Abstract
Background:
Lycium chinense fruit (LCF) is widely distributed in East Asia that has been used traditionally for antiaging purposes. This study was performed to examine the effects of LCF on attention and cognitive function in healthy young people.
Materials and Methods:
An 11-week, double-blind, randomized, placebo-controlled, crossover trial of 74 patients was conducted and its data were collected on Kyung-Hee University Korean Medical Hospital, Seoul, Korea. In crossover treatment, LCF or placebo was administered three times a day, total 3600 mg as two capsules of 600 mg once for 4 weeks with 3-week washout each. The computerized neurocognitive function test (CNT), the Korean version of the attention-deficit/hyperactivity disorder rating scale-IV, the clinical global impression rating scale, and the Frankfurt attention inventory (FAIR) for two groups were conducted 0 week before and 4 week, 11 week after the experiment, and significant mean changes of these tests for within group or two groups were measured by paired t-test or unpaired t-test.
Results:
The administration of LCF or placebo crossover for 8 weeks in healthy young people presented significant improvement in the verbal learning test, digit span forward test, digit span backward test, auditory continuous performance task of CNT, and FAIR-performance value compared with the placebo group (each group n = 43, P < 0.05).
Conclusion:
Thus, the consumption of LCF might be beneficial to increase learning and memory through attention and cognitive enhancing effect in normal young people, at an average age of 18 years of age.
Keywords: Attention, cognitive function, crossover trial, Lycium chinense, young adults
INTRODUCTION
Memory may be conceptualized into three phases of information manipulation: those processes that handle the encoding of, storage of, and drawing on learned and witnessed facts and events, which is encoded physiologically for humans.[1] Almost every aspect of behavior and cognition requires learning. Learning is connected to memory. Learning may be defined as the acquisition of new information, whereas memory is the capacity for storing and retrieving this material.[2] In addition, learning-related executive functions contain attentional control, inhibition, and working memory. It is known that these executive functions have a distinct role in academic performance.[3,4,5] Particularly, deficiency of working memory skills is closely related to problems in learning and poor classroom behavior.[6,7] Working memory presents the function of actively seizing in mind and adjusting information relevant to a goal.[8] Individuals with poor working memory functioning are at risk of poor educational progress, and over 85% of children with poor working memory have problems in reading or mathematics. Recently, studies have shown academic performance and achievement of children with attention-deficit/hyperactivity disorder (ADHD), and/or learning disabilities were significantly improved by training of their working memory.[9] However, such improving agents of working memory have not been developed so far.
There have been many studies to search memory or cognition increasing agents to enforce learning and memory.[10] Lycium chinense fruit (LCF) is another memory or cognition booster. LCF is widely spread in East Asia and has been in demand traditionally for aging prevention uses.[11] LCF includes abundant polysaccharides, such as Lycium barbarum polysaccharides (LBPs), comprising 5%–8% of the dried fruits, scopoletin (6-methoxy-7-hydroxycoumarin, also named chrysatropic acid, ecopoletin, gelseminic acid, and scopoletol), the glucosylated precursor, and stable Vitamin C analog 2-O-β-d-glucopyranosyl-l-ascorbic acid, carotenoids (zeaxanthin and β-carotene), betaine, cerebroside, β-sitosterol, flavonoids, amino acids, minerals, and vitamins (in particular, riboflavin, thiamin, and ascorbic acid).[12]
LCF has been used as a traditional Chinese medicine (TCM) to enforce the liver and kidney and light the eye. Among various components, a group of polysaccharides (LBP) with a Glycan-O-Ser glycopeptide structure and betaine have been most investigated and regarded to be distinct for the efficacy of LCF.[12,13] Studies presented effects of LCF on aging, neuroprotection, general well-being, fatigue/endurance, metabolism/energy expenditure, glucose regulation in diabetes mellitus, glaucoma, oxygen-free radical properties, immunomodulation, tumor inhibition, and cytoprotection.[14] In addition to TCM, LCF can be circulated as a dietary supplement or classified as a food based on the long and safe traditional use.[14] Recently, the neuroprotective effects of LCF have been published by Shi et al. and Zhang et al.,[15,16] and our results showed that LCF had a protective effect against trimethyltin (TMT)-induced neuronal and cognitive impairments in an experiment using the Morris water maze task and the choline acetyltransferase (ChAT) and cyclic adenosine monophosphate (cAMP) of rats with TMT-induced neuronal and cognitive impairments.[17] Furthermore, our recent results presented that LCF significantly ameliorated learning and memory deficits in AD mice, as shown by increased time spent in the target zone during probe tests. In addition, LCF significantly decreased amyloid-β (Aβ) deposits, increased NeuN-positive cells, and upregulated the expression of BDNF and TrkB in the 3x Tg AD mice.[18]
However, there have been few reported clinical studies or trials involving Alzheimer's disease (AD) patients and even normal individuals receiving treatment with LCF on attention and cognitive function. In our present study, the computerized neurocognitive function test (CNT), the Korean version of the ADHD, rating scale-IV (K-ADHD-RS-IV), the clinical global impression (CGI) rating scale, and the Frankfurt attention inventory (FAIR) were measured to assess the effect of LCF on attention and cognitive function in normal young human subjects, especially in teenagers with an average age of 18 years of age to prevent from earlier deficiency of attention and cognitive function in young adults.
MATERIALS AND METHODS
Subjects and study design
Participants were 14–24 years old, mostly teenagers with an average age of 18 years old; they were recruited via advertisements.
Entry criteria were the following: (a) age between 14 and 24 years old, (b) male or female, and (c) ability to understand the objectives of the study and agreed to abide by the required rules during the study. If participants were aged between 14 and 20 years old, they and their parents had to provide informed consent.
Exclusion criteria were the following: (a) diagnosis of ADHD (any subtype) according to the Diagnostic and Statistical Manual of Mental Disorders criteria, (b) diagnosis of a developmental disorder, (c) pregnant or breastfeeding women and women with the possibility of getting pregnant, (d) gastrointestinal disease or history of gastrointestinal surgery, which might affect the absorption of study materials, (e) significant neurological (epilepsy, mental retardation, or stroke) or medical illnesses (diabetes, hypertension, or cardiovascular diseases), (f) participation in other clinical studies during the 4 weeks preceding the start of the study, and (g) more than 1.5 times normal limit of alanine transaminase or aspartate transaminase.
If participants were aged between 14 and 20 years old, parents were initially screened by phone, and their children were included in the study if the inclusion criteria were met. Written informed consent was obtained from the participants.
This study was ethically approved by the Kyung-Hee Korean Medical Center Institutional Review Board (Approved number: KOMCGIRB-2013-88). This study was an 11-week, double-blind, randomized, placebo-controlled crossover experiment. The trial was conducted at Kyung-Hee University Korean Medical Hospital from November 1, 2011, to April 30, 2013. The trial was performed in accordance with the Declaration of Helsinki and Good Clinical Practice.[19] During the screening period, a physical examination, medical history, basic laboratory tests, psychiatric interview, and neuropsychological tests were conducted. In eligible individuals, data for cognitive tests and blood tests were obtained at baseline and after the 11-week treatment period. Participants were then randomly allocated to either the LCF (3600 mg) or the placebo condition.
The random assignment was performed using stratified blocked randomization and envelop method. In this, participating clinicians are given randomly generated treatment allocations within sealed opaque envelopes. Once a patient has consented to enter a trial, an envelope is opened, and the patient is then offered the allocated treatment regimen.[20] Subject numbers of placebo and LCF were 38 and 36 each initially, when each group randomly allocated [Figure 1]. However, placebo and LCF were switched to each other and administrated for another 4 week treatment after 4-week treatment and 3-week washout because of crossover trial [Figure 1]. A crossover trial is a trial design in which individuals function as their own control and are assigned to receive the investigational product and controls in an order determined by randomizations, typically with a washout period between the two products. In analysis, data of 43 participants of placebo or LCF except lost to follow-up were measured. Participants were told to not use any supplements or herbal medicine during the course of the study, abstain from alcohol consumption, refrain from vigorous exercise, and get sufficient sleep one day before the baseline and endpoint. Compliance was checked at each visit. This trial was registered at ClinicalTrials.gov (NCT number 03439111; www.clinicaltrials.gov).
Figure 1.
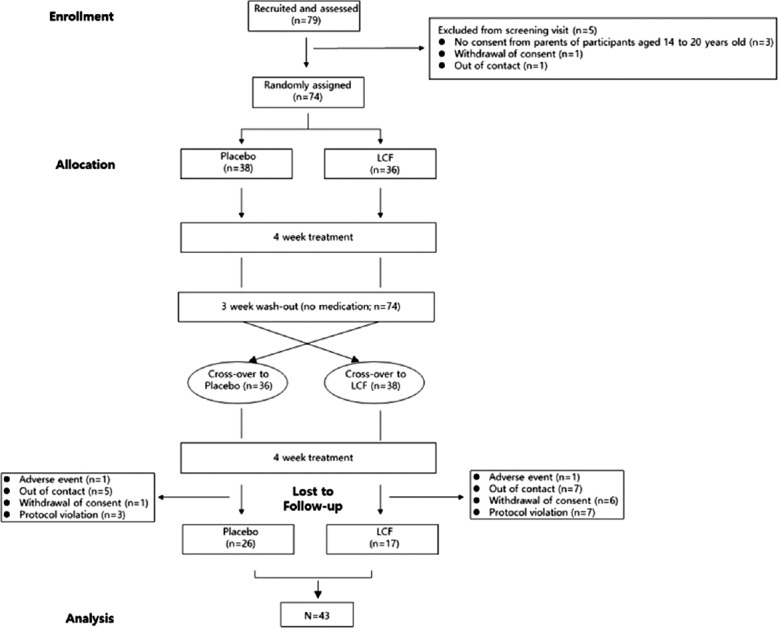
Flow chart of the 11-week procedure and randomization of double-blind, placebo-controlled crossover study, with all individuals receiving both treatments. n = Group size
Preparation and administration of Lycium chinense fruit
All dried water extracts and capsules of the LCF and LCF-matched placebo capsules used in the present study were manufactured and provided by the BIOMIX Company (Ilsan City, Korea). The fruit of Lycium chinensis Miller was collected in Cheongyang-gun, Republic of Korea and provided by Chungcheongnam-do Agricultural Research and Extension Services. The samples were authenticated by Professor Hyunsu Bae and deposited with Herbal Voucher No 342 (KHUKMP) at Department of Physiology, College of Korean Medicine, Kyung-Hee University. The dried fruits were extracted with distilled water for 3 h at 100°C by reflux extraction method. The extract was filtered using Whatman filter paper no. 2 (Toyo Rushi Kaisha. Ltd, Japan), evaporated in a rotary evaporator, and lypophilized. The powder obtained (LCF, yield 40%) was kept at −20°C until use.[18]
For study, we used capsules of batch number 11,001 (exp. 10/31/2013). 600 mg of the LCF capsule was composed of 384 mg of LCF dried water extract whereas six hundred milligrams of the LCF-matched placebo capsule was only composed of starch. Placebo capsules were made using the same capsule preparation method without LCF. LCF-matched placebo capsules were identical to LCF capsules in color, shape, and size.
The LCF capsule was standardized based on the amount of betaine determined by high-performance liquid chromatography-evaporative light scattering detection. By considering the amount of betaine in LCF capsule, the calibration curve was composed of five different concentration levels ranging from 12.4 to 296.5 μg/ml.
According to quantitative analysis, the concentration of 3.41 mg/g of betaine was included in the LCF capsule. Individuals in the LCF treatment group received two opaque capsules, each containing 384 mg of LCF, three times a day after breakfast, lunch, and dinner for 8 weeks (56 days). Individuals in the placebo group received the equivalent amount of starch capsules and administered with the same schedule. An effective day was defined as a day in which more than four LCF or placebo capsules were administered per person. The results from participants experiencing 39 effective days (70% of 56 days) were used for the final analysis.
Measurements
Those measurements were performed at weeks 0, 4, and 11.
The computerized neurocognitive function test
The CNT was used at weeks 0, 4, and 11. CNT is extremely sensitive to virtually all the clinical conditions associated with cognitive dysfunction. It is capable of calculating reaction times with millisecond accuracy and can generate massive amounts of precise data.[21]
Korean version of attention-deficit/hyperactivity disorder rating scale-IV
The K-ADHD-RS is an 18-item standardized, valid, reliable instrument for the diagnosis of ADHD and is also a weekly assessment tool for checking the treatment response in children and adolescents with ADHD. Each item in the instrument describes one of the symptoms of ADHD rated on a three-point Likert scale (0 = never or rarely, 1 = sometimes, 2 = often, and 3 = very often).[21]
The clinical global impression rating scale
The CGI rating scale is also a commonly used measure of symptom severity, treatment response, and the efficacy of treatments in treatment studies of patients with mental disorders.[21]
The Frankfurt attention inventory
The FAIR measures concentration behavior and consists of 640 stimuli with high similarity that has to be discriminated against each other within 6 min.
Statistical analysis
The results are presented as the mean values ± standard deviation of the two groups, and statistical significance was determined using paired t-test for changes in CNT, K-ADHD-RS-IV, CGI, and FAIR by trial period within each group. Changes in mean differences of measurements during the 11-week study period were obtained by calculating the difference between the pre- and post-intervention measurements for each group. The statistical significance of these mean differences between LCF and placebo was measured by unpaired t-test. Results with P < 0.05 were considered as statistically significant.
Sample size calculation
The sample size calculation was based on an α-level type I error of 0.05 for a two tailed test, a β-level type II error of 0.2, and a statistical power of 80%. Following research on neurocognitive effect of dietary S-allyl-L-cysteine in patients with mild cognitive impairment, the digit span forward score of CNT, and considering a difference of 0.18 as clinically significant (estimated mean standard error, 0.14), compared to placebo, the sample size was calculated as 16 participants in each group. A dropout rate of 20% was assumed, and at least 20 participants were enrolled in each group.[22]
RESULTS
Participant flow
A total of 79 participants were recruited. Seventy-four were eligible and randomized to either the placebo or LCF group. Of the 74 test individuals who were enrolled and allocated in the present study, 43 (58.1%) completed the 11-week trial, including 17/38 individuals in the initial placebo group (44.7%) and 26/36 individuals in the initial LCF group (72.2%). The participants underwent their initial treatments and underwent their crossover treatments after 3 weeks. Most of the participants who dropped out did so because of personal reasons, and two participants were omitted due to adverse events, enteritis in the placebo group, and a menstrual irregularity in the LCF group [Figure 1]. The total number of samples was 86 because 43 participants had treatments with both LCF and placebo [Figure 1].
Baseline characteristics
The key baseline characteristics of the study participants (n = 43) who completed the two 4-week intervention periods (placebo and LCF) of this double-blind, randomized crossover study are presented in Table 1.
Table 1.
Selected baseline characteristics of the study participants (n=43) who completed both the two 4-week intervention periods (Placebo and Lycium chinense fruit) of this double-blind, randomized, crossover study
| Parameters | Total (n=43) |
|---|---|
| Age (year) | 19.52±2.29 |
| Gender, n (%) | |
| Male | 14 (32.6) |
| Female | 29 (67.4) |
| Education (year) | 11.43±1.32 |
Data are presented as means±SD, n (%). SD=Standard deviation
Efficacy results
Figures 2 and 3 present the efficacy outcome variables by visits and group, explaining only the data of individuals who finished the trial (n = 43, respectively, for LCF group and placebo group). After 11 weeks, there were significant differences compared with baseline in the following parameters: (1) verbal learning test (VLT) and digit span forward test of CNT, (2) CGI-S and I of CGI as well as (3) FAIR-performance value (FAIR-PV) and continuity value (CV) of FAIR in the LCF group; however, in the placebo group, only the parameter (2) and (3) were statistically significant. However, FAIR-PV and CV of FAIR in the LCF group were more significant than the placebo group after the 11-week trial.
Figure 2.
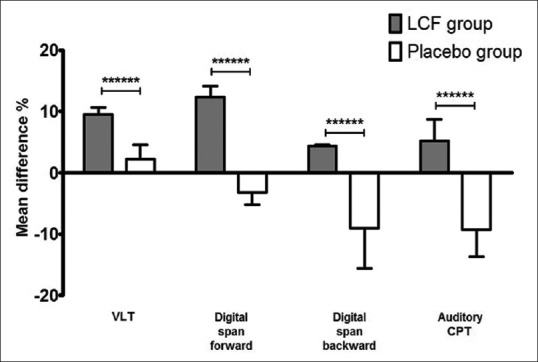
Mean differences of verbal learning test, digit span forward and backward tests, and auditory CPT of CNT test significant in LCF and placebo groups after 11-week treatment. Histogram of verbal learning test, digit span forward and backward tests, and auditory CPT of CNT test is shown. Results are presented as mean ± standard deviation. Mean difference percent were obtained from the mean value percent change of each score before and after the 11-week treatment. P value represents the difference between LCF and placebo (unpaired t-test, ******P < 0.0001). n = 43 for each group. CPT = Continuous performance task; CNT = Computerized neurocognitive function test; LCF = Lycium chinense fruit
Figure 3.
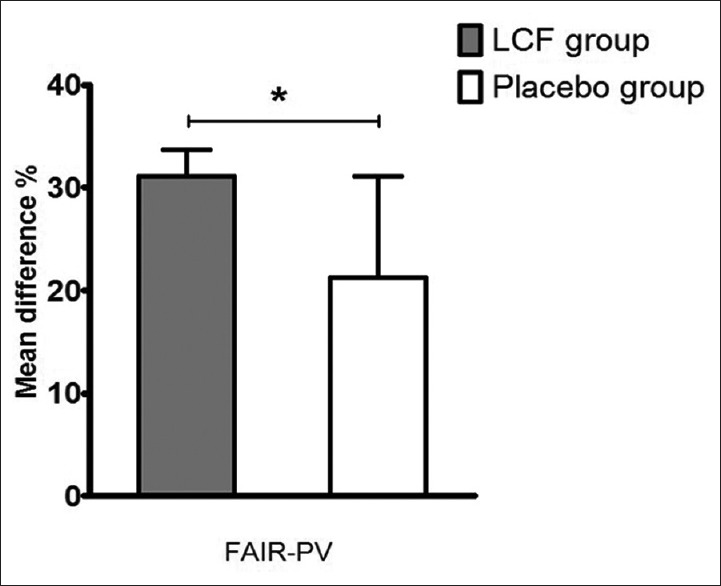
Mean differences of FAIR-PV significant in LCF and placebo groups after 11-week treatment. Histogram of FAIR-PV is shown. Results are presented as mean ± standard deviation. Mean difference percent were obtained from the mean value percent change of each score before and after the 11-week treatment. P value represents the difference between LCF and placebo (unpaired t-test, *P < 0.05). n = 43 for each group. LCF = Lycium chinense fruit
The following measures demonstrated significant mean differences from baseline to week 11: the VLT [mean difference percent in the LCF group was approximately four times higher than that of the placebo group, 9.54% ± 1.11% vs. 2.29% ± 2.24%, n = 43 for each group, Figure 2]; digit span forward test [mean difference percent in the LCF group was approximately fifteen times higher than that of the placebo group, 12.33% ± 1.80% vs. −3.19% ± 1.98%, n = 43 for each group, Figure 2]; digit span backward test [mean difference percent in the LCF group was approximately fourteen times higher than that of the placebo group, 4.42% ± 0.15% vs. −9.03% ± 6.56%, n = 43 for each group, Figure 2]; auditory continuous performance task (CPT) of CNT [mean difference percent in the LCF group was approximately fourteen times higher than that of the placebo group, 5.16% ± 3.54% vs. −9.28% ± 4.39%, n = 43 for each group, Figure 2]; and FAIR-PV (mean difference percent in the LCF group was approximately 1.5 times higher than that of the placebo group, 31.08% ± 2.60% vs. 21.22% ± 9.90%, n = 43 for each group) of the FAIR [Figure 3].
DISCUSSION
This study was performed to examine the effects of LCF on attention and cognitive function in healthy young people as an 11-week, double-blind, randomized, placebo-controlled, crossover trial. The administration of LCF or placebo crossover for 8 weeks in healthy young people presented significant improvement in the VLT, digit span forward test, digit span backward test, auditory CPT of CNT, and FAIR-PV compared with the placebo group (P < 0.05). The consumption of LCF might be beneficial to increase learning and memory through attention and cognitive-enhancing effect in normal young people. Our previous results presented a protective effect against TMT-induced neuronal and cognitive impairments in an experiment using the Morris water maze task and the ChAT and cAMP of rats with TMT-induced neuronal and cognitive impairments.[17] Positive resolving effects of LBPs were also shown on memory and neurogenesis impairments in scopolamine-treated rats.[23] In the other hand, Lycium barbarum water extract decreased escape latency time in the Morris water maze test. In addition, the levels of acetylcholine and ChAT were significantly increased in the serum and hypothalamus in a mouse model of AD, induced by the combination of AlCl3 and Dgalactose.[24] Furthermore, our recent results presented that LCF significantly ameliorated learning and memory deficits in AD mice, as shown by increased time spent in the target zone during probe tests. In addition, LCF significantly decreased Aβ deposits, increased NeuN-positive cells, and upregulated the expression of BDNF and TrkB in the 3x Tg AD mice.[18] Based on this evidence, this study was planned to investigate the effects of LCF on attention and cognitive function in healthy young people, especially teenagers with an average age of 18 years to prevent from earlier deficiency of attention and cognitive function in young adults.
In the CNT, LCF significantly enhanced the scores of the VLT, digit span forward or backward test, and auditory CPT compared with placebo [Figure 2]. CNTs are able to measure mild degrees of cognitive impairment and can investigate the effectiveness of a treatment. Among CNT, the VLT is a clinical assessment of verbal learning and memory that represents relevance to measuring the effects of numerous neurological conditions, including ADHD, and the digit span task is used to assess working memory's number storage capacity.[21] A continuous performance task, or CPT, is any of several types of neuropsychological tests that assesses a person's sustained and selective attention.
There are various CPTs, and the commonly used measurements are the Integrated Visual and Auditory CPT (IVA-2), Test of Variables of Attention, and the Conners' CPT-II. These attention assessments are often employed as part of a battery of measurements to comprehend a person's executive functioning or their capacity to classify and keep information.[12] Such enhanced scores of VLT, digit span forward or backward test, and auditory CPT with treatment of LCF might help normal young people attain better verbal learning and memory, and working memory's number storage capacity which are reflections of cognitive function.
In the FAIR measurement, LCF treatment was closely related to a significant increase of FAIR-PV compared with the control group [Figure 3]. The FAIR investigates attention as an ability to focus (i.e., accurately and quickly distinguish visually resembling signs while ignoring irrelevant information). PV assesses the amount of attentively processed test items during a defined test period, and the CV reflects the extent of continuously upheld concentration during the entire test.[12] Thus, LCF may help normal young people obtain better concentration by increasing scores of FAIR, especially for FAIR-PV, which increases the amount of attention processing during a defined period.
Executive functions contain attentional control, inhibition, and working memory. It is known that these executive functions have a distinct role in academic performance.[3,4,5] Particularly, deficiency of working memory skills is closely related to problems in learning and poor classroom behavior.[6,7] Working memory presents the function of actively seizing in mind and adjusting information relevant to a goal.[8] Individuals with poor working memory functioning are at risk of poor educational progress, and over 85% of children with poor working memory have problems in reading or mathematics. Recently, studies have shown academic performance and achievement of children with ADHD and/or learning disabilities were significantly improved by training of their working memory.[9]
Thus, collectively, LCF may help normal young people obtain better learning ability and academic achievement by enhancing working memory and attention and cognitive function, resulting in increased executive functions.
It is reported that LCF has its rich sources of various nutraceuticals and phytochemicals, such as organic acids, sugars, and phenolic compounds. Among those constituents, betaine is distinct component of LCF.[25] It was known that betaine restores neuronal damage due to homocysteine-induced AD such as pathological cascade, including tau hyperphosphorylation and Aβ deposition and decreased Aβ levels, by changing APP processing in N2a cells presenting the Swedish mutant of APP.[26] Furthermore, supplementation of betaine reduced the homocysteine-induced memory deficits in rats, and its resolving mechanism was ascribed to the Hcy-induced tau hyperphosphorylation at multiple AD-related sites through activation protein phosphatase-2A (PP2A) with decreased inhibitory demethylated PP2AC at Leu309 and phosphorylated PP2AC at Tyr307 and decreased Aβ production with decreased presenilin-1 protein levels. It is also found that supplementation of betaine ameliorated the Hcy-induced memory deficits, enhanced long-term potentiation, and increased dendritic branches numbers and the density of the dendritic spines, with upregulation of NR1, NR2A, synaptotagmin, synaptophysin, and phosphorylated synapsin I protein levels.[27] Furthermore, such memory deficit resolving effect and its mechanism were demonstrated by a clinical trial. In that clinical trial, betaine decreased the levels of phosphorylated tau, elevated PP2Ac activity, inhibited Aβ accumulation, effectively suppressed inflammation as well as trigger an increase in memory-related proteins and increased cognitive function significantly in AD patients.[28] The amounts of betaine for a day administration (3, 6, 12 mg/60 kg human/day) were fitted to our study (7.86 mg betaine/LCF 2,304 mg/day). Based on these studies, it is speculated that betaine of LCF might help normal young people obtain better learning ability and academic achievement by enhancing working memory and attention and cognitive function, resulting in increased executive function by reduction of homocysteine, inhibitions of tau hyperphosphorylation and Aβ accumulation, and suppression of inflammation as well as induction of memory-related proteins. However, further studies should be performed to further elucidate the reduction of homocysteine, inhibitions of tau hyperphosphorylation and Aβ accumulation, and suppression of inflammation as well as induction of memory-related proteins of LCF in clinical trial. This study has some limitations and strengths. This study has some strengths. This study was an well-planned clinical trial based on double-blind, randomized, placebo-controlled, crossover trial to prevent any possible bias and used good presenting measurements for attention and cognitive function, CNT, K-ADHD-RS-IV, CGI, and FAIR, different from previous measurements for attention and cognitive function. However, this study has some limitations, which are no control of nutritional and social environments, not enough patient numbers and no biomarkers in this clinical trial. Furthermore, we did not measure carryover effect and the effect of time and intervention for crossover trial. We will perform study overcoming those limitations in the next study.
CONCLUSION
This study was performed to examine the effects of LCF on attention and cognitive function in healthy young people. An 11-week, double-blind, randomized, placebo-controlled, crossover trial was conducted. Cognitive functions were assessed using various cognitive scale tests. The administration of LCF presented significant improvement in most cognitive parameters compared with the placebo group. Thus, the consumption of LCF might be beneficial to increase learning and memory through attention and cognitive enhancing effect in normal young people of an average of 18 year old through presumably reduction of homocysteine, inhibitions of tau hyperphosphorylation and Aβ accumulation, and suppression of inflammation as well as induction of memory-related proteins by possibly betaine, LCF major component.
Financial support and sponsorship
This work was supported by the Technology development R&D program (S1079170) funded by the Small and Medium Business Administration (SMBA, Korea).
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Izquierdo I, Medina JH, Izquierdo LA, Barros DM, de Souza MM, Mello e Souza T. Short – And long-term memory are differentially regulated by monoaminergic systems in the rat brain. Neurobiol Learn Mem. 1998;69:219–24. doi: 10.1006/nlme.1998.3825. [DOI] [PubMed] [Google Scholar]
- 2.Karilampi U, Helldin L, Hjärthag F, Norlander T, Archer T. Verbal learning in schizopsychotic outpatients and healthy volunteers as a function of cognitive performance levels. Arch Clin Neuropsychol. 2007;22:161–74. doi: 10.1016/j.acn.2006.12.003. [DOI] [PubMed] [Google Scholar]
- 3.Barkley RA. Behavioral inhibition, sustained attention, and executive functions: Constructing a unifying theory of ADHD. Psychol Bull. 1997;121:65–94. doi: 10.1037/0033-2909.121.1.65. [DOI] [PubMed] [Google Scholar]
- 4.Willcutt EG, Doyle AE, Nigg JT, Faraone SV, Pennington BF. Validity of the executive function theory of attention-deficit/hyperactivity disorder: A meta-analytic review. Biol Psychiatry. 2005;57:1336–46. doi: 10.1016/j.biopsych.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 5.Bull R, Scerif G. Executive functioning as a predictor of children's mathematics ability: Inhibition, switching, and working memory. Dev Neuropsychol. 2001;19:273–93. doi: 10.1207/S15326942DN1903_3. [DOI] [PubMed] [Google Scholar]
- 6.Aronen ET, Vuontela V, Steenari MR, Salmi J, Carlson S. Working memory, psychiatric symptoms, and academic performance at school. Neurobiol Learn Mem. 2005;83:33–42. doi: 10.1016/j.nlm.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 7.Gathercole SE, Pickering SJ. Working memory deficits in children with low achievements in the national curriculum at 7 years of age. Br J Educ Psychol. 2000;70(Pt 2):177–94. doi: 10.1348/000709900158047. [DOI] [PubMed] [Google Scholar]
- 8.Baddeley A. The fractionation of working memory. Proc Natl Acad Sci U S A. 1996;93:13468–72. doi: 10.1073/pnas.93.24.13468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Donk ML, Hiemstra-Beernink AC, Tjeenk-Kalff AC, van der Leij AV, Lindauer RJ. Interventions to improve executive functioning and working memory in school-aged children with AD (H) D: A randomised controlled trial and stepped-care approach. BMC Psychiatry. 2013;13:23. doi: 10.1186/1471-244X-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tyagi A, Delanty N. Herbal remedies, dietary supplements, and seizures. Epilepsia. 2003;44:228–35. doi: 10.1046/j.1528-1157.2003.19902.x. [DOI] [PubMed] [Google Scholar]
- 11.Jin M, Huang Q, Zhao K, Shang P. Biological activities and potential health benefit effects of polysaccharides isolated from Lycium barbarum L. Int J Biol Macromol. 2013;54:16–23. doi: 10.1016/j.ijbiomac.2012.11.023. [DOI] [PubMed] [Google Scholar]
- 12.Gao Y, Wei Y, Wang Y, Gao F, Chen Z. Lycium barbarum: A traditional Chinese herb and A promising anti-aging agent. Aging Dis. 2017;8:778–91. doi: 10.14336/AD.2017.0725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ibi D, Tsuchihashi A, Nomura T, Hiramatsu M. Involvement of GAT2/BGT-1 in the preventive effects of betaine on cognitive impairment and brain oxidative stress in amyloid β peptide-injected mice. Eur J Pharmacol. 2019;842:57–63. doi: 10.1016/j.ejphar.2018.10.037. [DOI] [PubMed] [Google Scholar]
- 14.Yao R, Heinrich M, Weckerle CS. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J Ethnopharmacol. 2018;212:50–66. doi: 10.1016/j.jep.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Shi Z, Wu D, Yao JP, Yao X, Huang Z, Li P, et al. Protection against oxygen-glucose deprivation/Reperfusion injury in cortical neurons by combining omega-3 polyunsaturated acid with Lycium barbarum polysaccharide. Nutrients. 2016;8 doi: 10.3390/nu8010041. pii: E41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Q, DU X, Xu Y, Dang L, Xiang L, Zhang J, et al. The effects of gouqi extracts on morris maze learning in the APP/PS1 double transgenic mouse model of Alzheimer's disease. Exp Ther Med. 2013;5:1528–30. doi: 10.3892/etm.2013.1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park HJ, Shim HS, Choi WK, Kim KS, Bae H, Shim I, et al. Neuroprotective effect of Lucium chinense fruit on trimethyltin-induced learning and memory deficits in the rats. Exp Neurobiol. 2011;20:137–43. doi: 10.5607/en.2011.20.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ye M, Moon J, Yang J, Hwa Lim H, Bin Hong S, Shim I, et al. The standardized Lycium chinense fruit extract protects against Alzheimer's disease in 3xTg-AD mice. J Ethnopharmacol. 2015;172:85–90. doi: 10.1016/j.jep.2015.06.026. [DOI] [PubMed] [Google Scholar]
- 19.World Medical Association. World medical association declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Organ. 2001;79:373–4. [PMC free article] [PubMed] [Google Scholar]
- 20.Beller EM, Gebski V, Keech AC. Randomisation in clinical trials. Med J Aust. 2002;177:565–7. doi: 10.5694/j.1326-5377.2002.tb04955.x. [DOI] [PubMed] [Google Scholar]
- 21.Hong SS, Cho SH. Acupuncture for attention deficit hyperactivity disorder (ADHD): Study protocol for a randomised controlled trial. Trials. 2011;12:173. doi: 10.1186/1745-6215-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shin A, Kim S, Chun HS, Kim JM, Chang N. Neurocognitive effect of dietary S-allyl-L-cysteine in patients with mild cognitive impairment (MCI) Faseb J. 2006;20(Suppl I):A186. [Google Scholar]
- 23.Chen W, Cheng X, Chen J, Yi X, Nie D, Sun X, et al. Lycium barbarum polysaccharides prevent memory and neurogenesis impairments in scopolamine-treated rats. PLoS One. 2014;9:e88076. doi: 10.1371/journal.pone.0088076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu X, Qu Y, Chu Q, Li W, He J. Investigation of the neuroprotective effects of Lycium barbarum water extract in apoptotic cells and Alzheimer's disease mice. Mol Med Rep. 2018;17:3599–606. doi: 10.3892/mmr.2017.8310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Potterat O. Goji (Lycium barbarum and L. chinense): Phytochemistry, pharmacology and safety in the perspective of traditional uses and recent popularity. Planta Med. 2010;76:7–19. doi: 10.1055/s-0029-1186218. [DOI] [PubMed] [Google Scholar]
- 26.Liu XP, Qian X, Xie Y, Qi Y, Peng MF, Zhan BC, et al. Betaine suppressed aβ generation by altering amyloid precursor protein processing. Neurol Sci. 2014;35:1009–13. doi: 10.1007/s10072-014-1630-y. [DOI] [PubMed] [Google Scholar]
- 27.Chai GS, Jiang X, Ni ZF, Ma ZW, Xie AJ, Cheng XS, et al. Betaine attenuates Alzheimer-like pathological changes and memory deficits induced by homocysteine. J Neurochem. 2013;124:388–96. doi: 10.1111/jnc.12094. [DOI] [PubMed] [Google Scholar]
- 28.Sun J, Wen S, Zhou J, Ding S. Association between malnutrition and hyperhomocysteine in Alzheimer's disease patients and diet intervention of betaine. J Clin Lab Anal. 2017;31:e22090. doi: 10.1002/jcla.22090. [DOI] [PMC free article] [PubMed] [Google Scholar]


