Abstract
Background:
Multiple sclerosis (MS) can involve cognitive entities, including memory, attention, performance, and information processing. Furthermore, MS causes depression and negatively affects the quality of life (QOL). This study was aimed to assess the efficacy of cognitive rehabilitation on cognitive entities of MS patients.
Materials and Methods:
This is a clinical trial study conducted on 56 MS patients in 2016–2017. Patients were randomly divided into two Groups of A (cognitive rehabilitation) and B (control group). Patients were evaluated in terms of memory, attention, QOL, and depression. Questionnaires included Abbreviated Mental Test, Prospective and Retrospective Memory Questionnaire, Everyday Memory Questionnaire, Digit Spam test for attention assessment, QOL-54 questionnaire, and Second version of Beck questionnaire assessing depression. They were filled through an interview before the study initiation, and then, the intervention group underwent ten sessions of cognitive rehabilitation and questionnaires refilled within 3 months after study initiation. Outcomes of the two groups were compared.
Results:
Memory, attention, QOL, and depression improved significantly following the intervention in cases (P < 0.05), while no significant change was observed among controls (P > 0.05). Comparison of cases and controls in the second evaluation showed a significant difference between cases and controls (P < 0.05).
Conclusion:
Ten sessions of cognitive rehabilitation could significantly improve MS patients' cognitive performance. Moreover, this approach affected their QOL and sense of depression in a decisive trend. It can be concluded that cognitive rehabilitation can successfully affect numerous aspects of MS patients, while numerous medical therapies may be required for treatment of each mere aspect. Further evaluations are strongly recommended.
Keywords: Attention, cognitive rehabilitation, depression, memory, multiple sclerosis, quality of life
INTRODUCTION
Multiple sclerosis (MS) is a chronic disabling inflammatory disease of the central nervous system (CNS) that occurs due to autoimmunity against the myelin sheath. MS mostly affects young individuals, predominantly among women. While different genetic and environmental factors have been suggested to play a role in MS etiology, it has remained unclear.[1]
The chronic nature of MS, along with the disabling symptoms, has led affected individuals to struggle with some levels of cognitive impairment, reported in 43%–70% of them.[2] Cognitive impairment in MS does not involve all the entities equally. Usually, attention, management functioning, processing velocity, and spatial vision are affected to a more extent while verbal and expressive lingual entities are preserved.[3] Cognitive manifestations may be present in the earliest stages of the disease or occur during more severe phases.[4]
Time and content are two entities of memory that can be affected by MS. Time categories of memory include short-term, long-term, and job memory, and content categories include conscious and unconscious memories. Over sixty percent of MS patients experience memorial dysfunction, and job memory and long-term conscious memory are the most affected areas.[3] On the other hand, MS causes depression, as the most common associated psychological disorder, and decreased quality of life (QOL).[5]
Variety of medical and behavioral therapies have been utilized to save, rehabilitate, and preserve memorial function, depression, and QOL among MS patients, resulting in uncertain outcomes.[6,7,8] Due to the inadequate and contradictory information about the best and most efficient means of improving MS-related psychological consequences, the current study aimed to assess the efficacy of cognitive rehabilitation on depression, memory, attention, and QOL in these patients.
MATERIALS AND METHODS
Study participants
The current presentation is a double-blinded clinical-trial study conducted from August 2016 to April 2017 on 56 patients out of 100 ones with MS who were eligible for the participation in the study and referred to Kashani MS Clinic affiliated to Isfahan University of Medical Sciences. Among the remained 44 ones, 30 ones were excluded as they did not participate in the sessions, ten ones either did not refer for the posttest completion or had more than 20% of incomplete data, and four other patients withdrew the study because of relapses occur during the study course [Figure 1].
Figure 1.
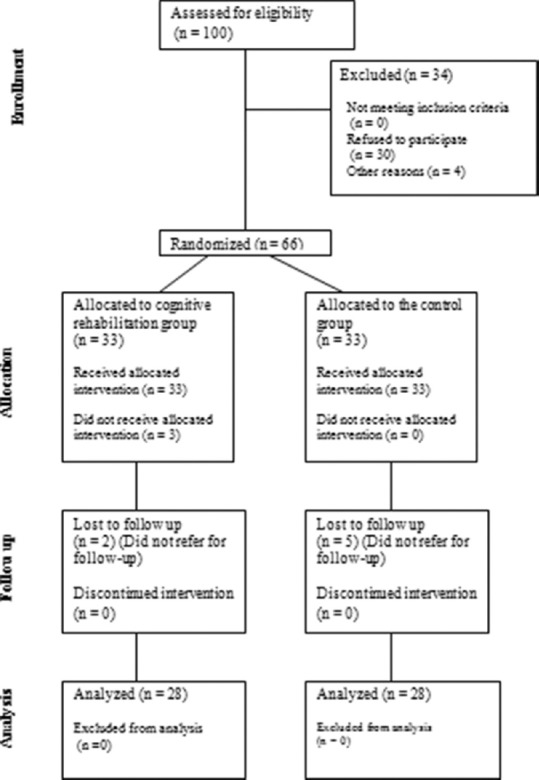
Consort diagram of the studied population
The inclusion criteria were defined as least ability of writing and reading, Extended Disability Severity Scale (EDSS) of ≤5.5 based on John Kurtzke criteria,[9] mild to moderate memorial impairment based on Everyday Memory Questionnaire (EMQ),[10] and mild to moderate depression status based on second version of Beck depression inventory.[11] Patients who denied participating in the primary psychologic and cognitive assessment were excluded. The Ethics Committee of Isfahan University of Medical Sciences approved the study protocol. Besides, the study protocol was enrolled in the Iranian Registry of Clinical Trials and performed based on the code number IRCT2016042227522N1.
All of the participants were requested to sign the written informed consents before the enrollment.
Sampling and randomization
Eligible patients were recruited and randomly divided into two groups of intervention (Group A) and control (Group B). The study population selection was performed through convenience sampling, and randomization was performed using Random Allocation Software. Therefore, each patient was provided with a particular number using the mentioned software that allocated him/her to either the control group or the intervention group. A random number was assigned to each patient, and individuals with even numbers were allocated to the intervention group. Patients and the psychologist who interpreted the questionnaires were blinded to assignments.
The sample size of the study was measured based on the Borm–Fransen–Lemmens formula[12] as follows:
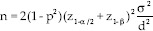
The test level was 0.05, the test power was 0.8, and the coefficient correlation between pretests and posttests was 0.7, and eventually, the 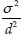 ratio was considered as 2.5. Therefore, the estimated required sample size was 28 for each of the groups.
ratio was considered as 2.5. Therefore, the estimated required sample size was 28 for each of the groups.
Assessment tools
This study was aimed to assess the efficacy of cognitive rehabilitation on cognitive function, QOL, and depression status of MS affected patients. These assessments have been done using varieties of questionnaires presented as follows:
Patients in both groups were evaluated using the validated Persian version of Abbreviated Mental Test (AMT),[13] Prospective and Retrospective Memory Questionnaire (PRMQ),[14] EMQ,[10] Digit Span test for attention assessment,[15] QOL-54 questionnaire,[16] and second version of Beck depression inventory.[11]
EMQ questionnaire is a 28-item questionnaire assessing general memory and attention aspects (reliability: 0.80 and Cronbach's alpha: 0.88).[17] Second version Beck depression inventory is a 21-item questionnaire (reliability: 0.74 and validity: 0.87).[11] AMT evaluates the mental status of patients and is shown to have the validity of 92.15% and reliability of 81.5%.[13] PRMQ is a 16-item questionnaire that was turned to Persian with the reliability of 0.84 for prospective and 0.80 for retrospective memory.[14] QOL-54 Persian version is a questionnaire containing 54 questions regarding the assessment of life quality in MS patients. This Persian questionnaire has high validity and reliability with a a-Cronbach coefficient of 0.96.[16]
Study procedure
In Group A, the therapeutic intervention was ten sessions of cognitive rehabilitation courses. Each session lasted for 2 h and was individualized for each case based on impaired function reconstruction and modulation. Sessions were held every 7–10 days. Generally, in each class, the therapist aimed for reinforcement and/or consolidation of previous cognitive abilities which have been impaired and tried to reinforce other remained abilities for compensation of impaired abilities. In this way, patients could rehabilitate their role in society and actively maintain their functions.[18]
Group B attended similar classes with regard to the number and duration of sessions; however, the content of the sessions was different and was not supporting cognitive rehabilitation. In these sessions, patients were requested to present their experiences of cognitive impairments, and cases with successful coping with new conditions were admired. At the end of the intervention, outcomes were compared between the two groups.
Cognitive rehabilitation
Cognitive rehabilitation schedule consisted of rehabilitation entities including attention, concentration, visual and auditory memory, and autobiography memory. The approaches were performed considering the severity of cognitive impairment and with the aim of optimization of the residual functions. To achieve the mentioned rehabilitative programs, the mnemonic approach was utilized which includes visual imagery, theological organization, and relational strategies including mnemonics of fiction, the clues about the first word, chain connection, and the technique of PQRST (Preview, Question, Read, Self-recitation, and Test).[19,20,21] The sessions were performed as follows:
Numbers of 10 sessions of group treatment were performed that each had a duration of 120 min. Memory and its disturbances in the daily life were explained for the participants; then, the autobiographical memory, its subtypes, and its disturbances were represented.
The technique of recalling positive memories through autobiographical memory was trained, and then, the psychologist presented several samples and requested the participants to recall and then present their positive memories.
Statistical analysis
Obtained data were analyzed using SPSS-22 software (The Statistical Package for Social Sciences; IBM; Chicago; The United States). Descriptive data were presented in means and percentages. In the purpose of analyzing data and hypothesis testing, MANCOVA model was used. T-test was utilized to compare pretest statuses of the two groups. P < 0.05 was considered as a significant level.
RESULTS
Initially, 100 MS patients were screened and invited to participate, and 56 cases were included at the end. All of the members of Group A and Group B fulfilled the study protocol, and none of them were eliminated from the study for any reason.
The mean age of participants was 31.33 years with a gender distribution of 39 females and 17 males. Participants were randomly divided into two groups including cases group consisted of 28 ones with mean age of 32.21 years and control group consisted of 28 patients with mean age of 30.46 years (P = 0.449). The distribution of gender in groups was as follows: female: male ratio 2.50 for Group A and 2.11 for Group B (P = 0.776). The mean baseline EDSS was not different between two groups (2.28 for cases versus 1.87 for controls; P = 0.284). Detailed information about patients' demographics has been presented in Table 1.
Table 1.
Demographic and clinical characteristics of the studied population
| Intervention group (%) | Control group (%) | P | |
|---|---|---|---|
| Age | 32.21 | 30.46 | 0.449 |
| Gender | |||
| Male | 8 (28.5) | 9 (32.1) | 0.776 |
| Female | 20 (71.4) | 19 (67.8) | |
| Extended disability status score | 2.28 | 1.87 | 0.287 |
| Duration of the disease | 7.46 | 7.07 | 0.639 |
| Relapse rate since the previous year | |||
| No | 18 (64.28) | 19 (67.8) | 0.949 |
| One | 7 (25) | 6 (21.4) | |
| Two | 3 (10.7) | 3 (10.7) | |
| Type of the MS | |||
| Relapsing remitting | 19 (67.8) | 20 (71.4) | 0.943 |
| Primary progressive | 3 (10.7) | 3 (10.7) | |
| Secondary progressive | 6 (21.4) | 5 (17.8) | |
| Drug treatments | |||
| Beta interferon | 19 (67.8) | 21 (75) | 0.822 |
| Fingolimod | 6 (21.4) | 5 (17.8) | |
| Rituximab | 3 (10.7) | 2 (7.1) | |
| Dominant presentation | |||
| Motor symptoms | 13 (46.4) | 10 (35.7) | 0.883 |
| Sensory symptoms | 4 (14.2) | 5 (17.85) | |
| Brainstem symptoms | 6 (21.4) | 6 (21.4) | |
| Sphincteric symptoms | 3 (10.7) | 3 (10.7) | |
| Ocular symptoms | 2 (7.14) | 4 (14.2) |
MS=Multiple sclerosis
The studied population pretests showed significant difference between cases and controls regarding physical (P = 0.011) and mental health (P = 0.014), while the other entities including EMQ (P = 0.994), PRMQ (P = 0.568), digit span test (P = 0.705), and depression were not statistically different (P = 0.062) [Table 2]. Table 2 presents information about patients' cognitive status over 3 months after the intervention. As it is presented, patients in Group A showed improved status of everyday memory, prospective and retrospective memory, digit span test, physical and mental health, and eventually better rehabilitation of their depression within 3 months after cognitive rehabilitation therapy (P < 0.05). In contrast, individuals in Group B experienced deterioration of everyday memory and prospective and retrospective memory, although statistically nonsignificant (P > 0.05).
Table 2.
Comparison of pretests-posttests derived from patients of two groups
| Variable | Control | Cognitive rehabilitation | Comparing two groups (before treatment) | ||||
|---|---|---|---|---|---|---|---|
| Mean±SD | P* | Mean±SD | P* | ||||
| Before the intervention | After the intervention | Before the intervention | After the intervention | ||||
| Everyday memory | 109.07±46.39 | 112.57±41.14 | 0.76 | 126.86±49.39 | 92.93±44.29 | <0.001 | 0.994 |
| Prospective and retrospective memory | 42.86±9.70 | 45.57±7.73 | 0.06 | 49.07±9.11 | 36.11±9.76 | <0.001 | 0.568 |
| Digit span memory | 12±2.62 | 11.54±2.41 | 0.23 | 10.14±3.54 | 12±2.95 | <0.001 | 0.705 |
| Physical health | 58.42±12.40 | 56.25±12.09 | 0.38 | 59.46±15.92 | 66.93±15.59 | <0.001 | 0.011 |
| Mental health | 52.18±12.70 | 50.90±15.32 | 0.75 | 50.53±17.09 | 67.77±15.12 | <0.001 | 0.014 |
| Depression | 20.89±6.59 | 20.64±5.69 | 0.83 | 20.8±6.59 | 11±6.86 | <0.001 | 0.062 |
*Paired sample t-test to compare before and after treatment. SD=Standard deviation
To assess the efficacy of the intervention, the multivariate covariance test has been used. In this term, the six posttest assessed variables were considered as the vectors of response variables, and the six pretest assessed variables were considered as the predictive variables. The outcomes of the spherical test were assessed using the Box test; therefore, the indices of the test, F = 1.235 and P = 0.210 showed no confounding role of sphericity. Besides, considering the Wilk's Lambda test, the intervention had a significant effect (F = 12.576, and Sig = 0.001), representing the significance of the intervention on only one of the response variables.
Thus, we found that cognitive rehabilitation was in direct association with improvement in cognitive statuses including everyday memory, prospective and retrospective memory, digit span test as an assessment of attention, physical, and mental health and moreover caused a significant improvement of depression. These findings are presented in Table 3.
Table 3.
Analysis of covariance for evaluating the effectiveness of cognitive rehabilitation on multiple sclerosis patients
| Source | Type III sum of squares | df | Mean square | F | Significant |
|---|---|---|---|---|---|
| Intervention | |||||
| Depression | 1010.968 | 1 | 1010.968 | 36.643 | 0.000 |
| PRMQ | 1932.555 | 1 | 1932.555 | 47.286 | 0.000 |
| EMQ | 10959.553 | 1 | 10959.553 | 7.255 | 0.010 |
| Digit span test | 36.195 | 1 | 36.195 | 11.944 | 0.001 |
| Physical health | 1285.000 | 1 | 1285.000 | 3.631 | 0.063 |
| Mental health | 4695.908 | 1 | 4695.908 | 3.267 | 0.077 |
| Error | |||||
| Depression | 1324.309 | 48 | 27.590 | ||
| PRMQ | 1961.730 | 48 | 40.869 | ||
| EMQ | 72513.120 | 48 | 1510.690 | ||
| Digit span test | 145.457 | 48 | 3.030 | ||
| Physical health | 16987.956 | 48 | 353.916 | ||
| Mental health | 68988.040 | 48 | 1437.251 |
PRMQ=Prospective and Retrospective Memory Questionnaire; EMQ=Everyday Memory Questionnaire
DISCUSSION
It has been well established that MS causes cognitive, motor, and behavioral disorders through the formation of inflammatory destructive sclerotic plaques in CNS. Based on previous findings, 50%–80% of MS patients would experience significant disabilities within a decade after their disease initiation. Of those, cognitive dysfunction plays a crucial role in the occurrence of disabilities in this vulnerable population.[22]
Although MS patients commonly experience cognitive impairment, this dysfunction cannot be easily diagnosed through routine neurological examinations. That is while cognitive impairment significantly disrupts individual and social functioning of patients.[23] From a physiologic point of view, memory impairment is mainly in association with the abnormality of the temporal lobe of the brain while other aspects of cognitive dysfunction (including information processing, attention, and concentration) are mainly attributed to the frontal region. Size and location of lesions may be responsible for memory dysfunction as well.[24] For instance, Rao et al.[25] and Swirsky-Sacchetti et al.[26] in two different studies presented that ventricular-brain ratio and corpus callosum size are directly in association with entities including verbal intelligence, attention, conceptual reasoning, and concentration.
Moreover, MS patients usually suffer from attention deficit as well as decreased processing velocity.[27] On the other hand, depression is known as the most common psychological disorders seen in MS. The chronic and disabling nature of the disease is recognized to be responsible for this finding, although brain lesions may also play a role by affecting self-esteem and confidence based on the damaged areas.[28]
Chiaravalloti et al. showed that autobiography memory would be affected by MS negatively. In that study, cognitive rehabilitation lead to alterations in the improvement of autobiography memory.[29] Further study by Raskin and Sohlberg showed similar results considering patents' memorial aspects.[30] Results from both studies are also comparable to ours.
Although the association of depression with neurological disorders has not been well established, the direct association of depression with memory, training, and ability of planning is determined. Depression affects cognitive function negatively, and treatment of depression among MS patients has been associated with better cognitive performance.[31] Furthermore, improvement of cognitive entities including attention, concentration, and information processing has reduced the anxiety of MS patients and thus has led to pleasure and increased confidence.[32] This study showed that cognitive rehabilitation could improve both depression and cognitive performance of MS patients, which is in line with previous reports.
With regard to the QOL, MS patients are usually affected significantly. The underlying reasons for decreased QOL include the progressive nature of MS, long duration of the disease, physical disabilities, fatigue, and depression.[33] In the current study, we observed significant improvement of patients' QOL following cognitive rehabilitation in comparison to control group. These findings were achieved in both physical and mental subscales of QOL.
Different therapeutic techniques have been suggested to treat, control, or prevent cognitive dysfunction. Medical therapy,[34] compensatory interventions,[35] cognitive rehabilitation,[36] and computerized cognitive rehabilitation[37] are the suggested interventions. We found that cognitive rehabilitation could improve various aspects of cognitive dysfunction. This outcome was achieved through classes in which we tried to make patients compensate their missed abilities by reinforcement of remained ones and also trying to develop their remained abilities, which could improve their self-esteem and confidence. Furthermore, QOL and depression improved significantly after cognitive rehabilitation approach. O'Brien et al. reviewed the effect of cognitive rehabilitation at the earliest days of its introduction and concluded that it is not able to change patients' cognitive performance considerably.[38]
In contrast, Birnboim and Miller found that cognitive rehabilitation improves cognitive performance.[39] Similarly, Mattioli et al. showed that 3-month computerized intensive cognitive rehabilitation could considerably improve attention, executing performance, and information processing among patients with relapsing-remitting MS. Furthermore, patients reported lower levels of depression after the intervention.[40] Our findings comply with most of the previous studies in this field.
CONCLUSION
Here, we found that ten sessions of cognitive rehabilitation could significantly improve cognitive performance in MS patients. Moreover, our approach affected their QOL and depression positively. It can be concluded that cognitive rehabilitation can successfully affect numerous cognitive and psychological aspects of MS patients and should be utilized more among these cases. Further evaluation of this issue is strongly recommended.
Financial support and sponsorship
Isfahan University of Medical Sciences sponsored this study.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors of this study offer their most appreciations toward nurses and officials of Kashani Hospital.
REFERENCES
- 1.Ascherio A, Munger KL, editors . Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention an Update Seminars in Neurology. Boston, Massachusetts: Thieme Medical Publishers; 2016. [DOI] [PubMed] [Google Scholar]
- 2.Cotter J, Vithanage N, Colville S, Lyle D, Cranley D, Cormack F, et al. Investigating domain-specific cognitive impairment among patients with multiple sclerosis using touchscreen cognitive testing in routine clinical care. Front Neurol. 2018;9:331. doi: 10.3389/fneur.2018.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rocca MA, Amato MP, De Stefano N, Enzinger C, Geurts JJ, Penner IK, et al. Clinical and imaging assessment of cognitive dysfunction in multiple sclerosis. Lancet Neurol. 2015;14:302–17. doi: 10.1016/S1474-4422(14)70250-9. [DOI] [PubMed] [Google Scholar]
- 4.Covey TJ, Shucard JL, Shucard DW. Event-related brain potential indices of cognitive function and brain resource reallocation during working memory in patients with multiple sclerosis. Clin Neurophysiol. 2017;128:604–21. doi: 10.1016/j.clinph.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 5.Lex H, Weisenbach S, Sloane J, Syed S, Rasky E, Freidl W. Social-emotional aspects of quality of life in multiple sclerosis (P4.417) AAN Enterp. 2018;23:411–23. doi: 10.1080/13548506.2017.1385818. [DOI] [PubMed] [Google Scholar]
- 6.Goverover Y, Chiaravalloti N, Genova H, DeLuca J. A randomized controlled trial to treat impaired learning and memory in multiple sclerosis: The self-GEN trial. Mult Scler. 2018;24:1096–104. doi: 10.1177/1352458517709955. [DOI] [PubMed] [Google Scholar]
- 7.Planche V, Moisset X, Morello R, Dumont E, Gibelin M, Charré-Morin J, et al. Improvement of quality of life and its relationship with neuropsychiatric outcomes in patients with multiple sclerosis starting treatment with natalizumab: A 3-year follow-up multicentric study. J Neurol Sci. 2017;382:148–54. doi: 10.1016/j.jns.2017.10.008. [DOI] [PubMed] [Google Scholar]
- 8.Calandri E, Graziano F, Borghi M, Bonino S. Improving the quality of life and psychological well-being of recently diagnosed multiple sclerosis patients: Preliminary evaluation of a group-based cognitive behavioral intervention. Disabil Rehabil. 2017;39:1474–81. doi: 10.1080/09638288.2016.1198430. [DOI] [PubMed] [Google Scholar]
- 9.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–52. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 10.Royle J, Lincoln NB. The everyday memory questionnaire-revised: Development of a 13-item scale. Disabil Rehabil. 2008;30:114–21. doi: 10.1080/09638280701223876. [DOI] [PubMed] [Google Scholar]
- 11.Ghassemzadeh H, Mojtabai R, Karamghadiri N, Ebrahimkhani N. Psychometric properties of a persian-language version of the beck depression inventory second edition: BDI-II-PERSIAN. Depress Anxiety. 2005;21:185–92. doi: 10.1002/da.20070. [DOI] [PubMed] [Google Scholar]
- 12.Borm GF, Fransen J, Lemmens WA. A simple sample size formula for analysis of covariance in randomized clinical trials. J Clin Epidemiol. 2007;60:1234–8. doi: 10.1016/j.jclinepi.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 13.Bakhtiyari F, Foroughan M, Fakhrzadeh H, Nazari N, Najafi B, Alizadeh M, et al. Validation of the persian version of abbreviated mental test (AMT) in elderly residents of kahrizak charity foundation. Iran J Diabetes Metab. 2014;13:487–94. [Google Scholar]
- 14.Zare H, Sahragard M, Khodamoradi S. Investigating of internal consistency and confirmatory factor analysis of prospective and retrospective memory in an Iranian sample. Iran J Cogn Educ. 2014;1:33–8. [Google Scholar]
- 15.Shaygannejad V, Janghorbani M, Ashtari F, Zanjani HA, Zakizade N. Effects of rivastigmine on memory and cognition in multiple sclerosis. Can J Neurol Sci. 2008;35:476–81. doi: 10.1017/s0317167100009148. [DOI] [PubMed] [Google Scholar]
- 16.Ghaem H, Borhani Haghighi A, Jafari P, Nikseresht AR. Validity and reliability of the persian version of the multiple sclerosis quality of life questionnaire. Neurol India. 2007;55:369–75. doi: 10.4103/0028-3886.33316. [DOI] [PubMed] [Google Scholar]
- 17.Efklides A, Yiultsi E, Kangellidou T, Kounti F, Dina F, Tsolaki M. Wechsler memory scale, rivermead behavioral memory test, and everyday memory questionnaire in healthy adults and Alzheimer's patients. Euro J Psychol Assess. 2002;18:63. [Google Scholar]
- 18.Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence-based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Arch Phys Med Rehabil. 2011;92:519–30. doi: 10.1016/j.apmr.2010.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Greenwood RJ, McMillan TM, Barnes MP, Ward CD. Handbook of Neurological Rehabilitation. Hove and New York: Psychology Press; 2005. [Google Scholar]
- 20.Wilson BA. Neuropsychological rehabilitation. Annu Rev Clin Psychol. 2008;4:141–62. doi: 10.1146/annurev.clinpsy.4.022007.141212. [DOI] [PubMed] [Google Scholar]
- 21.Wilson BA. Memory Rehabilitation: Integrating Theory and Practice The Guilford Press. New York and London: Guilford Press; 2009. [Google Scholar]
- 22.Cadden M, Arnett P. Factors associated with employment status in individuals with multiple sclerosis. Int J MS Care. 2015;17:284–91. doi: 10.7224/1537-2073.2014-057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Eijlers AJ, Meijer KA, van Geest Q, Geurts JJ, Schoonheim MM. Determinants of cognitive impairment in patients with multiple sclerosis with and without atrophy. Radiology. 2018;288:544–51. doi: 10.1148/radiol.2018172808. [DOI] [PubMed] [Google Scholar]
- 24.Crouch TA. Cognitive Functioning, Depression, and Strengths as Predictors of Quality of Life in Multiple Sclerosis. Dissertation. 2019 [Google Scholar]
- 25.Rao SM, Leo GJ, Haughton VM, St Aubin-Faubert P, Bernardin L. Correlation of magnetic resonance imaging with neuropsychological testing in multiple sclerosis. Neurology. 1989;39:161–6. doi: 10.1212/wnl.39.2.161. [DOI] [PubMed] [Google Scholar]
- 26.Swirsky-Sacchetti T, Mitchell DR, Seward J, Gonzales C, Lublin F, Knobler R, et al. Neuropsychological and structural brain lesions in multiple sclerosis: A regional analysis. Neurology. 1992;42:1291–5. doi: 10.1212/wnl.42.7.1291. [DOI] [PubMed] [Google Scholar]
- 27.Denney DR, Gallagher KS, Lynch SG. Deficits in processing speed in patients with multiple sclerosis: Evidence from explicit and covert measures. Arch Clin Neuropsychol. 2011;26:110–9. doi: 10.1093/arclin/acq104. [DOI] [PubMed] [Google Scholar]
- 28.Berzins SA, Bulloch AG, Burton JM, Dobson KS, Fick GH, Patten SB, et al. Determinants and incidence of depression in multiple sclerosis: A prospective cohort study. J Psychosom Res. 2017;99:169–76. doi: 10.1016/j.jpsychores.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Chiaravalloti ND, Genova HM, DeLuca J. Cognitive rehabilitation in multiple sclerosis: The role of plasticity. Front Neurol. 2015;6:67. doi: 10.3389/fneur.2015.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Raskin SA, Sohlberg MM. Prospective memory intervention: A review and evaluation of a pilot restorative intervention. Brain Impair. 2009;10:76–86. [Google Scholar]
- 31.Charboneau J. Cognitive Impairment, Depression, Anxiety, and Personality and MS Patient Estimations of Memory Function. Dissertations. 2017 [Google Scholar]
- 32.Klein OA, Drummond A, Mhizha-Murira JR, Mansford L, dasNair R. Effectiveness of cognitive rehabilitation for people with multiple sclerosis: a meta-synthesis of patient perspectives. Neuropsychol Rehabil. 2017;29:1–22. doi: 10.1080/09602011.2017.1309323. [DOI] [PubMed] [Google Scholar]
- 33.Chehreh-Negar N, Shams F, Zarshenas S, Nikseresht A. Correlation between working memory and quality of life in multiple sclerosis patients. Feyz J Kashan Univ Med Sci. 2012;16:337–45. [Google Scholar]
- 34.Christodoulou C, MacAllister WS, McLinskey NA, Krupp LB. Treatment of cognitive impairment in multiple sclerosis: Is the use of acetylcholinesterase inhibitors a viable option? CNS Drugs. 2008;22:87–97. doi: 10.2165/00023210-200822020-00001. [DOI] [PubMed] [Google Scholar]
- 35.Lincoln NB, Dent A, Harding J, Weyman N, Nicholl C, Blumhardt LD, et al. Evaluation of cognitive assessment and cognitive intervention for people with multiple sclerosis. J Neurol Neurosurg Psychiatry. 2002;72:93–8. doi: 10.1136/jnnp.72.1.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hubacher M, DeLuca J, Weber P, Steinlin M, Kappos L, Opwis K, et al. Cognitive rehabilitation of working memory in juvenile multiple sclerosis-effects on cognitive functioning, functional MRI and network related connectivity. Restor Neurol Neurosci. 2015;33:713–25. doi: 10.3233/RNN-150497. [DOI] [PubMed] [Google Scholar]
- 37.Pérez-Martín MY, González-Platas M, Eguía-Del Río P, Croissier-Elías C, Sosa AJ. Efficacy of a short cognitive training program in patients with multiple sclerosis. Neuropsychiatr Dis Treat. 2017;13:245–52. doi: 10.2147/NDT.S124448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.O'Brien AR, Chiaravalloti N, Goverover Y, Deluca J. Evidenced-based cognitive rehabilitation for persons with multiple sclerosis: A review of the literature. Arch Phys Med Rehabil. 2008;89:761–9. doi: 10.1016/j.apmr.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 39.Birnboim S, Miller A. Cognitive rehabilitation for multiple sclerosis patients with executive dysfunction. J Cogn Rehabil. 2004;22:8–11. [Google Scholar]
- 40.Mattioli F, Stampatori C, Zanotti D, Parrinello G, Capra R. Efficacy and specificity of intensive cognitive rehabilitation of attention and executive functions in multiple sclerosis. J Neurol Sci. 2010;288:101–5. doi: 10.1016/j.jns.2009.09.024. [DOI] [PubMed] [Google Scholar]


