Abstract
Chocolate is well known for its fine flavor, and its history began in ancient times, when the Maya considered chocolate (a cocoa drink prepared with hot water) the “Food of the Gods”. The food industry produces many different types of chocolate: in recent years, dark chocolate, in particular, has gained great popularity. Interest in chocolate has grown, owing to its physiological and potential health effects, such as regulation of blood pressure, insulin levels, vascular functions, oxidation processes, prebiotic effects, glucose homeostasis, and lipid metabolism. However, further translational and epidemiologic studies are needed to confirm available results and to evaluate other possible effects related to the consumption of cocoa and chocolate, verifying in humans the effects hitherto demonstrated only in vitro, and suggesting how best to consume (in terms of dose, mode, and time) chocolate in the daily diet.
Keywords: chocolate, cocoa, Food of the Gods, Theobroma cacao, nitric oxide, cardiovascular effects
1. Background
The history of chocolate began with the Maya, who were probably the first people in South America to cultivate the cocoa plant [1]. For the Maya, chocolate was a cocoa drink prepared with hot water and often flavored with cinnamon and pepper. It was called the “Food of the Gods” and was presented at the table of Emperor Moctezuma II by the Aztecs [1].
In 1502, Christopher Columbus was the first European to encounter cocoa. He captured a canoe that contained cocoa beans, which were considered “mysterious-looking almonds” and identified as a form of currency in Mesoamerica [2,3].
Cocoa appeared in Europe in 1528, when the Spanish conquistador Hernán Cortés brought samples of cocoa to King Charles of Spain, spreading the great effects of the beverage prepared from this “brown gold” [3,4]. It was in 1753 that the Swedish scientist Carl Linnaeus named the cocoa plant Theobroma cacao, from the Latin name Theobroma [literally ‘food of the Gods’], and the Aztec word xocolatl [i.e., xococ (bitter) and atl (water)] [5].
The characteristics of chocolate were long ignored in Europe owing to difficulties with an environment unfavorable to its growth. The natural habitat of the cocoa tree is the lower level of an evergreen rain forest. Cocoa plants respond well to relatively high temperatures (with a maximum annual average of 30–32 °C and minimum average of 18–21 °C) and generally high relative humidity: often as much as 100% during the day, falling to 70–80% at night [6]. According to the latest published data of the International Cocoa Organization (ICCO), the total world production of cocoa beans in 2016–17 was 4,739,000 tons, principally from Africa (3,622,000 tons) [7].
Demand for organic cocoa products is also expanding, as consumers are increasingly concerned about food security and other environmental issues. However, the organic cocoa market still represents a very small share of the total cocoa market, estimated at less than 0.5% of total production [8].
In this review, we will discuss the main evidence relating to cocoa and chocolate, exploring the possible effects on human health related to their consumption.
2. Chocolate Varieties
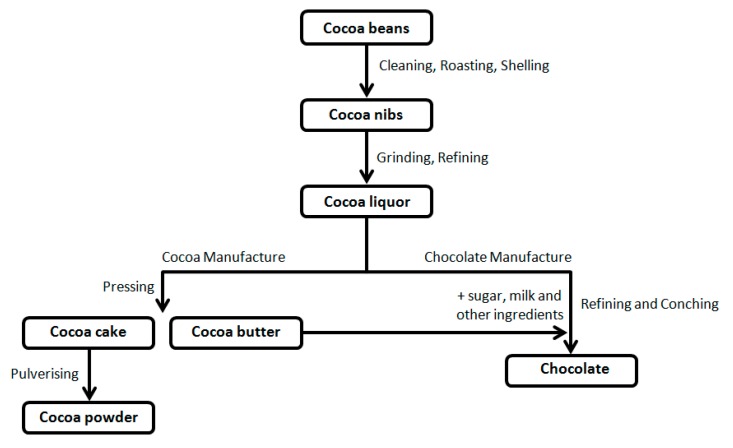
Starting from cocoa beans, through various processes of transformation (Figure 1), the food industry produces different types of chocolate with defined ingredients and characteristics [1,9,10,11].
Figure 1.
The processing of chocolate from cocoa beans.
(1) Dark chocolate contains cocoa bean solids (up to 80% of the total weight) and cocoa butter. With the intense, persistent aroma of cocoa, it melts in the mouth, leaving a pleasant, bitter aftertaste. Its quality depends on the percentage of cocoa. Most of the health benefits attributable to chocolate are associated with consuming the dark type.
(2) Gianduja chocolate is a combination of hazelnuts, cocoa, and sugar; it is brown.
(3) Milk chocolate contains cocoa butter, sugar, milk powder, lecithin, and cocoa (the latter not less than 20–25%). With a bright appearance, it has an intense, persistent aroma and sweet taste with a slightly bitter accent of cocoa.
(4) White chocolate contains cocoa butter, milk, and sugar with no cocoa solids; it has a sweet, pleasant taste.
3. Nutritional Aspects
Cocoa, the basic ingredient in chocolate, contains a significant amount of fat (40–50% as cocoa butter, with approximately 33% oleic acid, 25% palmitic acid, and 33% stearic acid). It also contains polyphenols, which constitute about 10% of a whole bean’s dry weight [12]. Cocoa bean is one of the best-known sources of dietary polyphenols, containing more phenolic antioxidants than most foods [13]. Three groups of polyphenols can be identified in cocoa beans: catechins (37%), anthocyanidins (4%), and proanthocyanidins (58%); these flavonoids are the most abundant phytonutrients in cocoa beans [14,15,16]. However, the bitterness caused by polyphenols makes unprocessed cocoa beans rather unpalatable. Manufacturers have, therefore, developed processing techniques for eliminating the bitterness. Such processes decrease the polyphenol content by up to 10-fold: for consumers the product is markedly different, mainly owing to the low-polyphenol content [12,15] and the other substances added during the processing phase (e.g., sugar, emulsifiers such as soy lecithin). It is well known that polyphenols are associated with beneficial effects, therefore cocoa (rich in polyphenols) and dark chocolate (with a high percentage of cocoa and higher phenolic antioxidant compounds compared to the other chocolate varieties [13]) have assumed significant importance [17].
The nitrogenous compounds of cocoa include both proteins and methylxanthines (theobromine and caffeine) [18]. Cocoa is also rich in minerals: potassium, phosphorus, copper, iron, zinc, and magnesium [18]. The nutritional values of cocoa and two types of chocolate appear in Table 1 [13,19,20].
Table 1.
Nutritional values per 100 g of cocoa and two types of chocolate.
| Chemical Composition | Cocoa | Dark Chocolate | Milk Chocolate |
|---|---|---|---|
| Water (g) | 2.5 | 0.5 | 0.8 |
| Protein (g) | 20.4 | 6.6 | 7.3 |
| Lipid (g) | 25.6 | 33.6 | 36.3 |
| Cholesterol (mg) | 0 | 0 | 10 |
| Carbohydrate (g) | 11.5 | 49.7 | 50.5 |
| Sugar (g) | traces | 49.7 | 50.5 |
| Total fiber (g) | - | 8 | 3.2 |
| Sodium (mg) | - | 11 | 120 |
| Potassium (mg) | - | 300 | 420 |
| Iron (mg) | 14.3 | 5 | 3 |
| Calcium (mg) | 51 | 51 | 262 |
| Phosphorus (mg) | 685 | 186 | 207 |
| Thiamin (mg) | 0.08 | 0.07 | 0.09 |
| Riboflavin (mg) | 0.3 | 0.07 | 0.39 |
| Niacin (mg) | 1.7 | 0.6 | 0.6 |
| Vitamin A (µg) | 7 | 9 | 25 |
| Phenolics (mg) | 996–3781 | 579 | 160 |
| Flavonids (mg) | - | 28 | 13 |
| Theobromine (mg) | - | 802 | 125 |
| Energy (kcal) | 355 | 515 | 545 |
| Energy (kJ) | 1486 | 2155 | 2281 |
4. Lights and Shadows in Chocolate and Cocoa Consumption
Chocolate consumption has recently increased around the world; dark chocolate, in particular, has become very popular for its high concentrations of cocoa and beneficial effects on human health compared with normal or milk chocolate [21,22,23,24]. In addition, milk chocolate could be associated with adverse effects due to its sugar content.
Therefore, only dark chocolate, with high percentages of cocoa, flavonoids, and theobromine and low content of sugar, differently from milk chocolate or other types of chocolate, would be associated with health-promoting effects [11], including the prevention of cardiovascular disease. Similarly, cocoa induces positive effects on blood pressure, insulin resistance, and vascular function. It increases production of nitric oxide (NO) and has antioxidant effects, e.g., delayed oxidation of low-density lipoprotein (LDL) cholesterol and inhibiting ultraviolet-induced DNA oxidation [25,26].
The advantages and disadvantages of chocolate and cocoa consumption are discussed in the following sections, according to in vivo or in vitro studies.
4.1. Cardiovascular Effects
A series of beneficial effects on the cardiovascular system might occur following regular intake of cocoa-containing foods and beverages. Benefits include effects on blood pressure, insulin resistance, and vascular and platelet function [25].
Polyphenols, abundant in cocoa and dark chocolate, activate endothelial NO synthase; that leads to generation of NO [27], which lowers blood pressure by promoting vasodilation [28,29,30,31,32,33]. Indeed, following the consumption of dark chocolate, effects include improvement of the pulse wave speed and of the atherosclerotic score index, with parietal relaxation of large arteries and dilation of small and medium-sized peripheral arteries. Higher concentrations of plasma epicatechins help release endothelium-derived vasodilators and increase the concentration of plasma procyanidins, which leads to greater NO production and bioavailability [32]. Once released, NO also activates the prostacyclin synthesis pathway, which acts as a vasodilator in synergy with NO, thereby contributing to thrombosis protection [17]. Further, the anti-inflammatory and vasoprotective properties of prostacyclin are enhanced by its ability to reduce plasma leukotrienes [17,34,35].
A meta-analysis of randomized trials report that both acute and chronic chocolate and cocoa ingestion effectively increased flow-mediated vasodilatation, reduced systolic and diastolic blood pressure, and reduced serum insulin levels [36]. In young and healthy adults, a daily ingestion of 20 g of higher cocoa chocolate (90%) for a 30-day period improved vascular function by reducing central brachial artery pressures and promoting vascular relaxation [37]. A Swedish prospective study linked chocolate consumption (≥3–4 servings/week) with lower risk of myocardial infarction and ischemic heart disease [38]. On the other hand, a large prospective study exploring data from 83,310 postmenopausal women free of pre-existing major chronic diseases found no association between chocolate consumption and risk of coronary heart disease, stroke, or both combined. Conversely, an increased risk existed among women less than 65 years, in the highest quintile of chocolate consumption [39]. A lack of association between chocolate intake and risk of atrial fibrillation was also reported in a large cohort of United States male physicians [40]. Another population-based, prospective study on 20,992 participants failed to demonstrate an association between high chocolate intake (up to 100 g/day) and incident heart failure [41]. A systematic review suggested that regular chocolate use (<100 g/week) may be linked with reduced cardiovascular risk, and that the most appropriate dose of chocolate consumption was 45 g/week, since higher levels might counteract the health benefits due to adverse effects linked with elevated sugar consumption [42]. These findings were similar to results from a large cohort of Swedish men, which showed a J-shaped association between chocolate consumption and incidence of heart failure, with protective effects absent in subjects consuming ≥1 serving per day [43].
Cocoa plays also a role in treating cerebral conditions, such as stroke; in fact, cocoa intake is associated with increased cerebral blood flow [44]. In the same way, daily chocolate consumption may reduce the likelihood of a stroke attack [18,45]. However, a large Japanese population-based, prospective cohort study reported an association between chocolate consumption and lower risk of stroke in women but not in men [26].
Table 2 shows the studies on cardiovascular effects related to cocoa or chocolate consumption.
Table 2.
Studies on cardiovascular effects related to cocoa or chocolate consumption, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Dong J-Y. et al. 2017 [26] | Prospective human cohort study | Chocolate | Inverse association between chocolate consumption and risk of developing stroke in women |
| Engler M.B. et al. 2004 [29] | Randomized controlled trial in human | Chocolate | Dark chocolate improved endothelial function and increased concentration of plasmatic epicatechins in healthy adults |
| Fisher N.D. & Hollenberg N.K. 2006 [30] | Randomized controlled trial in human | Cocoa | Cocoa enhanced several measures of endothelial function (nitric oxide-dependent) to a greater degree among older, in whom endothelial function is more disturbed, than younger healthy subjects |
| Fisher N.D. et al. 2003 [31] | Randomized controlled trial in human | Cocoa | Cocoa induced vasodilation via activation of the nitric oxide system, providing a plausible mechanism for the protection that flavanol-rich foods induce against coronary events |
| Murphy K.J. et al. 2003 [33] | Randomized, double-blind, placebo-controlled study | Cocoa | Cocoa flavanol and procyanidin supplementation significantly increased plasma epicatechin and catechin concentrations and significantly decreased platelet function |
| Schramm D.D. et al. 2003 [34] | Randomized controlled trial in human | Cocoa | Valuating the food effects on the absorption and pharmacokinetics of cocoa flavanols, carbohydrates increased oral flavanol absorption |
| Schwab U.S. et al. 1996 [35] | Randomized crossover trial in human | Cocoa | Palmitic acid-enriched diet (using palm oil) increased serum lipids, lipoproteins and plasma cholesteryl ester transfer protein activity compared with the stearic acid-enriched diet (using cocoa butter) |
| Pereira T. et al. 2019 [37] | Randomized double-blind trial in human | Chocolate | Cocoa-rich chocolate improved vascular function by reducing central brachial artery pressures and promoting vascular relaxation in young, healthy adults |
| Larsson S.C. et al. 2016 [38] | Prospective human study | Chocolate | Chocolate consumption was associated with lower risk of myocardial infarction and ischemic heart disease |
| Greenberg J.A. et al. 2018 [39] | Prospective human study | Chocolate | No association between chocolate intake and risk of coronary heart disease, stroke, or both combined was observed |
| Khawaja O. et al. 2015 [40] | Randomized double-blind controlled human study | Chocolate | No support to association between chocolate consumption and risk of atrial fibrillation among male physicians |
| Kwok C.S. et al. 2016 [41] | Prospective human study | Chocolate | Habitual chocolate consumption was not associated with the risk of incident heart failure among healthy men and women |
| Steinhaus D.A. et al. 2017 [43] | Prospective cohort human study | Chocolate | J-shaped relationship between chocolate consumption and heart failure incidence |
| Francis S.T. et al. 2006 [44] | Randomized controlled trial in human | Cocoa | Measurements of arterial spin labeling cerebral blood flow demonstrated an increase in blood flow after ingestion of flavanol-rich cocoa, suggesting its potential use for treatment of vascular impairment |
| Walters M.R. et al. 2013 [45] | Randomized controlled trial in human | Chocolate | Chocolate consumption is associated with an acute change in cerebral vasomotor reactivity, independent of metabolic and hemodynamic parameters. |
4.2. Glucose Homeostasis
Cocoa components offer potential as antidiabetic agents, especially with type 2 diabetes mellitus (T2D). This aspect is of particular relevance owing to the emerging worldwide epidemic of metabolic syndrome, including obesity, T2D, and dyslipidemia [46].
Cocoa and flavonols improve glucose homeostasis by slowing carbohydrate digestion and absorption in the gut [47,48]. Indeed, cocoa extracts and procyanidins dose-dependently inhibit pancreatic α-amylase, pancreatic lipase, and secreted phospholipase A2 [48,49]. Cocoa and its flavonols improve insulin sensitivity by regulating glucose transport and insulin signaling proteins in insulin-sensitive tissues (liver, adipose tissue, and skeletal muscle) preventing in these tissues oxidative and inflammatory damage associated with the disease [47]. In younger and normal body-weight men, the results from the Physicians’ Health Study reported an inverse relation of chocolate consumption with incident diabetes [50]. In a multiethnic United States cohort, authors found a lower risk of developing T2D in subjects with the highest intake of chocolate products and cocoa-derived flavonoids [51]. A dose-response meta-analysis, however, suggested a nonlinear association between chocolate consumption and the risk of T2D, with a peak protective effect at 2 servings/week and no benefit recorded when increasing consumption was above 6 servings/week [52].
A prospective study in a large number of Japanese pregnant women also showed a lower risk of gestational diabetes in subjects in the highest quartile of chocolate consumption [53].
The observed effects on glucose homeostasis seem to be strongly dependent on the amount of polyphenols. In fact, a single-blind randomized placebo-controlled cross-over study showed, after 4 weeks, negative metabolic effects (i.e., raised fasting insulin, insulin resistance, and salivary cortisol) in subjects consuming 20 g/day dark chocolate with negligible polyphenol content but not in those consuming the same amount of polyphenol-rich (500 mg) chocolate [54].
Therefore, the daily consumption of small quantities of flavonols from cocoa or chocolate, associated with a dietary intake of flavonoids, would constitute a natural and economic approach to prevent or potentially contribute to the treatment of T2D with minimal toxicity and negative side effects [47]. However, most commercially available soluble cocoa products or chocolates contain low amount of flavonols and are rich in sugar and calories. Therefore, high consumption of chocolate will induce paradoxical consequences, i.e., weight gain and impaired glucose homeostasis, especially in T2D patients and obese individuals [48].
Table 3 shows the studies on glucose homeostasis effects related to cocoa or chocolate use.
Table 3.
Studies on glucose homeostasis effects related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Gu Y. et al. 2011 [49] | In vitro porcine study | Cocoa | Cocoa extracts and cocoa procyanidins inhibited enzymes for digestion of carbohydrates and lipids, suggesting a role in body weight management in conjunction with a low calorie diet |
| Matsumoto C. et al. 2015 [50] | Randomized human study | Chocolate | Inverse relation of chocolate intake with incident diabetes mellitus in younger and normal–body weight men |
| Maskarinec G. et al. 2019 [51] | Cohort human study | Chocolate products | Participants with higher chocolate consumption and higher flavanol intake from cocoa products experienced a lower risk of developing type-2 diabetes |
| Yuan S. et al. 2017 [52] | Prospective human study | Chocolate | Chocolate consumption was associated with decreased risks of coronary heart disease, stroke, and diabetes |
| Dong J-Y et al. 2019 [53] | Prospective cohort human study | Chocolate | Chocolate consumption was associated with a lower risk of gestational diabetes mellitus |
| Almoosawi S. et al. 2012 [54] | Single-blind randomized placebo-controlled cross-over human study | Chocolate | Metabolic benefits of consuming polyphenol-rich dark chocolate and possibility of adverse effects occurring with polyphenol-poor chocolate |
4.3. Cancer
Results regarding the effects of cocoa/chocolate consumption on cancer are rather controversial. Early studies suggested that excess chocolate intake could be a predisposing factor to tumor development (as colorectal and breast cancer) [55,56].
According to other in vitro studies, cocoa inhibits the growth of cancer cells; however, the exact anticancer mechanisms are poorly understood [57,58].
Some authors demonstrated that cocoa liquor procyanidins significantly reduced the incidence and multiplicity of lung carcinomas and decreased thyroid adenomas developed in male rats, and inhibited mammary and pancreatic tumorigenesis in female rats [59,60]. Cocoa procyanidins also reduced vascular endothelial growth factor activity and angiogenic activity associated with tumor, determining down-regulation of tyrosine kinase ErbB2 [61].
In the last years, the treatment of different ovarian cancer cell lines with various concentrations of cocoa procyanidin-rich extract, inducing cytotoxicity and chemosensitization, showed a significant percentage of cells in sub-G1/G0 (hypodiploid) phase, which increased with increasing concentration, and a significant accumulation of cells in the S phase was seen [62]. This effect is probably due to an increase in intracellular levels of reactive oxygen species (ROS) [63]. In an animal study, a diet containing dark chocolate reduced the total number of aberrant crypt foci in the colon. The effect was associated with down-regulation in the transcription levels of both COX-2 and ReIA [64]. In addition, cocoa significantly decreased the tumor incidence and size in mice with colitis-associated cancer [65].
At present, further translational and prospective studies need to explore the intrinsic mechanisms of cocoa’s anticancer action to support its use as a co-adjuvant in preventing and treating cancer [18].
Table 4 shows the studies on cancer related to cocoa or chocolate use.
Table 4.
Studies on cancer related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Boutron-Ruault M.C. et al. 1999 [55] | Randomized controlled trial in human | Chocolate | Chocolate intake resulted a risk factor to colorectal tumor development |
| Carnesecchi S. et al. 2002 [57] | In vitro human study | Cocoa | Cocoa polyphenols interfered with polyamine metabolism, showing an important anti-proliferative effects |
| Yamagishi M. et al. 2002 [59] | In vitro and in vivo rat study | Cocoa | Cocoa liquor proanthocyanidins inhibited mutagenicity of 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) and rat pancreatic carcinogenesis in the initiation stage, but not mammary carcinogenesis induced by PhIP |
| Yamagishi M. et al. 2003 [60] | In vivo rat study | Cocoa | Cocoa liquor proanthocyanidins exerted chemopreventive effects in the lung, decreasing the incidence and multiplicity of carcinomas, and the quantitative values of adenomas in a dose-dependent manner in the thyroid |
| Kenny T. et al. 2004 [61] | In vitro human study | Cocoa | Down-regulation of tyrosine kinase ErbB2 and inhibition of human aortic endothelial cell growth by cocoa procyanidins |
| Taparia S. & Khanna A. 2016 [62] | In vitro human study | Cocoa | Treatment of ovarian cancer cell lines with cocoa procyanidin-rich extract showed a significant percentage of cells in sub-G1/G0 phase and a significant accumulation of cells in the S phase |
| Taparia S.S. & Khanna A. 2016 [63] | In vitro human study | Cocoa | Procyanidin-rich extract of natural cocoa powder caused ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines |
| Hong M.Y. et al. 2013 [64] | In vitro rat study | Chocolate | Chocolate diet-fed animals downregulated transcription levels of COX-2 and RelA and lowered the proliferation index |
| Saadatdoust Z. et al. 2015 [65] | In vitro mice study | Cocoa | Cocoa diet suppresses colitis-associated cancer tumorigenesis |
4.4. Obesity and Lipid Metabolism
Recently, some studies have investigated the preventive or therapeutic effects of cocoa and cocoa constituents against obesity and metabolic syndrome [66]. Administering cocoa to rats decreased visceral adipose tissue [67]. DNA analysis conducted on the liver and mesenteric fat tissue provided interesting clues. In that study, the authors observed decreased expression of various genes associated with fatty acid transport and synthesis in the liver and mesenteric fat as well as increased expression of genes associated with thermogenesis [18,67].
In a clinical study, smelling dark chocolate was assessed to evaluate an appetite response. Chocolate produced a satiation response and reduced appetite; thus, it could be helpful in preventing weight gain [68]. Further, flavonoids can produce metabolic events that induce reduction of lipogenesis, induction of lipolysis, and increased adiponectin secretion; such events reduce lipid deposition and insulin resistance, thus mitigating obesity [17].
A study reported a significantly greater and dose-dependent weight gain over time in subjects with more frequent chocolate consumption. However, no information was provided about the consumer profile of enrolled subjects and the type of chocolate consumed (in particular, the specific amount of dark chocolate) [69].
A recent meta-analysis reported the lack of effects of cocoa or dark chocolate on weight, body mass index (BMI), and waist circumference. However, a subgroup analysis showed reduced weight and BMI following cocoa/dark chocolate supplementation ≥ 30 g chocolate per day in trials between 4–8 weeks, pointing to the relevant role of the consumed dose and trial duration [70].
Dark chocolate might also operate in combination with other nutraceuticals, and have positive effects on lipid profile. Our group has recently reported distinct effects of 24 g almond varieties on organoleptic features and on gastrointestinal function (gallbladder and gastric emptying, orocecal transit) in healthy subjects [71]. One 4-week crossover feeding trial among 31 overweight or obese adults determined that daily consumption of almonds (42 g/day) alone or combined with dark chocolate was beneficial for total cholesterol, low-density (LDL) lipoprotein cholesterol, and apolipoprotein B concentrations. The authors concluded that incorporating almonds, dark chocolate, and cocoa into a diet without exceeding energy needs could reduce the risk of coronary heart disease [72].
A meta-analysis showed that, in the short term (2–12 weeks), dark chocolate/cocoa consumption can significantly lower total and LDL cholesterol levels, but has no effect on high-density lipoprotein HDL and triglycerides [73]. Similar results derive from a placebo-controlled cross-over study, in which daily consumption of cocoa flavonol-containing dark chocolate bars with added plant sterols significantly reduced serum total and LDL cholesterol [74].
Normal weight obese syndrome consists of an excessive body fat associated with a normal BMI, and a higher risk for cardiovascular morbidity and mortality. A group of normal weight obese women consuming dark chocolate (100 g/day, 70% cocoa) for a short period (one week) displayed a rise in the HDL cholesterol levels, and a decrease of the LDL/HDL cholesterol ratio and abdomen circumference. The authors concluded that the regular consumption of dark chocolate would help in maintaining a good atherogenic profile, due to the favorable effects on HDL cholesterol, lipoprotein ratios, and possibly on inflammation markers [75].
Table 5 shows the studies on obesity and lipid metabolism related to cocoa or chocolate use.
Table 5.
Studies on obesity and lipid metabolism related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Gu Y. et al. 2014 [66] | In vitro mice study | Cocoa | Dietary supplementation with cocoa in obese mice ameliorates obesity-related inflammation, insulin resistance, and fatty liver disease |
| Matsui N. et al. 2005 [67] | In vivo rat study | Cocoa | Cocoa ingestion decreased fatty acid synthesis and transport in liver and white adipose tissues, determining a body weight, mesenteric white adipose tissue weight and serum triacylglycerol concentrations lower in rats fed the cocoa diet than in those fed the mimetic cocoa diet |
| Massolt E.T. et al. 2010 [68] | Randomized controlled trial in human | Chocolate | Smell or ingestion of dark chocolate determined suppression of appetite because of the changes in ghrelin. |
| Greenberg J.A. et al. 2013 [69] | Prospective human cohort study | Chocolate | Habitual chocolate consumption was associated with long-term weight gain, in a dose-response manner |
| Lee Y. et al. 2017 [72] | Randomized controlled trial in human | Chocolate and cocoa | Consumption of almonds alone or combined with dark chocolate under controlled-feeding conditions improved lipid profiles |
| Allen R.R. et al. 2008 [74] | Double-blind placebo-controlled cross-over human study | Chocolate | Regular consumption of chocolate bars containing plant sterols and cocoa flavanols as part of a low-fat diet supported cardiovascular health by lowering cholesterol and improving blood pressure |
| Di Renzo L. et al. 2013 [75] | Case-control human study | Chocolate | Regular consumption of dark chocolate determined favourable effects on HDL cholesterol, lipoprotein ratios and inflammation markers in normal weight obese women |
4.5. Intestinal Microbiota
In recent years, there is a growing interest in the study of intestinal microbiota and its changes as result of a particular diet. The human gut harvests the intestinal microbiota, a huge collection of microbes with a key role in energy storage and metabolic disorders [76]. Whereas flavonol monomers and dimers are absorbed in the small intestine, procyanidins undergo metabolization by colonic microbiota, with production of phenolic acids, subsequently absorbed, metabolized in the liver, and eliminated in the urine or in feces [77,78,79,80]. Thus, gut microbiota is responsible for the metabolization of polyphenols in other bio-active compounds (i.e., valerolactones [81], and various phenolic acids [82]) with potential anti-inflammatory properties [17].
A study conducted on rats fed with cocoa diet for 6 weeks highlighted a significant reduction of percent of Bacteroides, Clostridium, and Staphylococcus, changes of tool-like reception expression, and a reduction of immunoglobulin A intestinal secretion, significantly correlated with the decrease in the proportion of the Clostridium and Streptococcus [78].
In pigs, cocoa consumption, in addition to determining changes in metabolites in biofluids and tissues, as the increase in O-methyl-epicatechin glucuronide conjugates in serum, urine, and visceral adipose tissue, induced a significant increase of the abundance of Lactobacillus species from the casei group in feces and Bifidobacterium species in proximal colon contents [83].
Tzounis et al. [79] conducted the first human-intervention study designed to investigate the influence of high cocoa flavanol intake on the growth of the human fecal microbiota. In particular, these authors assessed that the intake of 494 mg of cocoa flavonoids/ day for 4 weeks had a significant effect on intestinal microbiota growth.
Table 6 shows the studies on intestinal microbiota related to cocoa or chocolate use.
Table 6.
Studies on intestinal microbiota related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Wiese S. et al. 2015 [77] | Randomized, double-blind, cross-over human study | Cocoa | Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols |
| Massot-Cladera M. et al. 2012 [78] | In vivo rat study | Cocoa | Cocoa intake affected the growth of certain species of gut microbiota in rats and changes in the toll-like receptor pattern and in the intestinal immune system |
| Tzounis X. et al. 2011 [79] | Randomized controlled double-blind crossover trial in human | Cocoa | Consumption of the high–cocoa flavanol drink modified the gut microflora, reducing the plasmatic triacylglycerol and C-reactive protein concentrations. |
| Urpi-Sarda M. et al. 2007 [82] | In vivo human and rat study | Cocoa | Sensitivity and recovery of epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in human and rat urine |
| Jang S. et al. 2016 [83] | In vivo and in vitro pig study | Cocoa | Consumption of cocoa powder enhanced the abundance of Lactobacillus and Bifidobacterium species and induced a reduction of tumor necrosis factor-α and toll-like receptor gene expression in intestinal tissues |
4.6. Immune System
In vivo and in vitro studies showed that cocoa has regulatory properties on the immune cells implicated in both innate and acquired immunity. In animals, these effects are present at systemic and intestinal level [84,85]. In Lewis rats a 10% cocoa diet or a 0.25% theobromine diet were both able, after one week, to lower serum concentrations of IgG, IgM, IgA, and intestinal IgA, as compared with control diet. Both cocoa and theobromine modified the thymocyte composition increasing CD4-CD8- and CD4+CD8- proportions, and changed the composition of mesenteric lymph node (reduced percentage of T-helper) and spleen (increased proportion of T-helper). Taken together, the data suggest that theobromine is the agent mediating the major immunoregulatory effects of cocoa [86]. Dark chocolate consumption was found having anti-inflammatory effects in a 4-week randomized clinical trial, which was especially visible in the reduced post-challenge responses of cytokines, vascular markers, white blood cells, and leukocyte-activation markers [87,88].
Regular cocoa consumption could be related to preventing or improving health imbalance induced by allergic processes [89]. The positive effects of cocoa flavonoids on the immune system (related to several allergic mechanisms) are known, such as reducing the release of mediators, restoring the balance of T-helper 1 and T-helper 2 cells [90], and down-regulation of IgE production [89,91]. By contrast, chocolate is one of the main potentially allergenic foods that is also capable of causing hypersensitivity reactions, manifesting different clinical symptoms (e.g., fatigue, irritability, insomnia, headache, asthma, and diarrhea) which appear in a few hours or days after food intake [92].
Table 7 shows the studies on the immune system related to cocoa or chocolate use.
Table 7.
Studies on immune system effects related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Ramiro-Puig E. et al. 2008 [85] | In vivo and in vitro rat study | Cocoa | Cocoa-enriched diet modulated intestinal immune responses in young rats |
| Camps-Bossacoma M. et al. 2018 [86] | In vivo and in vitro rat study | Cocoa | Theobromine in cocoa was responsible for systemic and intestinal antibody concentrations and for modifying lymphocyte composition in young healthy rats |
| Esser D. et al. 2014 [87] | Randomized double blind crossover human study | Chocolate | Dark chocolate consumption improved leukocyte adhesion factors and vascular function in overweight men |
| Rodríguez-Lagunas M.J. et al. 2019 [89] | Cross-sectional observational human study | Cocoa | Consumption of cocoa was inversely correlated with physical activity and allergies. Moderate cocoa consumers had less frequency of chronic disease than the low consumers |
| Abril-Gil M. et al. 2012 [91] | In vivo rat study | Cocoa | Cocoa-enriched diet produced an immunomodulatory effect that prevented anti-allergen IgE synthesis |
4.7. Central Nervous System
There is evidences of some beneficial effects on the central nervous system, but larger, prospective studies are missing, so far.
In healthy volunteers, the ingestion of 100 g dark chocolate (72% cocoa) increased [18F] fluorodeoxyglucose (18F-FDG) uptake in the visual cortex, in somatosensory, motor, and pre-frontal cortices, as shown by combined positron emission tomography-computed tomography (PET-CT) [22]. These findings point to dark chocolate-dependent acute effects on cerebral function [22]. The polyphenols in dark chocolate could act on the central nervous system (CNS) and neurological functions through the production of NO [11,17]. Vasodilation and increased cerebral blood flow provide oxygen and glucose to neurons, leading to increased formation of blood vessels in the hippocampus [11,93]. The polyphenol-dependent antioxidant potential could contribute to amelioration of some neurodegenerative disorders [11,93,94]. This inference is based on the fact that age-related cognitive impairment and disorders, such as Alzheimer’s and Parkinson’s diseases, are related to the accumulation of reactive oxygen species in the brain [11,94,95].
The effect of cocoa bioactives on signaling pathways in neurocytes may provide another support for linking dark chocolate with regulation of brain function [11]. Cocoa flavonols and methylxanthines can activate the cascade pathways of such molecules as rapamycin that play a crucial role in synaptic function, neuronal growth, memory mechanisms, and the pathogenesis of neurodegenerative disorders [96].
A prospective study on elderly subjects (age ≥65 years) with normal mini-mental state examination at entry showed that chocolate intake was linked with a decreased risk of cognitive decline during a median follow up of 48 months [97]. Results from a cross-sectional analysis in subjects aged 23–98 years showed a better cognitive performance in those consuming chocolate more frequently. However, following a prospective observation, a relationship between cognitive function and chocolate intake was not confirmed when measured up to 18 years later [98].
4.8. Psychological Aspects
The social and psychological context of everyday life affects metabolic health, emotions, and moods; it can play a role in determining dietary choices [99,100]. In some cases, chocolate consumption can be indirectly associated with a form of depression: hysteroid dysphoria. This condition involves frequent episodes of depression in response to feeling inadequate or socially rejected, which culminates in true bulimic attacks for confectionery and chocolate. A true chocolate addiction (being chocoholic) is akin to alcoholism and nicotine dependence; it affects 40% of the female and 15% of the male population in Western countries [101]. The symptoms involve being responsive to drugs that enhance serotonin transmission; this suggests that central serotonin pathways may be involved in chocolate consumption. The presence of serotonin could explain why sugar and confectionery are strongly desired during chocolate bulimic crises. The ingestion of carbohydrates (e.g., bread and chocolate) increases the relationship between plasma tryptophan and other neutral amino acids; consequently, the transport of tryptophan through the blood–brain barrier is activated, with an increase in cerebral serotonin synthesis, which produces a feeling of energy and pleasure [102].
4.9. Sexual Aspects
Chocolate exerts several effects on human sexuality, mainly acting as an aphrodisiac [103]. Cocoa powder and chocolate contain three unsaturated N-acylethanolamines, which, acting as cannabinoid mimics, could activate cannabinoid receptors or increase anandamide concentrations [103,104]. The latter, in conjunction with other components of chocolate (such as caffeine and theobromine), produces a transient feeling of well-being. Anandamide enhances sexual performance in male rats [103,105]. Moreover, serotonin has been found in several regions of the female genital tract in humans and other animals, where it acts on vasoconstriction and vasodilatation. The principal component of sexual arousal is peripheral vasocongestion of genital tissues; thus, serotonin could be involved in the process of sexual stimulation [103].
Table 8 shows the studies on the nervous system, and psychological and sexual aspects related to cocoa or chocolate use.
Table 8.
Studies on the nervous system, and psychological and sexual aspects related to cocoa or chocolate use, included in this review.
| Study | Study Design | Food Type | Main Outcomes |
|---|---|---|---|
| Fox M. et al. 2019 [22] | Randomized controlled trial human study | Chocolate | Dark chocolate with a high cocoa content has effects on colonic and cerebral function in healthy volunteers |
| Madhavadas S. et al. 2016 [94] | In vivo and in vitro rat study | Chocolate | Dark chocolate enhanced cognitive function and cholinergic activity in the hippocampus and corrected metabolic disturbances of rats |
| Moreira A. et al. 2016 [97] | Prospective cohort human study | Chocolate | Chocolate intake was associated with a lower risk of cognitive decline |
| Chrichton G.E. et al. 2016 [98] | Longitudinal human study | Chocolate | Chocolate intake was associated with better cognitive function |
| Martin F.I. et al. 2012 [100] | Randomized controlled trial in human | Chocolate | Snacks differing in sensory properties and presentation differently influenced postprandial anxiety, energy and emotional states |
| Salonia A. et al. 2006 [103] | Observational human study | Chocolate | Positive association between daily chocolate intake and sexual function. |
5. Conclusions
Cocoa and chocolate act as functional foods, since both carry a number of substances contributing to beneficial health effects. Chocolate combines some organoleptic characteristics with aphrodisiac and antidepressant properties, extending its effects beyond the cardiovascular system, metabolic diseases, CNS diseases, and psychological profiles.
We should stress that several studies evaluated the health-promoting properties of cocoa and not of chocolate itself.
Moreover, because in chocolate processing, cocoa loses some of the polyphenol compounds (the main constituents responsible for the beneficial effects on health), we think that the role of chocolate on human health cannot be completely compared to that of cocoa. Despite the availability of a number of in vitro and experimental reports, epidemiological studies assessing possible beneficial effects of chocolate (in particular dark chocolate) are still scarce. One should keep in mind the presence of a number of confounders (i.e., other diet components, lifestyle, environmental exposures, exact consumption of chocolate, chocolate composition, duration of observation, and other risk factors). Such conditions strongly limit the strength of evidences.
In conclusion, further translational studies need to evaluate all possible effects related to consuming chocolate and to verify in humans the effects hitherto demonstrated only in vitro and on animals. This approach could suggest how best to consume (in terms of dose, mode, and time) chocolate in the daily diet, considering eating habits and lifestyle.
Acknowledgments
We thank the Edanz Group (www.edanzediting.com/ac) for editing a draft of this manuscript.
Author Contributions
M.T.M., G.C., and P.P. conceived the study and reviewed the manuscript; G.D., F.T., O.D.G., and G.R.C. analyzed the scientific literature and wrote the manuscript; A.D.C. reviewed the manuscript. All authors read and approved the final manuscript.
Funding
This study received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Verna R. The history and science of chocolate. Malays. J. Pathol. 2013;35:111–121. [PubMed] [Google Scholar]
- 2.Coe S.D., Coe M.D. The True History of Chocolate. 1st ed. Thames and Hudson; London, UK: 1996. [Google Scholar]
- 3.Dillinger T.L., Barriga P., Escárcega S., Jimenez M., Salazar Lowe D., Grivetti L.E. Food of the gods: Cure for humanity? A cultural history of the medicinal and ritual use of chocolate. J. Nutr. 2000;130(Suppl. 8S):2057S–2072S. doi: 10.1093/jn/130.8.2057S. [DOI] [PubMed] [Google Scholar]
- 4.Lippi D. Chocolate in history: Food, medicine, medi-food. Nutrients. 2013;5:1573–1584. doi: 10.3390/nu5051573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moramarco S., Nemi L. Nutritional and Health Effects of Chocolate. In: Squicciarini M.P., Swinner J., editors. The Economics of Chocolate. 1st ed. Oxford University Press; New York, NY, USA: 2016. pp. 134–156. [Google Scholar]
- 6.International Cocoa Organization (ICCO) [(accessed on 10 July 2019)]; Available online: https://www.icco.org/about-cocoa/growing-cocoa.html.
- 7.International Cocoa Organization (ICCO) [(accessed on 10 July 2019)]; Available online: https://www.icco.org/about-us/icco-news/408-may-2019-quarterly-bulletin-of-cocoa-statistics.html.
- 8.International Cocoa Organization (ICCO) [(accessed on 21 October 2019)]; Available online: https://www.icco.org/about-cocoa/chocolate-industry.html.
- 9.Beckett S.P. The Science of Chocolate. 2nd ed. The Royal Society of Chemistry; Cambridge, UK: 2008. [Google Scholar]
- 10.Corti R., Perdrix J., Flammer A.J., Noll G. Dark or white chocolate? Cocoa and cardiovascular health. Rev. Med. Suisse. 2010;6:499–504. [PubMed] [Google Scholar]
- 11.Petyaev I.M., Bashmakov Y.K. Dark chocolate: Opportunity for an alliance between medical science and the food industry? Front. Nutr. 2017;4:43. doi: 10.3389/fnut.2017.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rusconi M., Conti A. Theobroma cacao L., the Food of the Gods: A scientific approach beyond myths and claims. Pharmacol. Res. 2010;61:5–13. doi: 10.1016/j.phrs.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 13.Meng C.C., Jalil A.M., Ismail A. Phenolic and theobromine contents of commercial dark, milk and white chocolates on the Malaysian market. Molecules. 2009;14:200–209. doi: 10.3390/molecules14010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wollgast J., Anklam E. Review on polyphenols in Theobroma cacao: Changes in composition during the manufacture of chocolate and methodology for identification and quantification. Food Res. Int. 2000;33:423–447. doi: 10.1016/S0963-9969(00)00068-5. [DOI] [Google Scholar]
- 15.Zugravu C., Otelea M.R. Dark chocolate: To eat or not to eat? A review. J. AOAC Int. 2019;102:1388–1396. doi: 10.5740/jaoacint.19-0132. [DOI] [PubMed] [Google Scholar]
- 16.Andújar I., Recio M.C., Giner R.M., Ríos J.L. Cocoa polyphenols and their potential benefits for human health. Oxid. Med. Cell. Longev. 2012;2012:906252. doi: 10.1155/2012/906252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Magrone T., Russo M.A., Jirillo E. Cocoa and dark chocolate polyphenols: From biologym to clinical applications. Front. Immunol. 2017;8:677. doi: 10.3389/fimmu.2017.00677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Latif R. Chocolate/cocoa and human health: A review. Neth. J. Med. 2013;71:63–68. [PubMed] [Google Scholar]
- 19.Istituto Nazionale di Ricerca per gli Alimenti e la Nutrizione (INRAN) [(accessed on 25 November 2019)]; Available online: http://www.clitt.it/contents/scienze-files/6160_rodato_quaderno-files/6160_TabelleComposAlim.pdf.
- 20.Urbańska B., Kowalska J. Comparison of the Total Polyphenol Content and Antioxidant Activity of Chocolate Obtained from Roasted and Unroasted Cocoa Beans from Different Regions of the World. Antioxidants. 2019;8:283. doi: 10.3390/antiox8080283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sumiyoshi E., Matsuzaki K., Sugimoto N., Tanabe Y., Hara T., Katakura H., Miyamoto M., Mishima S., Shido O. Sub-Chronic Consumption of Dark Chocolate Enhances Cognitive Function and Releases Nerve Growth Factors: A Parallel-Group Randomized Trial. Nutrients. 2019;11:2800. doi: 10.3390/nu11112800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fox M., Meyer-Gerspach A.C., Wendebourg M.J., Gruber M., Heinrich H., Sauter M., Woelnerhanssen B., Koeberle D., Juengling F. Effect of cocoa on the brain and gut in healthy subjects: A randomised controlled trial. Br. J. Nutr. 2019;121:654–661. doi: 10.1017/S0007114518003689. [DOI] [PubMed] [Google Scholar]
- 23.Pruijm M., Hofmann L., Charollais-Thoenig J., Forni V., Maillard M., Coristine A., Stuber M., Burnier M., Vogt B. Effect of dark chocolate on renal tissue oxygenation as measured by BOLD-MRI in healthy volunteers. Clin. Nephrol. 2013;80:211–217. doi: 10.5414/CN107897. [DOI] [PubMed] [Google Scholar]
- 24.Grassi D., Lippi C., Necozione S., Desideri G., Ferri C. Short-term administration of dark chocolate is followed by a significant increase in insulin sensitivity and a decrease in blood pressure in healthy persons. Am. J. Clin. Nutr. 2005;81:611–614. doi: 10.1093/ajcn/81.3.611. [DOI] [PubMed] [Google Scholar]
- 25.Corti R., Flammer A.J., Hollenberg N.K., Lüscher T.F. Cocoa and cardiovascular health. Circulation. 2009;119:1433–1441. doi: 10.1161/CIRCULATIONAHA.108.827022. [DOI] [PubMed] [Google Scholar]
- 26.Dong J.Y., Hiroyasu I., Kazumasa Y., Norie S., Shoichiro T., Japan Public Health Center-based Prospective Study Group Chocolate consumption and risk of stroke among men and women: A large population-based, prospective cohort study. Atherosclerosis. 2017;260:8–12. doi: 10.1016/j.atherosclerosis.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 27.Mancia G., de Backer G., Dominiczak A. Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Hypertension (ESH) and of the European Society of Cardiology (ESC) J. Hypertens. 2007;25:1105–1187. doi: 10.1097/HJH.0b013e3281fc975a. [DOI] [PubMed] [Google Scholar]
- 28.Desch S., Schmidt J., Kobler D., Sonnabend M., Eitel I., Sareban M., Rahimi K., Schuler G., Thiele H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am. J. Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 29.Engler M.B., Engler M.M., Chen C.Y., Malloy M.J., Browne A., Chiu E.Y., Kwak H.K., Milbury P., Paul S.M., Blumberg J., et al. Flavonoid-rich dark chocolate improves endothelial function and increases plasma epicatechin concentrations in healthy adults. J. Am. Coll. Nutr. 2004;23:197–204. doi: 10.1080/07315724.2004.10719361. [DOI] [PubMed] [Google Scholar]
- 30.Fisher N.D., Hollenberg N.K. Aging and vascular responses to flavanol-rich cocoa. J. Hypertens. 2006;24:1575–1580. doi: 10.1097/01.hjh.0000239293.40507.2a. [DOI] [PubMed] [Google Scholar]
- 31.Fisher N.D., Hughes M., Gerhard-Herman M., Hollenberg N.K. Flavanol rich cocoa induces nitric-oxide-dependent vasodilation in healthy humans. J. Hypertens. 2003;21:2281–2286. doi: 10.1097/00004872-200312000-00016. [DOI] [PubMed] [Google Scholar]
- 32.Gammone M.A., Efthymakis K., Pluchinotta F.R., Bergante S., Tettamanti G., Riccioni G., D’Orazio N. Impact of chocolate on the cardiovascular health. Front. Biosci. 2018;23:852–864. doi: 10.2741/4620. [DOI] [PubMed] [Google Scholar]
- 33.Murphy K.J., Chronopoulos A.K., Singh I., Francis M.A., Moriarty H., Pike M.J., Turner A.H., Mann N.J., Sinclair A.J. Dietary flavanols and procyanidin oligomers from cocoa (Theobroma cacao) inhibit platelet function. Am. J. Clin. Nutr. 2003;77:1466–1473. doi: 10.1093/ajcn/77.6.1466. [DOI] [PubMed] [Google Scholar]
- 34.Schramm D.D., Karim M., Schrader H.R., Holt R.R., Kirkpatrick N.J., Polagruto J.A., Ensunsa J.L., Schmitz H.H., Keen C.L. Food effects on the absorption and pharmacokinetics of cocoa flavanols. Life Sci. 2003;73:857–869. doi: 10.1016/S0024-3205(03)00373-4. [DOI] [PubMed] [Google Scholar]
- 35.Schwab U.S., Maliranta H.M., Sarkkinen E.S., Savolainen M.J., Kesäniemi Y.A., Uusitupa M.I. Different effects of palmitic and stearic acid-enriched diets on serum lipids and lipoproteins and plasma cholesteryl ester transfer protein activity in healthy young women. Metabolism. 1996;45:143–149. doi: 10.1016/S0026-0495(96)90044-X. [DOI] [PubMed] [Google Scholar]
- 36.Hooper L., Kay C., Abdelhamid A., Kroon P.A., Cohn J.S., Rimm E.B., Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am. J. Clin. Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 37.Pereira T., Bergqvist J., Vieira C., Gruner Svealv B., Castanheira J., Conde J. Randomized study of the effects of cocoa-rich chocolate on the ventricle-arterial coupling and vascular function of young, healthy adults. Nutrition. 2019;63–64:175–183. doi: 10.1016/j.nut.2019.02.017. [DOI] [PubMed] [Google Scholar]
- 38.Larsson S.C., Akesson A., Gigante B., Wolk A. Chocolate consumption and risk of myocardial infarction: A prospective study and meta-analysis. Heart. 2016;102:1017–1022. doi: 10.1136/heartjnl-2015-309203. [DOI] [PubMed] [Google Scholar]
- 39.Greenberg J.A., Manson J.E., Neuhouser M.L., Tinker L., Eaton C., Johnson K.C., Shikany J.M. Chocolate intake and heart disease and stroke in the Women’s Health Initiative: A prospective analysis. Am. J. Clin. Nutr. 2018;108:41–48. doi: 10.1093/ajcn/nqy073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khawaja O., Petrone A.B., Kanjwal Y., Gaziano J.M., Djousse L. Chocolate Consumption and Risk of Atrial Fibrillation (from the Physicians’ Health Study) Am. J. Cardiol. 2015;116:563–566. doi: 10.1016/j.amjcard.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok C.S., Loke Y.K., Welch A.A., Luben R.N., Lentjes M.A., Boekholdt S.M., Pfister R., Mamas M.A., Wareham N.J., Khaw K.T., et al. Habitual chocolate consumption and the risk of incident heart failure among healthy men and women. Nutr. Metab. Cardiovasc. Dis. NMCD. 2016;26:722–734. doi: 10.1016/j.numecd.2016.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ren Y., Liu Y., Sun X.Z., Wang B.Y., Zhao Y., Liu D.C., Zhang D.D., Liu X.J., Zhang R.Y., Sun H.H., et al. Chocolate consumption and risk of cardiovascular diseases: A meta-analysis of prospective studies. Heart. 2019;105:49–55. doi: 10.1136/heartjnl-2018-313131. [DOI] [PubMed] [Google Scholar]
- 43.Steinhaus D.A., Mostofsky E., Levitan E.B., Dorans K.S., Hakansson N., Wolk A., Mittleman M.A. Chocolate intake and incidence of heart failure: Findings from the Cohort of Swedish Men. Am. Heart J. 2017;183:18–23. doi: 10.1016/j.ahj.2016.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Francis S.T., Head K., Morris P.G., Macdonald I.A. The effect of flavanol-rich cocoa on the fMRI response to a cognitive task in healthy young people. J. Cardiovasc. Pharmacol. 2006;47(Suppl. 2):S221–S223. doi: 10.1097/00005344-200606001-00018. [DOI] [PubMed] [Google Scholar]
- 45.Walters M.R., Williamson C., Lunn K., Munteanu A. Chocolate consumption and risk of stroke: A prospective cohort of men and meta-analysis. Neurology. 2013;80:1173–1174. doi: 10.1212/01.wnl.0000428365.81656.e0. [DOI] [PubMed] [Google Scholar]
- 46.Vecchie A., Dallegri F., Carbone F., Bonaventura A., Liberale L., Portincasa P., Fruhbeck G., Montecucco F. Obesity phenotypes and their paradoxical association with cardiovascular diseases. Eur. J. Intern. Med. 2018;48:6–17. doi: 10.1016/j.ejim.2017.10.020. [DOI] [PubMed] [Google Scholar]
- 47.Martín M.A., Goya L., Ramos S. Antidiabetic actions of cocoa flavanols. Mol. Nutr. Food Res. 2016;60:1756–1769. doi: 10.1002/mnfr.201500961. [DOI] [PubMed] [Google Scholar]
- 48.Ramos S., Martín M.A., Goya L. Effects of cocoa antioxidants in type 2 diabetes mellitus. Antioxidants. 2017;6:84. doi: 10.3390/antiox6040084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gu Y., Hurst W.J., Stuart D.A., Lambert J.D. Inhibition of key digestive enzymes by cocoa extracts and procyanidins. J. Agric. Food. Chem. 2011;59:5305–5311. doi: 10.1021/jf200180n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Matsumoto C., Petrone A.B., Sesso H.D., Gaziano J.M., Djousse L. Chocolate consumption and risk of diabetes mellitus in the Physicians’ Health Study. Am. J. Clin. Nutr. 2015;101:362–367. doi: 10.3945/ajcn.114.092221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maskarinec G., Jacobs S., Shvetsov Y., Boushey C.J., Setiawan V.W., Kolonel L.N., Haiman C.A., Le Marchand L. Intake of cocoa products and risk of type-2 diabetes: The multiethnic cohort. Eur. J. Clin. Nutr. 2019;73:671–678. doi: 10.1038/s41430-018-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yuan S., Li X., Jin Y., Lu J. Chocolate Consumption and Risk of Coronary Heart Disease, Stroke, and Diabetes: A Meta-Analysis of Prospective Studies. Nutrients. 2017;9:688. doi: 10.3390/nu9070688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dong J.Y., Kimura T., Ikehara S., Cui M., Kawanishi Y., Yamagishi K., Ueda K., Iso H., Japan Environment. Children’s Study Group Chocolate consumption and risk of gestational diabetes mellitus: The Japan Environment and Children’s Study. Br. J. Nutr. 2019;122:936–941. doi: 10.1017/S0007114519001806. [DOI] [PubMed] [Google Scholar]
- 54.Almoosawi S., Tsang C., Ostertag L.M., Fyfe L., Al-Dujaili E.A. Differential effect of polyphenol-rich dark chocolate on biomarkers of glucose metabolism and cardiovascular risk factors in healthy, overweight and obese subjects: A randomized clinical trial. Food Func. 2012;3:1035–1043. doi: 10.1039/c2fo30060e. [DOI] [PubMed] [Google Scholar]
- 55.Boutron-Ruault M.C., Senesse P., Faivre J., Chatelain N., Belghiti C., Meance S. Foods as risk factors for colorectal cancer: A case-control study in Burgundy (France) Eur. J. Cancer Prev. 1999;8:229–235. doi: 10.1097/00008469-199906000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Richardson S., Gerber M., Cenee S. The role of fat, animal protein and some vitamin consumption in breast cancer: A case control study in southern France. Int. J. Cancer. 1991;48:1–9. doi: 10.1002/ijc.2910480102. [DOI] [PubMed] [Google Scholar]
- 57.Carnesecchi S., Schneider Y., Lazarus S.A., Coehlo D., Gosse F., Raul F. Flavanols and procyanidins of cocoa and chocolate inhibit growth and polyamine biosynthesis of human colonic cancer cells. Cancer Lett. 2002;175:147–155. doi: 10.1016/S0304-3835(01)00731-5. [DOI] [PubMed] [Google Scholar]
- 58.Kozikowski A.P., Tuckmantel W., Bottcher G., Romanczyk L.J., Jr. Studies in polyphenol chemistry and bioactivity. 4.(1) Synthesis of trimeric, tetrameric, pentameric, and higher oligomeric epicatechin-derived procyanidins having all-4beta, 8-interflavan connectivity and their inhibition of cancer cell growth through cell cycle arrest. J. Org. Chem. 2003;68:1641–1658. doi: 10.1021/jo020393f. [DOI] [PubMed] [Google Scholar]
- 59.Yamagishi M., Natsume M., Osakabe N., Nakamura H., Furukawa F., Imazawa T., Nishikawa A., Hirose M. Effects of cacao liquor proanthocyanidins on PhIP-induced mutagenesis in vitro, and in vivo mammary and pancreatic tumorigenesis in female Sprague-Dawley rats. Cancer Lett. 2002;185:123–130. doi: 10.1016/S0304-3835(02)00276-8. [DOI] [PubMed] [Google Scholar]
- 60.Yamagishi M., Natsume M., Osakabe N., Okazaki K., Furukawa F., Imazawa T., Nishikawa A., Hirose M. Chemoprevention of lung carcinogenesis by cacao liquor proanthocyanidins in a male rat multi-organ carcinogenesis model. Cancer Lett. 2003;191:49–57. doi: 10.1016/S0304-3835(02)00629-8. [DOI] [PubMed] [Google Scholar]
- 61.Kenny T., Keen C., Jones P., Kung H., Schmitz H., Gershwin M. Pentameric procyanidins isolated from Theobroma cacao seeds selectively downregulate ErbB2 in human aortic endothelial cells. Exp. Biol. Med. 2004;229:255–263. doi: 10.1177/153537020422900306. [DOI] [PubMed] [Google Scholar]
- 62.Taparia S., Khanna A. Effect of procyanidin-rich extract from natural cocoa powder on cellular viability, cell cycle progression, and chemoresistance in human epithelial ovarian carcinoma cell lines. Pharmacogn. Mag. 2016;12(Suppl. 2):S109–S115. doi: 10.4103/0973-1296.182164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Taparia S.S., Khanna A. Procyanidin-rich extract of natural cocoa powder causes ROS-mediated caspase-3 dependent apoptosis and reduction of pro-MMP-2 in epithelial ovarian carcinoma cell lines. Biomed. Pharmacother. 2016;83:130–140. doi: 10.1016/j.biopha.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 64.Hong M.Y., Nulton E., Shelechi M., Hernandez L.M., Nemoseck T. Effects of dark chocolate on azoxymethane-induced colonic aberrant crypt foci. Nutr. Cancer. 2013;65:677–685. doi: 10.1080/01635581.2013.789542. [DOI] [PubMed] [Google Scholar]
- 65.Saadatdoust Z., Pandurangan A.K., Ananda Sadagopan S.K., Mohd Esa N., Ismail A., Mustafa M.R. Dietary cocoa inhibits colitis associated cancer: A crucial involvement of the IL-6/STAT3 pathway. J. Nutr. Biochem. 2015;26:1547–1558. doi: 10.1016/j.jnutbio.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 66.Gu Y., Yu S., Lambert J.D. Dietary cocoa ameliorates obesity-related inflammation in high fat-fed mice. Eur. J. Nutr. 2014;53:149–158. doi: 10.1007/s00394-013-0510-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Matsui N., Ito R., Nishimura E., Yoshikawa M., Kato M., Kamei M., Shibata H., Matsumoto I., Abe K., Hashizume S. Ingested cocoa can prevent high-fat diet-induced obesity by regulating the expression of genes for fatty acid metabolism. Nutrition. 2005;21:594–601. doi: 10.1016/j.nut.2004.10.008. [DOI] [PubMed] [Google Scholar]
- 68.Massolt E.T., van Haard P.M., Rehfeld J.F., Posthuma E.F., van der Veer E., Schweitzer D.H. Appetite suppression through smelling of dark chocolate correlates with changes in ghrelin in young women. Regul. Pept. 2010;161:81–86. doi: 10.1016/j.regpep.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 69.Greenberg J.A., Buijsse B. Habitual chocolate consumption may increase body weight in a dose-response manner. PLoS ONE. 2013;8:e70271. doi: 10.1371/journal.pone.0070271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kord-Varkaneh H., Ghaedi E., Nazary-Vanani A., Mohammadi H., Shab-Bidar S. Does cocoa/dark chocolate supplementation have favorable effect on body weight, body mass index and waist circumference? A systematic review, meta-analysis and dose-response of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2019;59:2349–2362. doi: 10.1080/10408398.2018.1451820. [DOI] [PubMed] [Google Scholar]
- 71.Diella G., Di Ciaula A., Lorusso M.P., Summo C., Caggiano G., Caponio F., Montagna M.T., Portincasa P. Distinct Effects of two Almond Cultivars on Agreeability and Gastrointestinal Motility in Healthy Subjects: More than mere Nutraceuticals. J. Gastrointest. Liver Dis. JGLD. 2018;27:31–39. doi: 10.15403/jgld.2014.1121.271.dll. [DOI] [PubMed] [Google Scholar]
- 72.Lee Y., Berryman C.E., West S.G., Chen C.O., Blumberg J.B., Lapsley K.G., Preston A.G., Fleming J.A., Kris-Etherton P.M. Effects of dark chocolate and almonds on cardiovascular risk factors in overweight and obese individuals: A randomized controlled-feeding trial. J. Am. Heart Assoc. 2017;6:e005162. doi: 10.1161/JAHA.116.005162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tokede O.A., Gaziano J.M., Djousse L. Effects of cocoa products/dark chocolate on serum lipids: A meta-analysis. Eur. J. Clin. Nutr. 2011;65:879–886. doi: 10.1038/ejcn.2011.64. [DOI] [PubMed] [Google Scholar]
- 74.Allen R.R., Carson L., Kwik-Uribe C., Evans E.M., Erdman J.W., Jr. Daily consumption of a dark chocolate containing flavanols and added sterol esters affects cardiovascular risk factors in a normotensive population with elevated cholesterol. J. Nutr. 2008;138:725–731. doi: 10.1093/jn/138.4.725. [DOI] [PubMed] [Google Scholar]
- 75.Di Renzo L., Rizzo M., Sarlo F., Colica C., Iacopino L., Domino E., Sergi D., De Lorenzo A. Effects of dark chocolate in a population of normal weight obese women: A pilot study. Eur. Rev. Med. Pharm. Sci. 2013;17:2257–2266. [PubMed] [Google Scholar]
- 76.Clarke S.F., Murphy E.F., Nilaweera K., Ross P.R., Shanahan F., O’Toole P.W., Cotter P.D. The gut microbiota and its relationship to diet and obesity: New insights. Gut Microbes. 2012;3:186–202. doi: 10.4161/gmic.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wiese S., Esatbeyoglu T., Winterhalter P., Kruse H.P., Winkler S., Bub A., Kulling S.E. Comparative biokinetics and metabolism of pure monomeric, dimeric, and polymeric flavan-3-ols: A randomized cross-over study in humans. Mol. Nutr. Food Res. 2015;59:610–621. doi: 10.1002/mnfr.201400422. [DOI] [PubMed] [Google Scholar]
- 78.Massot-Cladera M., Pérez-Berezo T., Franch A., Castell M., Pérez-Cano F.J. Cocoa modulatory effect on rat faecal microbiota and colonic crosstalk. Arch. Biochem. Biophys. 2012;527:105–112. doi: 10.1016/j.abb.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 79.Tzounis X., Rodriguez-Mateos A., Vulevic J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am. J. Clin. Nutr. 2011;93:62–72. doi: 10.3945/ajcn.110.000075. [DOI] [PubMed] [Google Scholar]
- 80.Tzounis X., Vulevic J., Kuhnle G.G., George T., Leonczak J., Gibson G.R., Kwik-Uribe C., Spencer J.P. Flavanol monomer-induced changes to the human faecal microflora. Br. J. Nutr. 2008;99:782–792. doi: 10.1017/S0007114507853384. [DOI] [PubMed] [Google Scholar]
- 81.Monagas M., Urpi-Sarda M., Sanchez-Patan F., Llorach R., Garrido I., Gomez-Cordoves C., Andres-Lacueva C., Bartolome B. Insights into the metabolism and microbial biotransformation of dietary flavan-3-ols and the bioactivity of their metabolites. Food Func. 2010;1:233–253. doi: 10.1039/c0fo00132e. [DOI] [PubMed] [Google Scholar]
- 82.Urpi-Sarda M., Monagas M., Khan N., Lamuela-Raventos R.M., Santos-Buelga C., Sacanella E., Castell M., Permanyer J., Andres-Lacueva C. Epicatechin, procyanidins, and phenolic microbial metabolites after cocoa intake in humans and rats. Anal. Bioanal. Chem. 2009;394:1545–1556. doi: 10.1007/s00216-009-2676-1. [DOI] [PubMed] [Google Scholar]
- 83.Jang S., Sun J., Chen P., Lakshman S., Molokin A., Harnly J.M., Vinyard B.T., Urban J.F., Jr., Davis C.D., Solano-Aguilar G. Flavanol-enriched cocoa powder alters the intestinal microbiota, tissue and fluid metabolite profiles, and intestinal gene expression in pigs. J. Nutr. 2016;146:673–680. doi: 10.3945/jn.115.222968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Ramiro-Puig E., Castell M. Cocoa: Antioxidant and immunomodulator. Br. J. Nutr. 2009;101:931–940. doi: 10.1017/S0007114508169896. [DOI] [PubMed] [Google Scholar]
- 85.Ramiro-Puig E., Perez-Cano F.J., Ramos-Romero S., Perez-Berezo T., Castellote C., Permanyer J., Franch A., Izquierdo-Pulido M., Castell M. Intestinal immune system of young rats influenced by cocoa-enriched diet. J. Nutr. Biochem. 2008;19:555–565. doi: 10.1016/j.jnutbio.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 86.Camps-Bossacoma M., Perez-Cano F.J., Franch A., Castell M. Theobromine Is Responsible for the Effects of Cocoa on the Antibody Immune Status of Rats. J. Nutr. 2018;148:464–471. doi: 10.1093/jn/nxx056. [DOI] [PubMed] [Google Scholar]
- 87.Esser D., Mars M., Oosterink E., Stalmach A., Müller M., Afman L.A. Dark chocolate consumption improves leukocyte adhesion factors and vascular function in overweight men. FASEB J. 2014;28:1464–1473. doi: 10.1096/fj.13-239384. [DOI] [PubMed] [Google Scholar]
- 88.Van den Brink W., van Bilsen J., Salic K., Hoevenaars F.P.M., Verschuren L., Kleemann R., Bouwman J., Ronnett G.V., van Ommen B., Wopereis S. Current and Future Nutritional Strategies to Modulate Inflammatory Dynamics in Metabolic Disorders. Front. Nutr. 2019;6:129. doi: 10.3389/fnut.2019.00129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Rodríguez-Lagunas M.J., Vicente F., Pereira P., Castell M., Pérez-Cano F.J. Relationship between cocoa intake and healthy status: A pilot study in university students. Molecules. 2019;24:812. doi: 10.3390/molecules24040812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gandhi G.R., Neta M.T.S.L., Sathiyabama R.G., Quintans J.S.S., de Oliveira E., Silva A.M., Araújo A.A.S., Narain N., Júnior L.J.Q., Gurgel R.Q. Flavonoids as Th1/Th2 cytokines immunomodulators: A systematic review of studies on animal models. Phytomedicine. 2018;44:74–84. doi: 10.1016/j.phymed.2018.03.057. [DOI] [PubMed] [Google Scholar]
- 91.Abril-Gil M., Massot-Cladera M., Pérez-Cano F.J., Castellote C., Franch A., Castell M. A diet enriched with cocoa prevents IgE synthesis in a rat allergy model. Pharmacol. Res. 2012;65:603–608. doi: 10.1016/j.phrs.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 92.Żukiewicz-Sobczak W.A., Wróblewska P., Adamczuk P., Kopczyński P. Causes, symptoms and prevention of food allergy. Postep. Dermatol. Alergol. 2013;30:113–116. doi: 10.5114/pdia.2013.34162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wasik A., Antkiewicz-Michaluk L. The mechanism of neuroprotective action of natural compounds. Pharmacol. Rep. 2017;69:851–860. doi: 10.1016/j.pharep.2017.03.018. [DOI] [PubMed] [Google Scholar]
- 94.Madhavadas S., Kapgal V.K., Kutty B.M., Subramanian S. The neuroprotective effect of dark chocolate in monosodium glutamate-induced nontransgenic Alzheimer disease model rats: Biochemical, behavioral, and histological studies. J. Diet. Suppl. 2016;13:449–460. doi: 10.3109/19390211.2015.1108946. [DOI] [PubMed] [Google Scholar]
- 95.Dubner L., Wang J., Ho L., Ward L., Pasinetti G.M. Recommendations for development of new standardized forms of cocoa breeds and cocoa extract processing for the prevention of Alzheimer’s disease: Role of cocoa in promotion of cognitive resilience and healthy brain aging. J. Alzheimers Dis. 2015;48:879–889. doi: 10.3233/JAD-150536. [DOI] [PubMed] [Google Scholar]
- 96.Wrigley S., Arafa D., Tropea D. Insulin-like growth factor 1: At the crossroads of brain development and aging. Front. Cell. Neurosci. 2017;11:14. doi: 10.3389/fncel.2017.00014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Moreira A., Diogenes M.J., de Mendonca A., Lunet N., Barros H. Chocolate Consumption is Associated with a Lower Risk of Cognitive Decline. J. Alzheimers Dis. 2016;53:85–93. doi: 10.3233/JAD-160142. [DOI] [PubMed] [Google Scholar]
- 98.Crichton G.E., Elias M.F., Alkerwi A. Chocolate intake is associated with better cognitive function: The Maine-Syracuse Longitudinal Study. Appetite. 2016;100:126–132. doi: 10.1016/j.appet.2016.02.010. [DOI] [PubMed] [Google Scholar]
- 99.Alonso C., Guilarte M., Vicario M., Ramos L., Ramadan Z., Antolin M., Martinez C., Rezzi S., Saperas E., Kochhar S., et al. Maladaptive intestinal epithelial responses to life stress may predispose healthy women to gut mucosal inflammation. Gastroenterology. 2008;135:163–172. doi: 10.1053/j.gastro.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 100.Martin F.P., Antille N., Rezzi S., Kochhar S. Everyday eating experiences of chocolate and non-chocolate snacks impact postprandial anxiety, energy and emotional states. Nutrients. 2012;4:554–567. doi: 10.3390/nu4060554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trogu E. Cioccolismo, MDD. [(accessed on 25 November 2019)];1998 Volume 3 Available online: https://it.wikipedia.org/wiki/Cioccolismo. [Google Scholar]
- 102.Silva N.R. Chocolate consumption and effects on serotonin synthesis. Arch. Intern. Med. 2010;170:1608–1609. doi: 10.1001/archinternmed.2010.331. [DOI] [PubMed] [Google Scholar]
- 103.Salonia A., Fabbri F., Zanni G., Scavini M., Fantini G.V., Briganti A., Naspro R., Parazzini F., Gori E., Rigatti P., et al. Chocolate and women’s sexual health: An intriguing correlation. J. Sex Med. 2006;3:476–482. doi: 10.1111/j.1743-6109.2006.00236.x. [DOI] [PubMed] [Google Scholar]
- 104.Di Tomaso E., Beltramo M., Piomelli D. Brain cannabinoids in chocolate. Nature. 1996;382:677–678. doi: 10.1038/382677a0. [DOI] [PubMed] [Google Scholar]
- 105.Martinez-Gonzalez D., Bonilla-Jaime H., Morales-Otal A., Henriksen S.J., Velazquez-Moctezuma J., Prospero-Garcia O. Oleamide and anandamide effects on food intake and sexual behavior of rats. Neurosci. Lett. 2004;364:1–6. doi: 10.1016/j.neulet.2004.03.080. [DOI] [PubMed] [Google Scholar]