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 56.Brahma R.K., McCleary R.J., Kini R.M., Doley R. Venom gland transcriptomics for identifying, cataloging, and characterizing venom proteins in snakes. Toxicon. 2015;93:1–10. doi: 10.1016/j.toxicon.2014.10.022. [DOI] [PubMed] [Google Scholar]
- 57.Donoso Barros R. Emponzoñamiento por ofidios chilenos. Rev. Pediatr. Clin. Soc. 1961;1:65–79. [Google Scholar]
- 58.Peichoto M.E., Zychar B.C., Tavares F.L., de Camargo Goncalves L.R., Acosta O., Santoro M.L. Inflammatory effects of patagonfibrase, a metalloproteinase from Philodryas patagoniensis (Patagonia Green Racer; Dipsadidae) venom. Exp. Biol. Med. (Maywood) 2011;236:1166–1172. doi: 10.1258/ebm.2011.011125. [DOI] [PubMed] [Google Scholar]
- 59.Siigur E., Tonismagi K., Trummal K., Samel M., Vija H., Aaspollu A., Ronnholm G., Subbi J., Kalkkinen N., Siigur J. A new tyrosine-specific chymotrypsin-like and angiotensin-degrading serine proteinase from Vipera lebetina snake venom. Biochimie. 2011;93:321–330. doi: 10.1016/j.biochi.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 60.He J., Chen S., Gu J. Identification and characterization of Harobin, a novel fibrino(geno)lytic serine protease from a sea snake (Lapemis hardwickii) FEBS Lett. 2007;581:2965–2973. doi: 10.1016/j.febslet.2007.05.047. [DOI] [PubMed] [Google Scholar]
- 61.Yamazaki Y., Morita T. Structure and function of snake venom cysteine-rich secretory proteins. Toxicon. 2004;44:227–231. doi: 10.1016/j.toxicon.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 62.Shikamoto Y., Suto K., Yamazaki Y., Morita T., Mizuno H. Crystal structure of a CRISP family Ca2+ -channel blocker derived from snake venom. J. Mol. Biol. 2005;350:735–743. doi: 10.1016/j.jmb.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 63.Suzuki N., Yamazaki Y., Brown R.L., Fujimoto Z., Morita T., Mizuno H. Structures of pseudechetoxin and pseudecin, two snake-venom cysteine-rich secretory proteins that target cyclic nucleotide-gated ion channels: Implications for movement of the C-terminal cysteine-rich domain. Acta. Crystallogr. D Biol. Crystallogr. 2008;64:1034–1042. doi: 10.1107/S0907444908023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fukuda K., Mizuno H., Atoda H., Morita T. Crystal structure of flavocetin-A, a platelet glycoprotein Ib-binding protein, reveals a novel cyclic tetramer of C-type lectin-like heterodimers. Biochemistry. 2000;39:1915–1923. doi: 10.1021/bi992134z. [DOI] [PubMed] [Google Scholar]
- 65.Atoda H., Kaneko H., Mizuno H., Morita T. Calcium-binding analysis and molecular modeling reveal echis coagulation factor IX/factor X-binding protein has the Ca-binding properties and Ca ion-independent folding of other C-type lectin-like proteins. FEBS Lett. 2002;531:229–234. doi: 10.1016/S0014-5793(02)03507-X. [DOI] [PubMed] [Google Scholar]
- 66.Peterfi O., Boda F., Szabo Z., Ferencz E., Baba L. Hypotensive Snake Venom Components-A Mini-Review. Molecules. 2019;24:2778. doi: 10.3390/molecules24152778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Vink S., Jin A.H., Poth K.J., Head G.A., Alewood P.F. Natriuretic peptide drug leads from snake venom. Toxicon. 2012;59:434–445. doi: 10.1016/j.toxicon.2010.12.001. [DOI] [PubMed] [Google Scholar]
- 68.Acosta de Perez O., Leiva de Vila L., Peichoto M.E., Marunak S., Ruiz R., Teibler P., Gay C., Rey L. Edematogenic and myotoxic activities of the Duvernoy’s gland secretion of Philodryas olfersii from the north-east region of Argentina. Biocell. 2003;27:363–370. [PubMed] [Google Scholar]
- 69.Sanchez M.N., Timoniuk A., Marunak S., Teibler P., Acosta O., Peichoto M.E. Biochemical and biological analysis of Philodryas baroni (Baron’s green racer; Dipsadidae) venom: relevance to the findings of human risk assessment. Hum. Exp. Toxicol. 2014;33:22–31. doi: 10.1177/0960327113493302. [DOI] [PubMed] [Google Scholar]
- 70.Peichoto M.E., Céspedez J.A., Pascual J.A. Report of a bite by the South American colubrid snake Philodryas olfersii latirostris (Squamata: Colubridae) Acta. Herpetologica. 2007;2:11–15. [Google Scholar]
- 71.Prado-Franceschi J., Hyslop S. South American colubrid envenomations. J. Toxicol. Toxin Rev. 2002;21:117–158. doi: 10.1081/TXR-120004744. [DOI] [Google Scholar]
- 72.Naik B.S. “Dry bite” in venomous snakes: A review. Toxicon. 2017;133:63–67. doi: 10.1016/j.toxicon.2017.04.015. [DOI] [PubMed] [Google Scholar]
- 73.Rayo F., Covarrubias R., Ruiz M. Mordedura de serpiente. Bol. Soc. Cirugía. (Rev. Méd. Chile) 1938;66:773–779. [Google Scholar]
- 74.Jorge M.T., Nishioka Sde A., de Oliveira R.B., Ribeiro L.A., Silveira P.V. Aeromonas hydrophila soft-tissue infection as a complication of snake bite: Report of three cases. Ann. Trop. Med. Parasitol. 1998;92:213–217. doi: 10.1080/00034983.1998.11813282. [DOI] [PubMed] [Google Scholar]
- 75.Angel M.F., Zhang F., Jones M., Henderson J., Chapman S.W. Necrotizing fasciitis of the upper extremity resulting from a water moccasin bite. South. Med. J. 2002;95:1090–1094. doi: 10.1097/00007611-200295090-00033. [DOI] [PubMed] [Google Scholar]
- 76.Johow A. Mordedura de culebra. Bol. Soc. Cirugía. (Rev. Méd. Chile) 1938;66:661–663. [Google Scholar]
- 77.Wiegmann A. Amphibien. In: Meyen, F.J.F. (Ed.), Beiträge zur Zoologie, gesammelt auf einer Reise um die Erde. Nova Acta Academiae Caesareae Leopoldino-Carolinae Germinicae Naturae Curiosorum, Halle. 1835;17:183–268. [Google Scholar]
- 78.Quijada B. Herpetolojía. Reptiles Chilenos I Estranjeros Conservados en el Museo Nacional de Historia Natural (I) Boletín del Museo Nacional de Historia Natural, Chile. 1916;9:22–47. [Google Scholar]
- 79.Schmidt K.P., Walker W.F.J. Peruvian Snakes from The University of Arequipa. Zoo. Zer. Field Museum Nat. His. 1943;24:279–296. [Google Scholar]
- 80.Donoso-Barros R. Nuevos reptiles y anfibios de Chile. Boletin. De. La. Sociedad. De. Biología. De. Concepción. 1974;48:217–229. [Google Scholar]
- 81.Jaksic F., Yáñez J., Schlatter R. Prey of the Harris´ Hawk in Central Chile. 1980:196–198. [Google Scholar]
- 82.Jaksic F.M., Delibes M. A comparative analysis of food-niche relationships and trophic guild structure in two assemblages of vertebrate predators differing in species richness: Causes, correlations, and consequences. Oecologia. 1987;71:461–472. doi: 10.1007/BF00378722. [DOI] [PubMed] [Google Scholar]
- 83.Jiménez J., Jaksic F. Historia natural del aguila Geranoaetus melanoleucus: Una revisión. El Hornero. Revista de Ornitología Neotropical. 1990;2:97–110. [Google Scholar]
- 84.Medel R., Marquet P., Fox S., Jaksic F. Depredacion sobre lagartijas en Chile central: Importancia relativa de atributos ecológicos y morfológicos. Revista Chilena de Historia Natural. 1990:261–266. [Google Scholar]
- 85.Nunez H. Geographical data of Chilean lizards and snakes in the Museo Nacional de Historia Natural, Santiago, Chile. Smithsonian Herpetological Information Service. 1992;1:1–29. doi: 10.5479/si.23317515.91.1. [DOI] [Google Scholar]
- 86.Mella J.E. Reptiles en el Monumento Natural El Morado (Region Metropolitana, Chile): Abundancia relativa, distribucion altitudinal y preferencia por rocas de distinto tamaño. Gayana (Concepción) 2007;71:16–26. doi: 10.4067/S0717-65382007000100003. [DOI] [Google Scholar]
- 87.Sallaberry-Pincheira N., Garin C.F., González-Acuña D., Sallaberry M.A., Vianna J.A. Genetic divergence of Chilean long-tailed snake (Philodryas chamissonis) across latitudes: conservation threats for different lineages. Diversity and Distributions. 2011;17:152–162. doi: 10.1111/j.1472-4642.2010.00729.x. [DOI] [Google Scholar]
- 88.Cañas J., Urra F. Philodryas chamissonis. Nocturnal activity. Herpetol. Rev. 2019;50:600. [Google Scholar]
- 89.Reyes-Olivares R., Sepulveda-Luna E., Labra A. Philodryas chamissonis (Chilean Green Racer). Diet. Herpetol. Rev. 2017;48:865–866. [Google Scholar]
- 90.Skewes O., Acuña L., San Martín-Órdenes J. Depredación de polluelos de chercán (Troglodytes aedon) por la culebra de cola larga (Philodryas chamissonis) Boletin Chileno de Ornitología. Unión de Ornitólogos de Chile. 2013;19:30–33. [Google Scholar]
- 91.Muñoz-Leal S., Ardiles K., Figueroa R.A., González-Acuña D. Philodryas chamissonis (Reptilia: Squamata: Colubridae) preys on the arboreal marsupial Dromiciops gliroides (Mammalia: Microbiotheria: Microbiotheriidae) Brazil. J. Biolo. 2013;73:15–17. doi: 10.1590/S1519-69842013000100003. [DOI] [PubMed] [Google Scholar]
- 92.Moreno R., Moreno J., Ortiz J., Victoriano P., Torres-Pérez F. Herpetofauna del Parque Nacional Llanos de Challe (III Región, Chile) Gayana (Concepción) 2002;66:7–10. doi: 10.4067/S0717-65382002000100002. [DOI] [Google Scholar]
- 93.Moreno R., Moreno J., Torres-Pérez F., Ortiz J., Breskovic A. Catálogo Herpetológico del Museo del Mar de la Universidad Arturo Prat de Iquique, Chile. Gayana (Concepción) 2001;65:149–153. doi: 10.4067/S0717-65382001000200006. [DOI] [Google Scholar]
- 94.Philodryas chamissonis (long-tailed snake) and Liolaemus nitidus. Predation determined by pit tag. Herpetol. Rev. 2009;40:358. [Google Scholar]
- 95.Jaksié F.M., Greene H.W., Yáñez J.L. The guild structure of a community of predatory vertebrates in central Chile. Oecologia. 1981;49:21–28. doi: 10.1007/BF00376893. [DOI] [PubMed] [Google Scholar]
- 96.Castro-Pastene C., Carrasco H., Troncoso-Palacios J. Lagartijas y serpientes del Parque Nacional Radal Siete Tazas. Boletín Chileno de Herpetología. 2015;2:12–16. [Google Scholar]
- 97.Escobar M., Vukasonic M. Depredación de Philodryas chamissonis (Serpentes: Colubridae) sobre polluelos de Aphrastura spinicauda (Passeriformes: Furnariidae): ¿Una culebra arborícola? Not. Men. Mus. Hist. Nat. 2003;352:18–20. [Google Scholar]
- 98.Girard C. Abstract of a report to Lieut. James M. Gilliss, U.S.N., upon the reptiles collected during the U.S.N. Astronomical Expedition to Chili. Proc. Proc. Acad. Nat. Sci. Philadelphia. 1955;7:226–227. [Google Scholar]
- 99.Bozinovic F., Rosenmann M. Energetics and food requirements of the female snake Phillodryas chamissonisduring the breeding season. Oecologia. 1988;75:282–284. doi: 10.1007/BF00378610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.