Abstract
Stroke is the second leading cause of death globally and the third leading cause disability. Acute ischemic stroke (AIS), resulting from occlusion of major vessels in the brain, accounts for approximately 87% of strokes. Despite this large majority, current treatment options for AIS are severely limited and available to only a small percentage of patients. Therapeutic hypothermia (TH) has been widely used for neuroprotection in the setting of global ischemia postcardiac arrest, and recent evidence suggests that hypothermia may be the neuroprotective agent that stroke patients desperately need. Several clinical trials using systemic or selective cooling for TH have been published, reporting the safety and feasibility of these methods. Here, we summarize the major clinical trials of TH for AIS and provide recommendations for future studies.
Keywords: Endovascular stroke therapy, infarct, ischemic stroke, penumbra, therapeutic hypothermia
Introduction
Stroke is the second leading cause of death globally and the third leading cause disability.[1] In the United States alone, health-care expenditures related to stroke cost an estimated $34 billion annually.[2] Over the past two decades, the global burden of stroke has increased dramatically, and with progressive population aging, this trend is likely to continue.[1,3] The risk of stroke increases dramatically for each successive decade past 55 years old.[4] Acute ischemic stroke (AIS), resulting from occlusion of major vessels in the brain, accounts for approximately 87% of strokes.[2] Despite this large majority, current treatment options for AIS are severely limited and available to only a small percentage of patients. Recanalization using alteplase, a recombinant tissue plasminogen activator (t-PA), within 4.5 h of symptom onset remains the first-line treatment for AIS.[4] However, recent estimates suggest that only about 25% of AIS patients arrive to the hospital within 3 h of symptom onset, and only about 6%–8% of AIS patients actually receive the therapy.[4]
Alternatively, in situ clot removal using mechanical thrombectomy has evolved in recent years and is a promising option for patients who are ineligible for alteplase. Mechanical thrombectomy is currently recommended in select AIS patients, but treatment with alteplase remains preferential.[4] Randomized trials of mechanical thrombectomy have shown variable findings when compared to standard medical care for AIS; however, modifications to imaging techniques and endovascular devices demonstrate encouraging results.[5,6,7] Indeed, a recent meta-analysis reported that modern mechanical thrombectomy devices and imaging improved reperfusion rates and disability at 90 days in broad spectrum of AIS patients, including those receiving alteplase.[8] This evidence suggests that modern mechanical thrombectomy may improve functional outcomes and reperfusion in AIS patients, thus providing hope for neuroprotective agents that may have been hindered by incomplete reperfusion in the past.
Therapeutic Hypothermia
The ischemic penumbra, a concept first introduced by Astrup et al., represents a region of hypoperfused but potentially salvageable tissue surrounding an initial site of infarct.[8] Neuroprotective strategies attempt to preserve this “at-risk tissue.” While recanalization techniques have demonstrated functional benefit in the setting of AIS, neuroprotective methods are still desperately needed in order to restore neuronal function in the after-stroke phase.
Therapeutic hypothermia (TH) has been widely used for neuroprotection in the setting of global ischemia postcardiac arrest with remarkable results. A sentinel study using TH for the treatment of patients with acute brain injury postcardiac arrest found an odds ratio of 5.25 for a good clinical outcome in favor of hypothermia compared to normothermia.[9] The hypothermia after cardiac arrest study group reported a favorable neurologic outcome in 55% of hypothermic patients compared to 29% in their normothermic counterparts and lower rates of mortality at 6 months for hypothermia-treated patients.[10] Pooled analysis of data from six randomized controlled trials of endovascular cooling in the setting of acute myocardial infarction reported a 30% reduction in infarct volumes in patients treated with <35°C hypothermia with anterior myocardial infarctions when compared with controls.[11]
TH also holds promise for neuroprotection in the setting of AIS [Figure 1]. TH can inhibit many of the deleterious processes that occur in the setting of cerebral ischemia, including neuroinflammation, blood–brain barrier disruption, apoptosis, and free radical production.[12] As evidence of this neuroprotection, meta-analysis results from animal studies indicate that hypothermia reduces infarct size by 44%.[13] A recent small animal study of AIS indicates that hypothermia may reduce the neuronal release of high-mobility group box 1 (HMGB-1), which is well established as a mediator of inflammation and damage during brain ischemia. The reduction in HMGB-1 release may play a role in the reduced infarct sizes seen with TH.[14] However, animal studies have clearly demonstrated the time-sensitive nature of TH neuroprotection. TH provides the best neuroprotection when initiated immediately after or even before ischemia occurs. However, when TH initiation is delayed be 30 min or more, it fails to provide adequate neuroprotection in small animal models.[15] Thus, rapid induction of hypothermia is of critical importance for the treatment of AIS patients. Recent clinical trials have demonstrated the safety and efficacy of TH in stroke patients.[16] Taken together, this evidence suggests that TH may be the neuroprotective agent that stroke patients so desperately need; however, a variety of factors have hindered its translation to clinical practice.
Figure 1.
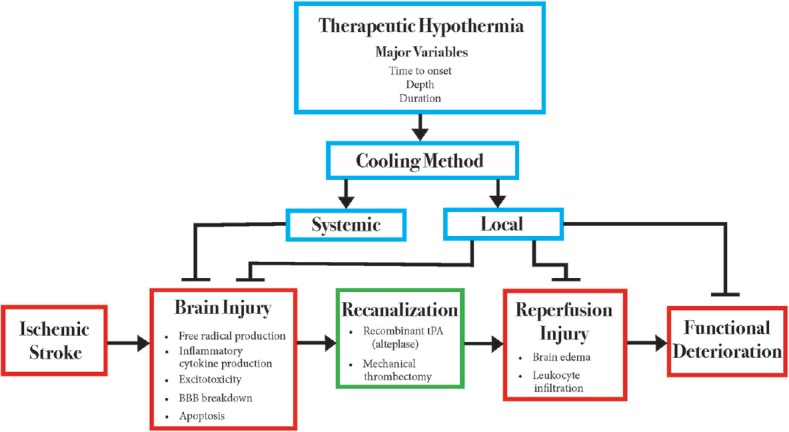
Theoretical interplay between therapeutic hypothermia and acute ischemic stroke. This figure summarizes the primary pathways by which acute ischemic stroke damages the brain parenchyma, resulting in functional deficits for stroke patients. Animal models of therapeutic hypothermia have demonstrated a significant benefit regarding stroke therapy, as indicated by inhibitory arrows in the figure. However, the mechanistic benefits of therapeutic hypothermia have not been investigated in clinical trials. Red boxes indicate related processes that occur during the acute ischemic stroke process. Blue boxes indicate factors related to therapeutic hypothermia. Inhibitory arrows indicate processes in which therapeutic hypothermia has shown efficacy in animal models
Systemic Therapeutic Hypothermia
Early studies on TH for AIS focused primarily on systemic hypothermia, using various methods to achieve global cooling [Table 1]. The Copenhagen Stroke Study and Cooling for Acute Ischemic Brain Damage (COOL AID) group demonstrated the safety and feasibility of TH with cooling blankets in awake patients with acute stroke.[17,18] In 2005, the Intravascular Cooling in the Treatment of Stroke (ICTuS) study investigated endovascular cooling techniques, concluding that TH could be achieved and maintained in this manner. ICTuS also developed a novel antishivering regimen to aid in tighter temperature control.[19] Subsequent studies have made it clear that endovascular techniques allow for a much quicker cooling time compared to noninvasive surface cooling, a desirable effect considering the narrow window for neuroprotection in AIS.
Table 1.
Summary information for clinical trials of therapeutic hypothermia in acute stroke patients
| Name, Year | Number in hypothermia cohort (n=) | Depth of hypothermia (°C) | Time from hypothermia onset to achievement of target temperature (hrs.) | Duration at target temperature (hrs.) | Cooling method |
|---|---|---|---|---|---|
| Kammersgaard et al., 2000 | 17 | 35.5 | 6.00 | N/A | Systemic - Surface |
| Kreiger et al., 2001 | 10 | 32 | 3.5±1.5 | 47.4±20.4 | Systemic - Surface |
| De Georgia et al., 2001 | 18 | 33 | 1.28±0.73 | 24 | Systemic - Infusion |
| Lyden et al., 2005 | 18 | 33 | N/A | 12-24 | Systemic - Infusion |
| Els et al., 2006 | 12 | 35 | 2.00±1.00 | 48 | Systemic - Infusion |
| Hemmen et al., 2010 | 28 | 33 | 1.11 | 24 | Systemic - Infusion |
| Ovesen et al., 2013 | 17 | 33 | 2.11 (endovascular), 3.27 (surface) |
24 | Systemic - Combination |
| Pilronen et al., 2014 | 18 | 35 | 4.50 | 10.5 | Systemic - Combination |
| Lyden et al., 2016 | 57 | <35 | 6.00 | 24 | Systemic - Infusion |
| Chen et al., 2016 | 26 | ~2 °C below baseline | 0.16 | N/A | Selective - Infusion |
| Peng et al., 2016 | 11 | N/A | 0.16 | N/A | Selective - Infusion |
| Geurts et al., 2017 | 22 | 34.5, 35 | 6.87 (34.5 °C), 7.36 (35 °C) | 24 | Systemic - Combination |
| Wu et al., 2017 | 45 | N/A | 0.16 | N/A | Selective - Infusion |
| Neugebauer et al., 2019 | 26 | 33±1 | N/A | 72 | Systemic - Combination |
A follow-up study from the COOL AID group used an endovascular device inserted into the inferior vena cava to achieve a target temperature of 33°C, with a mean cooling time of 77 ± 44 min.[20] ICTuS-L (n = 59), a randomized multi-center trial, achieved hypothermia at 33°C in 71.4% of patients, with a mean time-to-target temperature of 67 min after catheter placement. The most significant side effect noted in the TH group was pneumonia, which did not affect the outcome at 90 days. In addition, ICTuS-L demonstrated the safety and feasibility of endovascular cooling after t-PA recanalization.[21] COOLAID Oresund (n = 31) compared the cooling capacity of endovascular and surface cooling methods in patients under general anesthesia, concluding that TH was feasible in anesthetized patients.[22] The mean induction time to a target temperature of 33°C was 105 min and 182.5 min in the endovascular and surface cooling groups, respectively. However, pneumonia was recorded in six of the TH patients versus one of the controls, a finding that may be in part due to mechanical ventilation rather than an independent effect of TH. More recently, Piironen et al. induced TH with a bolus of cold saline (4°C–6°C) for 2 h before maintaining temperatures with standard cooling blankets. Using this method, target temperatures of <35.5°C were achieved in 89% of TH-treated patients within 4.5 h, a longer time period than with endovascular cooling alone.[23]
In response to previously reported pneumonia, ICTuS-2 (n = 120) was proposed in an attempt to lower pneumonia rates in TH-treated patients using a precise definition of pneumonia, strict surveillance for the condition, and a novel antishivering regimen. However, as a result of other ongoing clinical trials, ICTuS-2 was stopped short of completion. Nonetheless, this investigation provided valuable information. ICTuS-2 initiated TH in patients using a femoral venous cooling system after t-PA administration, again demonstrating the efficacy of this procedure. Cooling was rapid, with most of the TH cohort reaching target temperatures (33°C) within 2 h. However, despite the measures taken to reduce the risk of pneumonia, rates were still higher in the TH cohort compared with the normothermic cohort.[24]
In 2017, the COOLIST study (n = 22) investigated the feasibility of cooling at 34°C, 34.5°C, and 35°C using a bolus of cold saline to initiate hypothermia, followed by surface cooling to maintain temperatures for 24 h. COOLIST, like ICTuS-2, was stopped short of completion due to rigorous guidelines for patient surveillance and care that could not be met in many cases. Despite a small sample size, COOLIST reported that 35°C cooling is feasible in awake patients with AIS, whereas cooling to 34°C and 34.5°C is not. In this study, 100% of patients in the 35°C cohort, 33% of the 34.5°C cohort, and 0% of patients in the 34°C cohort completed the assigned treatment. Cooling times varied reported at 412 min and 442 min in the 34.5°C and 35°C cohorts, respectively. In addition, eight cooled patients were diagnosed with pneumonia compared to one patient in the standard treatment group.[25]
Systemic TH has also garnered attention as a combination therapy with decompressive hemicraniectomy for the treatment of malignant middle cerebral artery ischemic stroke. Els et al. initially investigated the safety and potential benefit of hemicraniectomy coupled with TH.[26] In this study, 25 patients were selected to receive either hemicraniectomy alone (n = 13) or in combination with mild TH at 35°C (n = 12). Ten patients in the combination cohort were treated with system endovascular infusion of cold saline into the inferior vena cava, whereas two patients were cooled with external cooling devices. In addition, TH patients underwent an active rewarming period at a rate of approximately 1°C/h. Mortality rates were reported at 8% and 15% in the combination and hemicraniectomy groups, respectively. No significant differences in adverse side effects were reported between the two groups, and the combination therapy group showed a trend toward better functional outcome at 6 months, warranting a future clinical trial.
In order to further elucidate the potential benefits of combination therapy, a recent randomized, controlled clinical trial was published (n = 50).[27] The primary endpoint of this study was the mortality rate at 14 days. All patients enrolled in the study received early hemicraniectomy within 48 h from symptom onset. In patients randomized to the combination therapy group (n = 26), TH was started within 12 h of hemicraniectomy. Fourteen (54%) of the patients in the combination group were cooled using endovascular infusion, ten received surface cooling, and the remaining two did not receive treatment. Eighty-eight percent of the combination patients reached a target temperature of 33°C ± 1°C and maintained it for a minimum of 72 h, and the remaining 12% were maintained at temperatures slightly above 34°C.
Safety measures including the incidence of severe adverse side effects (i.e., bleeding events, thrombotic events, infections, and liver or renal dysfunction)[28] were recorded for all patients. After 14 days, severe adverse effects were found in 46% of the combination group compared with 29% in the hemicraniectomy group. Nineteen percent of these side effects were associated with temperature management in the combination group. No significant difference was found between the two groups for the primary end, 14-day mortality rate. At the 12-month time point, 80% of the combination group and 43% of the control group were found to have severe adverse effects. This trial was stopped for safety reasons, largely because of the alarming increase in adverse events seen in the combination group. These results suggest that TH does not improve patient care in combination with hemicraniectomy for the treatment of malignant middle cerebral artery stroke.
Although decompressive craniectomy is an effective life-saving treatment for patients with massive cerebral infarctions, not all patients are eligible for this procedure. As an alternative method of therapy, TH has been suggested as an adjunct to standard care for patients with massive cerebral infarction, who are ineligible for craniectomy. Su et al. recently performed a randomized controlled trial looking at the benefits of TH for these patients.[29] In this study, 16 patients with massive cerebral hemispheric infarction were placed into a hypothermia group, whereas 17 were placed in a normothermia group. All patients received standard medical care in the neurointensive care unit immediately following enrollment in the study. In addition to this standard care, patients in the hypothermia cohort received cooling using a catheter placed in the inferior vena cava which included a lumen with three balloons. These balloons were perfused with a cold saline solution using a closed-loop tubing system. A Foley temperature catheter was used to maintain a bladder temperature of 33°C–34°C. Hypothermia was maintained for a minimum of 24 h and as long as 72 h depending on the physician discretion. At 6 months, 87.5% of the hypothermic cohort achieved good neurological outcomes, compared with 40% of the normothermic patients. Mortality rates were not significantly different between the two groups. Since this trial only included patients with contraindications for craniectomy procedures, it is possible that the results are not generalizable to other patient populations. However, these findings suggest that TH may have benefits regarding neurological outcomes in patients with massive cerebral infarctions who are ineligible for craniectomy interventions.
Current State of Systemic Therapeutic Hypothermia
Early studies using systemic cooling methods in the setting of AIS have provided valuable information about the safety and feasibility of this treatment option but also raised important points that need to be addressed. Previous studies have reported a wide range of time-to-target temperature, in some cases as long as 7 h. The neuroprotective window for AIS is only 4.5 h; thus, therapeutic options requiring a longer time span are likely not suitable for therapy. Methods for more rapid cooling are needed to maximize the efficacy of TH in acute stroke patients. Furthermore, shivering/dermal vasoconstriction continues to hinder the maintenance of target temperatures, despite measures to better control for this factor. Increased risks of pneumonia in patients treated with TH have also been consistently reported despite recent efforts to minimize the risk for pneumonia. Clinical trials have also reported cardiac, vascular, and respiratory abnormalities in patients treated with systemic TH. In addition, data regarding combination therapy using TH with hemicraniectomy procedures for the treatment of malignant middle cerebral artery stroke demonstrated no additional benefit to patients, and higher rates of adverse side effects compared to hemicraniectomy along, suggesting that systemic TH may be detrimental in some populations.
Currently, there is no consensus as to the optimal target temperature for TH in patients with AIS. Previous clinical trials have used various target temperatures, generally ranging from 33°C to 35°C. In an attempt to provide data on this matter, COOLIST compared three temperatures but was underpowered to provide substantial evidence for future investigations. Nielsen et al. reported that TH at 33°C was seen to confer no additional benefit when compared with 36°C in the setting of cardiac arrest.[30] However, investigations regarding TH for cardiac arrest are not generalizable to studies in the context of AIS. Further investigations are needed to optimize and standardize target temperatures.
While TH represents a promising neuroprotective strategy, it is currently not recommended for acute stroke therapy unless in the setting of clinical trials. The 2018 Guidelines for Early Management of Patients with Acute Ischemic Stroke implicates the increased risk of infection, especially pneumonia as a major issue with TH.[4] The European Stroke Organization guidelines for the management of temperature in patients with AIS do not recommend routine induction of hypothermia for improving functional outcomes or survival of patients with AIS.[31]
Selective Therapeutic Hypothermia
Many clinical trials have focused on systemic cooling for patients with AIS [Table 1]. However, selective endovascular infusion of cold saline directly into the brain parenchyma holds promise for more rapid induction of target temperatures. The brain represents a small volume of tissue, and as such, it will likely cool down more rapidly and with less fluid than is needed for whole-body cooling. In addition, this method minimizes the systemic effects of TH since fluids are directed to a localized region of the brain. From a practicality standpoint, selective hypothermia should be easy to induce by perfusing cold saline through preexisting angiographic catheters, which are commonly used for endovascular recanalization procedures. Early small animal studies using selective TH for AIS reported a 65% reduction in infarct volume and a 61% reduction in leukocyte infiltration to brain parenchyma.[32] Selective TH administered prereperfusion was reported to reduce infarct volumes by 75%–90% and conserve motor function after stroke in a murine model.[33,34] Postreperfusion studies have also reported improvements in infarct volume and functional recovery in rats, though not to the extent demonstrated with prereperfusion therapy.[35,36]
In 2007, a three-dimensional, mathematical model of selective cooling using intracarotid cold saline infusion was performed. This theoretical study modeled a normal brain, and a brain with stroke (ischemic core with surrounding penumbra) in order to estimate adequate flow rates, and time needed to achieve adequate cooling of brain tissue. This model suggested that localized infusion was safe in humans and much faster than systemic methods. The study concluded that an infusion of cold saline at 30 ml/min would be adequate to induce hypothermia (33°C–34°C) of the ipsilateral hemisphere in 10 min. Localized infusion of cold saline dramatically reduced cooling time when compared with endovascular systemic cooling (10-20 times faster) and systemic cooling with noninvasive techniques (18-42 times faster).[37]
Subsequently, Choi et al. investigated the feasibility of selective brain cooling in humans (n = 18) using a two-part study, in which Part 1 was used to establish the safety of infusion, whereas Part 2 would include infusion of saline at lower temperatures. Nine patients undergoing routine cerebral angiography were enrolled for Part 1 of the study, in which cold saline of 12°C–17°C was infused into one internal carotid artery at 33 ml/min for 10 min. In Part 2, nine patients were infused with saline at 4°C–7°C using the same protocol. Jugular venous bulb temperature, used as a proxy for brain parenchymal temperature, dropped approximately 0.85°C ± 0.13°C over the course of the infusion. Although brain parenchymal temperature could not be measured directly, it was suggested that it may have dropped as much as 2.8°C.[38] In a follow-up investigation from the same group, mathematical modeling was used to more accurately predict how brain temperatures may have changed in the pilot study. Using the previously developed three-dimensional model, it was estimated that the ipsilateral anterior circulation territory decreased by about 2°C as a result of the 10-min infusion, a comparable degree of cooling to clinical trials on systemic TH but with a much faster time to reach hypothermia.[39] Taken together, these results suggest that selective infusions of cold saline directly into brain tissue are safe and feasible and allow for progression-to-target temperatures in minutes, compared to the hours needed to achieve similar results with systemic methods.
Recently, a pilot study (n = 26) on selective brain cooling in AIS was published, providing the first evidence of feasibility and safety of this method in stroke patients.[40] Incorporating compelling evidence from prereperfusion cooling in rat models, this investigation includes both pre- and postrecanalization infusion of cold saline. Prereperfusion flushing may augment the neuroprotective capabilities of TH by removing harmful toxins and biochemical products that accumulate in ischemic brain tissues. Thus, before recanalization, the ischemic regions were infused with 50 ml of cold saline (4°C) at 10 ml/min through a microcatheter that was put across the thrombus. After recanalization using mechanical thrombectomy, 4°C saline was infused at 30 ml/min for 10 min. Due to the invasive nature of brain parenchymal temperature probes, no direct temperature data were recorded, a major limitation of this study. Systemic temperature, measured using a rectal probe, was reduced by a maximum of 0.3°C as a result of the infusions. Both pre- and postrecanalization local cooling were deemed safe and feasible in humans.[40]
In response to difficulties with brain temperature measurements, a recent investigation utilized Rhesus monkeys to determine the efficacy of selective brain cooling. This study investigated cooling using selective and systemic methods. For the selective cooling cohort (n = 4), a microcatheter was introduced into the middle cerebral artery, and then, 100 ml of iced Ringer's solution was infused at a rate of 5 ml/min for 20 min. For the systemic cooling cohort (n = 4), 100 ml of iced Ringer's solution was infused into the cephalic vein at a rate of 5 ml/min for 20 min. Brain temperature was monitored by thermistor probes, introduced through two cranial burr holes, and placed within the parenchyma of the basal ganglia and parietal lobe. Mild hypothermia (<35°C) was achieved at 10 min and maintained for approximately 20 min using selective infusion. Systemic infusion was not able to reduce temperatures below 36°C during the 20 min period.[41]
Peng et al. combined TH with arterial thrombectomy for the treatment of AIS due to middle cerebral artery occlusion (n = 26).[42] Fifteen patients were randomly placed into a normothermia group, whereas the remaining 11 were placed into a mild hypothermia group (28°C–33°C). Following arterial thrombolysis using a microcatheter, 500 ml of Ringer's solution at 4°C was pumped into brain tissue at 50 ml/min for 10 min through the microcatheter in order to induce mild hypothermia. Infarct sizes in both the normothermic and hypothermic groups were determined by magnetic resonance imaging at 24 h and 7 days following treatment, and infarct volumes were calculated using the Streiner formula. The infarct volume in the hypothermic cohort showed a significant reduction compared to the normothermic cohort, decreasing from 25 to 13 cm3 over the first 24 h. In addition, neurological deficit scores in the hypothermic cohort were significantly reduced compared to the normothermic group. These results indicate promising hypothermic neuroprotection in the setting of middle cerebral artery occlusions. However, it is not clear if target temperatures were actually measured or achieved in this study, or if TH was maintained for any amount of time, making these results difficult to generalize or replicate.
In 2017, a prospective, nonrandomized control study (n = 113) investigated the feasibility of selective TH in AIS patients when combined with mechanical thrombectomy (n = 45) versus a control group that underwent standard treatment (n = 68).[16] In the cooling cohort, ischemic regions were infused with 50 ml of cold saline (4°C) at 10 ml/min before recanalization, utilizing a microcatheter that was put across the thrombus. After recanalization with mechanical thrombectomy, 4°C saline was infused at 30 ml/min for 10 min. Computed tomography imaging taken at 3–7 days posttreatment indicated a significant reduction in the final infarct volume of patients in the cooling cohort compared to patients in the standard treatment cohort. In addition, the cooling cohort showed a nonsignificant trend toward a higher percentage of patients with good clinical outcomes; however, due to the limited sample size, these findings will require further verification. This study was not able to directly measure brain temperatures, a primary limitation to all selective TH clinical trials up to this point. Despite this, these results provide enough data to warrant future randomized clinical trials.
Perspective and Prospective
Previous clinical trials regarding TH for the treatment of AIS have shown variable results and also demonstrated the challenge of implementing this therapy into human populations. Although many trials have shown the safety and feasibility of TH, there is a paucity of data regarding its efficacy in reducing ischemic damage. More clinical investigations regarding the potential neurological and functional benefits of TH for AIS patients will be necessary in order to build a robust body of knowledge and better understand the biochemical changes that occur with TH in human populations. Furthermore, many of the current clinical trials have focused on systemic cooling methods. While these methods are easily implemented, they present serious drawbacks regarding the time needed to reach target temperatures; in many cases, target temperatures are not reached until after the 4.5-h therapeutic window for AIS likely attenuating the benefits provided to patients. In addition, side effects such as severe shivering, pneumonia, and cardiac abnormalities make it difficult to implement clinical trials in a safe and controlled manner. Finally, almost all clinical trials to date have included a small sample size, making them underpowered to report on significant trends that may occur with TH for AIS patients.
Future clinical trials of TH for AIS patients should aim to homogenizing study methodology. Previous clinical investigations have utilized a wide range of cooling methods, both systemic and selective, and target temperatures. These differences make it difficult to generalize data from clinical trials and to build upon the preexisting body of knowledge. In addition, investigations into selective brain cooling, which have received less attention than systemic cooling up to this point, are warranted going forward. The rapid induction of hypothermia and reduction in systemic symptoms make selective cooling an attractive option. Selective cooling may provide a novel treatment that can circumvent many of the widespread objections to the use of hypothermia after stroke.
Despite remarkable findings with selective cooling methods, the 2018 Guidelines for Early Management of Patients with Acute Ischemic Stroke did not factor these studies in its guidelines. Thus, efforts should be taken to improve recognition and clinical acceptance of selective TH in patients after AIS. TH is a potent neuroprotectant and holds a significant promise for AIS patients.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–48. doi: 10.1161/CIRCRESAHA.116.308413. [DOI] [PubMed] [Google Scholar]
- 2.Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, et al. Heart disease and stroke statistics-2017 update: A report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–11. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- 4.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49:e46–110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 5.Ciccone A, Valvassori L, Nichelatti M, Sgoifo A, Ponzio M, Sterzi R, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med. 2013;368:904–13. doi: 10.1056/NEJMoa1213701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med. 2018;378:708–18. doi: 10.1056/NEJMoa1713973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;372:1009–18. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 8.Astrup J, Siesjö BK, Symon L. Thresholds in cerebral ischemia – The ischemic penumbra. Stroke. 1981;12:723–5. doi: 10.1161/01.str.12.6.723. Doi: 10.1161/01.STR.12.6.723. [DOI] [PubMed] [Google Scholar]
- 9.Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346:557–63. doi: 10.1056/NEJMoa003289. [DOI] [PubMed] [Google Scholar]
- 10.Hypothermia after Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346:549–56. doi: 10.1056/NEJMoa012689. [DOI] [PubMed] [Google Scholar]
- 11.Dae M, O'Neill W, Grines C, Dixon S, Erlinge D, Noc M, et al. Effects of endovascular cooling on infarct size in ST-segment elevation myocardial infarction: A patient-level pooled analysis from randomized trials. J Interv Cardiol. 2018;31:269–76. doi: 10.1111/joic.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37:S186–202. doi: 10.1097/CCM.0b013e3181aa5241. [DOI] [PubMed] [Google Scholar]
- 13.van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: A systematic review and meta-analysis. Brain. 2007;130:3063–74. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- 14.Lee JH, Yoon EJ, Seo J, Kavoussi A, Chung YE, Chung SP, et al. Hypothermia inhibits the propagation of acute ischemic injury by inhibiting HMGB1. Mol Brain. 2016;9:81. doi: 10.1186/s13041-016-0260-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao H, Steinberg G. Limited therapeutic time windows of mild-to-moderate hypothermia in a focal ischemia model in rat. Stroke Res Treat. 2011;2011:131834. doi: 10.4061/2011/131834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wu C, Zhao W, An H, Wu L, Chen J, Hussain M, et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J Cereb Blood Flow Metab. 2018;38:2251–60. doi: 10.1177/0271678X18790139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kammersgaard LP, Rasmussen BH, Jørgensen HS, Reith J, Weber U, Olsen TS. Feasibility and safety of inducing modest hypothermia in awake patients with acute stroke through surface cooling: A case-control study: The Copenhagen Stroke Study. Stroke. 2000;31:2251–6. doi: 10.1161/01.str.31.9.2251. [DOI] [PubMed] [Google Scholar]
- 18.Krieger DW, De Georgia MA, Abou-Chebl A, Andrefsky JC, Sila CA, Katzan IL, et al. Cooling for acute ischemic brain damage (COOL AID): An open pilot study of induced hypothermia in acute ischemic stroke. Stroke. 2001;32:1847–54. doi: 10.1161/01.str.32.8.1847. [DOI] [PubMed] [Google Scholar]
- 19.Lyden PD, Allgren RL, Ng K, Akins P, Meyer B, Al-Sanani F, et al. Intravascular cooling in the treatment of stroke (ICTuS): Early clinical experience. J Stroke Cerebrovasc Dis. 2005;14:107–14. doi: 10.1016/j.jstrokecerebrovasdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- 20.De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for acute ischemic brain damage (COOL AID): A feasibility trial of endovascular cooling. Neurology. 2004;63:312–7. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- 21.Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): Final results. Stroke. 2010;41:2265–70. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ovesen C, Brizzi M, Pott FC, Thorsen-Meyer HC, Karlsson T, Ersson A, et al. Feasibility of endovascular and surface cooling strategies in acute stroke. Acta Neurol Scand. 2013;127:399–405. doi: 10.1111/ane.12059. [DOI] [PubMed] [Google Scholar]
- 23.Piironen K, Tiainen M, Mustanoja S, Kaukonen KM, Meretoja A, Tatlisumak T, et al. Mild hypothermia after intravenous thrombolysis in patients with acute stroke: A randomized controlled trial. Stroke. 2014;45:486–91. doi: 10.1161/STROKEAHA.113.003180. [DOI] [PubMed] [Google Scholar]
- 24.Lyden P, Hemmen T, Grotta J, Rapp K, Ernstrom K, Rzesiewicz T, et al. Results of the ICTuS 2 trial (intravascular cooling in the treatment of stroke 2) Stroke. 2016;47:2888–95. doi: 10.1161/STROKEAHA.116.014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Geurts M, Petersson J, Brizzi M, Olsson-Hau S, Luijckx GJ, Algra A, et al. COOLIST (cooling for ischemic stroke trial): A multicenter, open, randomized, phase II, clinical trial. Stroke. 2017;48:219–21. doi: 10.1161/STROKEAHA.116.014757. [DOI] [PubMed] [Google Scholar]
- 26.Els T, Oehm E, Voigt S, Klisch J, Hetzel A, Kassubek J. Safety and therapeutical benefit of hemicraniectomy combined with mild hypothermia in comparison with hemicraniectomy alone in patients with malignant ischemic stroke. Cerebrovasc Dis. 2006;21:79–85. doi: 10.1159/000090007. [DOI] [PubMed] [Google Scholar]
- 27.Neugebauer H, Schneider H, Bösel J, Hobohm C, Poli S, Kollmar R, et al. Outcomes of hypothermia in addition to decompressive hemicraniectomy in treatment of malignant middle cerebral artery stroke: A randomized clinical trial. JAMA Neurol. 2019;76:571–9. doi: 10.1001/jamaneurol.2018.4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Neugebauer H, Kollmar R, Niesen WD, Bösel J, Schneider H, Hobohm C, et al. DEcompressive surgery plus hypothermia for space-occupying stroke (DEPTH-SOS): A protocol of a multicenter randomized controlled clinical trial and a literature review. Int J Stroke. 2013;8:383–7. doi: 10.1111/ijs.12086. [DOI] [PubMed] [Google Scholar]
- 29.Su Y, Fan L, Zhang Y, Zhang Y, Ye H, Gao D, et al. Improved neurological outcome with mild hypothermia in surviving patients with massive cerebral hemispheric infarction. Stroke. 2016;47:457–63. doi: 10.1161/STROKEAHA.115.009789. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med. 2013;369:2197–206. doi: 10.1056/NEJMoa1310519. [DOI] [PubMed] [Google Scholar]
- 31.Ntaios G, Dziedzic T, Michel P, Papavasileiou V, Petersson J, Staykov D, et al. European Stroke Organisation (ESO) guidelines for the management of temperature in patients with acute ischemic stroke. Int J Stroke. 2015;10:941–9. doi: 10.1111/ijs.12579. [DOI] [PubMed] [Google Scholar]
- 32.Ding Y, Li J, Rafols JA, Phillis JW, Diaz FG. Prereperfusion saline infusion into ischemic territory reduces inflammatory injury after transient middle cerebral artery occlusion in rats. Stroke. 2002;33:2492–8. doi: 10.1161/01.str.0000028237.15541.cc. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y, Yao B, Zhou Y, Park H, McAllister JP, 2nd, Diaz FG. Prereperfusion flushing of ischemic territory: A therapeutic study in which histological and behavioral assessments were used to measure ischemia-reperfusion injury in rats with stroke. J Neurosurg. 2002;96:310–9. doi: 10.3171/jns.2002.96.2.0310. [DOI] [PubMed] [Google Scholar]
- 34.Ding Y, Li J, Luan X, Lai Q, McAllister JP, 2nd, Phillis JW, et al. Local saline infusion into ischemic territory induces regional brain cooling and neuroprotection in rats with transient middle cerebral artery occlusion. Neurosurgery. 2004;54:956–64. doi: 10.1227/01.neu.0000114513.96704.29. [DOI] [PubMed] [Google Scholar]
- 35.Ji YB, Wu YM, Ji Z, Song W, Xu SY, Wang Y, et al. Interrupted intracarotid artery cold saline infusion as an alternative method for neuroprotection after ischemic stroke. Neurosurg Focus. 2012;33:E10. doi: 10.3171/2012.5.FOCUS1215. [DOI] [PubMed] [Google Scholar]
- 36.Ji Y, Hu Y, Wu Y, Ji Z, Song W, Wang S, et al. Therapeutic time window of hypothermia is broader than cerebral artery flushing in carotid saline infusion after transient focal ischemic stroke in rats. Neurol Res. 2012;34:657–63. doi: 10.1179/1743132812Y.0000000061. [DOI] [PubMed] [Google Scholar]
- 37.Konstas AA, Neimark MA, Laine AF, Pile-Spellman J. A theoretical model of selective cooling using intracarotid cold saline infusion in the human brain. J Appl Physiol (1985) 2007;102:1329–40. doi: 10.1152/japplphysiol.00805.2006. [DOI] [PubMed] [Google Scholar]
- 38.Choi JH, Marshall RS, Neimark MA, Konstas AA, Lin E, Chiang YT, et al. Selective brain cooling with endovascular intracarotid infusion of cold saline: A pilot feasibility study. AJNR Am J Neuroradiol. 2010;31:928–34. doi: 10.3174/ajnr.A1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neimark MA, Konstas AA, Lee L, Laine AF, Pile-Spellman J, Choi J. Brain temperature changes during selective cooling with endovascular intracarotid cold saline infusion: Simulation using human data fitted with an integrated mathematical model. J Neurointerv Surg. 2013;5:165–71. doi: 10.1136/neurintsurg-2011-010150. [DOI] [PubMed] [Google Scholar]
- 40.Chen J, Liu L, Zhang H, Geng X, Jiao L, Li G, et al. Endovascular hypothermia in acute ischemic stroke: Pilot study of selective intra-arterial cold saline infusion. Stroke. 2016;47:1933–5. doi: 10.1161/STROKEAHA.116.012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang B, Wu D, Dornbos Iii D, Shi J, Ma Y, Zhang M, et al. Local cerebral hypothermia induced by selective infusion of cold lactated Ringer's: A feasibility study in rhesus monkeys. Neurol Res. 2016;38:545–52. doi: 10.1080/01616412.2016.1187827. [DOI] [PubMed] [Google Scholar]
- 42.Peng X, Wan Y, Liu W, Dan B, Lin L, Tang Z. Protective roles of intra-arterial mild hypothermia and arterial thrombolysis in acute cerebral infarction. Springerplus. 2016;5:1988. doi: 10.1186/s40064-016-3654-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


