Abstract
Hypothermia is the most reliably effective neuroprotectant, and yet systemic complications limit application. A large body of animal data suggests that hypothermia is effective for focal cerebral ischemia, namely acute ischemic stroke. In order to apply hypothermia effectively, a selective approach is required to maximize the effect on the brain while minimizing systemic side effects. Due to poor transferability of promising findings in rodent models to human clinical trials for neuroprotection, the focus of this review is large animal gyrencephalic models. Unlike rodent data which favor mild hypothermia, the majority of large animal studies on selective hypothermia support moderate-to-deep hypothermia (<30°C). Cold blood perfusion produces the rapid rate of temperature reduction and depth of hypothermia required to produce meaningful neuroprotection. Further studies of selective hypothermia in acute ischemic stroke require attention to duration and rate of cooling to optimize the neuroprotection offered by this technique.
Keywords: Perfusion, selective hypothermia, stroke
Introduction
Interest in hypothermia as a protection against hypoxic-ischemic injury during surgery dates back to the 1950s.[1,2,3] At that time, efforts to improve surgery on both the heart and brain led to investigations as to how to improve tolerance to circulatory arrest and resultant hypoxia. For cardiac surgeons, the issue was with direct surgery on the heart, which would require cardiac standstill, and which without some form of protection, would result in cerebral ischemia.[1] For neurosurgeons, the tantalizing possibility lay in being able to operate in a bloodless field in the depths of the cranium to precisely reconstruct diseased blood vessels. This led to interest in methods to arrest cerebral blood flow while improving the brain's tolerance to ischemia.[2,3].
These lines of investigation were focused on two different forms of cerebral ischemia. The cardiac surgeons were interested in the prevention of global ischemia due to cardiac standstill. For the neurosurgeons, the interest was in preventing focal ischemia due to temporary occlusion of a single brain artery.[3,4,5] Temporary occlusion provides the advantage of softening of the brain aneurysm sac, allowing manipulation and obliteration with a reduced risk of aneurysm rupture. However, temporary occlusion comes at the cost of potential stroke in the territory supplied by the artery. Hypothermia could decrease the metabolic demand of neural tissue and decrease the risk of stroke.
It should be noted that numerous case reports and even case series of survivors of accidental deep hypothermia are described in the literature.[6] These patients have arrived in full cardiac arrest and undergone resuscitation via extracorporeal circulation. A surprising number of survivors have excellent long-term outcomes.[7]
The metabolic effect of hypothermia was established in canine experiments the 1950s by Lougheed and Kahn. They demonstrated who found a 50% reduction in cerebral metabolic rate at 28°C and a 75% reduction at 25°C.[3] This was reproduced by Michenfelder again in a canine model with very similar findings.[8,9] Given the degree to which cerebral metabolism was reduced by moderate hypothermia, it seemed logical that it would be protective during induced cerebral ischemia. Initially, cooling was performed by whole body ice water bath, but cardiac dysrhythmias limited the degree of cooling to 30°C.[2] Later, human cases utilized full cardiac bypass with profound hypothermia (10°C–15°C), but bleeding was a significant problem.[4,5]
Early Experience with Selective Hypothermia
The recognition of these systemic side effects led to interest in focal or selective hypothermia applied to the area at risk for ischemia. Lougheed and Kahn first proposed local or selective hypothermia in 1955 as a way of circumventing the cardiac arrhythmia problem.[3] Using a bypass pump attached to a carotid artery in dogs, he was able to reduce brain temperature to 20°C in 20 min, while the systemic body temperature only dropped to 35°C. However, every subject developed fatal arrhythmia, and the approach was abandoned. Additional canine experiments overcame this issue with impressive clinical results.[10] Of 16 animals treated with femoral-carotid bypass hypothermia and concurrent ischemia, all survived and only two had permanent deficits, whereas none of the five normothermic controls survived.
Subsequently, a human trial of selective catheter-based brain cooling during surgery for aneurysms and tumors was undertaken in the 1960s.[11] Although ten patients achieved deep hypothermia of <20°C, bleeding complications led to a 50% mortality rate. It was concluded that selective brain cooling was inferior to open chest cardiopulmonary bypass techniques. However, the excessive mortality may have been due in part to the technique. The surgical procedure involved the isolation of all four arteries to the brain in order to perfuse the brain through one of them, the other three being clamped. Supranormal infusion pressures were then used to achieve total brain cooling, which probably contributed to the complication rate.
Contemporary Animal Models and Results
Models of global ischemia
Despite these early challenges, the scientific allure of hypothermia for brain ischemia continues, due to repeated demonstrations of reduced stroke volume in animal studies. These have included both global and focal ischemic models, in both small and large animals, and using different timing of induction of hypothermia in relation to the onset of ischemia. In a global ischemic model in swine with a full 20-min period of cardiac arrest, femoral-to-carotid bypass using cooled blood for 12 h led to brain temperatures of 32°C–34°C with drops in core temperature to only 35°C.[12,13] Histological grading in the CA1 region of the hippocampus, an area known for ischemia-sensitive neurons, demonstrated significantly better scores in animals that received hypothermia. In a canine model of global ischemia, clamping off three vessels and perfusing the brain solely through the right vertebral artery with cold Ringer's lactate led to marked drops in brain temperature with no evidence of cerebral ischemic damage either clinically or histologically.[14]
Models of focal ischemia
The above studies were of global ischemia produced by cardiac arrest. However, with neuroscientists' interest in focal ischemia, many studies have used focal ischemic models instead. Two meta-analyses, the first from van der Worp et al. in 2007 and a follow-up by Dimitrascu in 2016, found similar reductions in infarct size (about 44%) due to hypothermia in animal models of focal ischemia. Not surprisingly, the vast majority of experiments were in rodents (>90%).[15,16] The most common rodent model utilizes occlusion of one middle cerebral artery (MCA) and the ipsilateral or both carotids. In many such studies, infarct volumes have been found to be smaller compared with normothermic controls.[17,18,19,20,21] These studies differ in the degree of hypothermia and in the timing of hypothermia in relation to the ischemic insult. Hypothermia was generally induced by whole-body cooling, and the ischemic insult, although considered focal, involves a more significant loss of blood flow to the whole brain than a focal model would in a larger animal. Furthermore, rats tolerate a greater degree of hypothermia without cardiac arrhythmias than higher-order mammals. In one study, whole-body cooling to <24°C was tolerated.[20]
The timing of hypothermia relative to the ischemic insult is controversial.[20] While ischemic insults applied during hypothermia have shown promise, delayed hypothermia also appears beneficial.[17,19,21] In other words, hypothermia can be induced hours after the ischemic insult or even during reperfusion, and stroke volumes will still be reduced compared to normothermic controls. Proposed mechanisms for this benefit include the protection of “penumbra” neurons at the peripheral zone of ischemia, suppression of excitatory neurotransmitters, and suppression of leukotrienes.[20] During reperfusion with hypothermia, there are measurable reductions in ischemic metabolites such as lactate and high-energy phosphates.[18] Hypothermia during reperfusion also leads to reduction in blood–brain barrier disruption.[17] Thus, based on rodent studies on focal ischemia, it would appear that hyperacute stroke may benefit from hypothermia applied during reperfusion.
Although rodents are easily handled, tolerate moderate to deep hypothermia with whole-body cooling, and have provided insight into the mechanisms behind hypothermia's benefit, the transferability of these findings to higher-order mammals is not clear. Humans do not tolerate temperatures much below 32°C without significant cardiac effect.[2] Whole-body cooling can take hours to induce, and thus, its applicability to acute stroke is limited, where even an hour's delay can be critical.[22] To bridge the gap between rodent models and the challenges posed by the known complications of hypothermia in humans, numerous large animal focal ischemic models have been reported.[23] These models have a wide variation in species, extravascular versus intravascular techniques, and transient versus permanent occlusion. We believe that large animals, rather than rodents, are key to resolving efficacy and safety issues that have plagued the neuroprotection drug development world.
Rebirth of selective hypothermia
The failure of selective brain cooling performed in humans during the 1960s has been noted above. However, based on animal studies, and the theoretical reduction in cardiac and bleeding complications, large animal investigations into this promising technique have continued. In a study in baboons, a femoral-to-carotid circuit was used to cool the ipsilateral cerebral hemisphere to under 25°C via one carotid artery. This was rapidly performed within a mean of 12 min and maintained for 3 h with only minimal systemic cooling to 34°C.[24] No hemodynamic complications were noted, although 2 of 12 animals died due to other complications. A second study utilized swine, first as a feasibility study, in which brain temperatures were reduced to 30°C in 30 min without reducing core temperature below 34°C.[13] Subsequently, the same group induced a global ischemic insult in the form of controlled cardiac arrest for 20 min, then randomly provided selective cooling for 12 h. There was a statistically significant improvement in histological scores in the cooled group compared with controls.[12] Brain temperatures in this study were more in the moderate range (32°C–34°C), while core temperatures dropped to 35°C. Most recently, a small study producing focal ischemia in baboons using selective hypothermia maintained over 12 h demonstrated significant reductions in stroke volume on magnetic resonance imaging (MRI).[25]
In a return to human utilization, Lownie et al. utilized femoral-carotid bypass to induce selective hypothermia during the evacuation and clipping of a giant MCA aneurysm.[26] Utilizing a perfusion setup with oxygenated blood flow from the femoral artery reinfused at reduced temperature into the ipsilateral carotid artery, profound but selective hypothermia was induced in the ipsilateral hemisphere during the clip reconstruction of the aneurysm. Both safety and utility were demonstrated in this case report, with temporary occlusion times of approximately 20 min, and a brain temperature of 22°C, without evidence of stroke, hemodynamic changes, or coagulopathy. The reduction in temperature to a target of 22°C took only 20 min using variations in flow to avoid overperfusion.
Hypothermia in acute ischemic stroke
Given the demonstrable effect of hypothermia in reducing stroke volumes in both small and large animal models, as well as significant human data suggesting that hypothermia prevents ischemic injury, the utility of hypothermia for treatment after a focal ischemic insult is an area of active research. In a rodent model, delaying hypothermia until 1.5 h after the ischemic insult still showed decreased infarct volumes, although not as dramatically as with concurrent hypothermia.[19] In clinical trials, hypothermia is beneficial after cardiac arrest.[27] There is some disagreement about the length of time required for benefit, but depth of hypothermia may not be as important.[28] In acute ischemic stroke, multiple authors suggest that hypothermia may be useful as a bridge to recanalization.[22,25,28,29]
Human Experience Using Systemic Hypothermia
One barrier to clinical utility is the rapidity of hypothermia induction. This is not an issue for rodent studies, but whole-body cooling in humans takes a significant amount of time. The rate of cooling may be an important determinant for clinical utility in stroke.[22] The COOLAID study demonstrated that endovascular cooling combined with a drug cocktail (to prevent shivering) achieved target temperature in just over an hour, compared with surface cooling technology which can take over 4 h.[30] Whole-body hypothermia was achieved utilizing a specialized cooling catheter placed into the inferior vena cava. This endovascular approach represented a significant change from prior surface cooling. Specifically, 13 of 18 patients randomized to hypothermia were able to achieve the target temperature of 33°C in a mean of 77 min.
A subsequent human clinical trial called ICTuS-L evaluated the combination of the Food and Drug Administration (FDA)-approved intravenous tissue plasminogen activator and endovascular hypothermia for the treatment of acute ischemic stroke.[31] This was the first trial to evaluate a demonstrated stroke therapy with hypothermia but unfortunately could not enroll enough patients to evaluate whether hypothermia might extend the treatment window for intravenous therapy. Here, the mean time to target temperature after catheter placement was 67 min, and this was achieved in 26/28 patients. Like COOLAID, there were no significant differences in clinical outcome or mortality, although pneumonia was more commonly seen in the hypothermia treatment arm. Another iteration was ReCCLAIM which evaluated mechanical thrombectomy with endovascular cooling to 33°C.[32] Taking a single-arm cohort of mid ASPECTS, high NIHSS patients, they were able to achieve a mean time to target temperature of 64 min. In comparison with historical controls, hypothermia appeared to be protective against intracerebral hemorrhage, a potential marker of reperfusion injury.
Unfortunately, follow-up trials to these promising findings were stopped early or aborted. ICTUS-2 was stopped early with limited recruitment. However, target temperatures appear were achieved in about 2 h with a combination of cold saline intravenous bolus and insertion of endovascular cooling device.[16] ReCCLAIM II was stopped due to lack of funding.
Despite neutral clinical results in COOLAID and ICTUS-L, endovascular methods would seem to be ideal for the application of hypothermia to acute ischemic stroke. Endovascular cooling is significantly more rapid than whole body or surface cooling methods. If utilized in a selective fashion, endovascular cooling should reduce or avoid the systemic side effects of cardiac dysfunction and coagulopathy, while providing a greater depth of hypothermia. Finally, endovascular techniques are already in use as recanalization strategies for stroke, so adding catheter-delivered hypothermia to the mix makes sense as a “neuroprotective bridge” to recanalization.
Despite decades of successful animal experimentation using hypothermia in both global and focal ischemia models, and recent clinical trials showing benefit in both adults and neonatal global ischemia, there is no evidence that supports the use of hypothermia for acute ischemic stroke in humans.[22,28,31] This could be due to the prolonged time to induce whole-body hypothermia, uncertainties regarding the depth and duration of hypothermia required for clinical benefit, and limitations to whole-body hypothermia imposed by systemic complications. While some of these factors could be addressed using selective hypothermia, the benefit of hypothermia in human stroke remains hypothetical. A large animal model could address these issues in a transferable way prior to further human trials. The ideal animal model would provide both a reproducible ischemic stroke and a relative size similar to humans for endovascular catheter work.
Endovascular selective hypothermia
New catheter technology to administer selective cooling has become available that allows the marriage of endovascular cooling with a selective transcarotid approach. The “Twin-Flo” dual-lumen catheter (Thermopeutix Inc., San Diego, CA) consists of a 9.5-French balloon catheter coaxially introduced through a 14-French outer catheter. The outer catheter is positioned in the aorta and is used to withdraw oxygenated blood to an extracorporeal perfusion unit, wherein the blood is cooled and then reinfused via the balloon catheter into the common carotid artery ipsilateral to the ischemic zone [Figure 1]. The Twin-Flo (originally called Duo-Flo) has received 510k approval for human use in 2010 from the US FDA.
Figure 1.
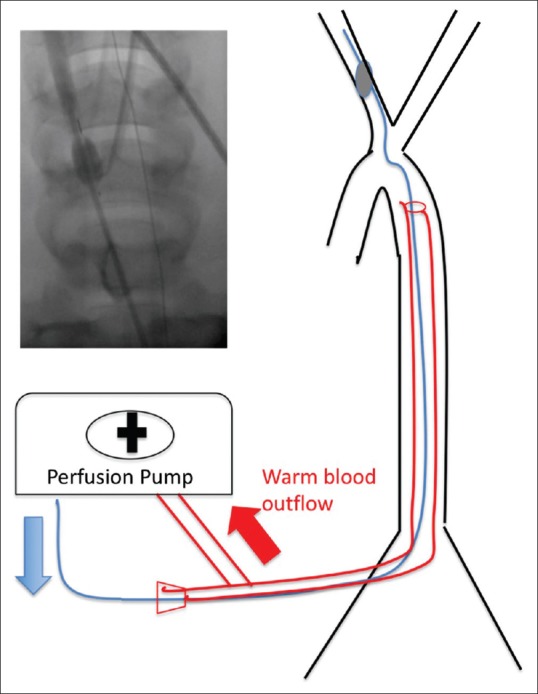
TwinFlo schematic. Blood is pulled from the aorta, cooled in a perfusion pump without oxygenator, and reinfused beyond the inflated balloon into the carotid artery. Inset shows angiogram with inflated balloon with dye beyond flowing into the right carotid artery
To test this device, we developed a new variation of a porcine model of ischemic stroke. We chose a porcine model for a number of reasons. Endovascular training models in pigs weighing 30–45 kg are well established.[33,34,35] Ischemic stroke models in pigs are also well established.[36,37,38] Pigs have a relatively thick skull that has led investigators to use either transorbital approaches or smaller specimens via a traditional craniotomy. Both approaches allow the study of permanent or transient MCA occlusion. Unlike rodents, no additional carotid clamping is required, making swine models much more like the human acute stroke situation. While small porcine models are considered advantageous for ease of handling and for craniotomy, their vasculature is too small to evaluate the use of endovascular tools designed for humans.[37] We note the same arguments are applicable to many large animal model species.[23]
It is in this milieu that we conducted our experiments.[39] The goal was to merge the advantages of endovascular cooling (speed) with placement into the carotid artery (selectivity) to efficiently reduce ipsilateral brain tissue temperature in a transient occlusion porcine stroke model through an entirely percutaneous approach. Our model is unique in that it allows for a traditional transcranial (rather than transorbital) approach for an adaptable occlusion of the MCAs in swine sized for endovascular device evaluation. It should be noted that swine have rete mirabile, so a precise MCA transcatheter occlusion is not possible. Thus, direct clipping of the MCA is the most appropriate way of inducing focal ischemia. In addition, swine have 2–5 MCA branches.[37] Other authors have performed thermocautery for a permanent occlusion of the MCA, but we demonstrated a transient ischemia model using commercially available aneurysm clips and microsurgical techniques familiar to neurosurgeons.
Our data have been previously published.[39] We demonstrated that selective and profound hypothermia could be established through an entirely percutaneous approach. This approach produced a statistically significant reduction in stroke volume by MRI and a similar trend on H and E histology. This was despite an almost 4.5 h delay from onset of ischemia to establishment of significant hypothermia, which we defined as hemicranial temperature <30°C. Our temperatures achieved (mean 26.5°C) and our “time to target temperature” were <30 min, which is clearly in line with other studies utilizing selective recirculated cold blood (cold blood perfusion, CBP) [Figure 2]. To date, this remains the largest paired large animal series evaluating hypothermia versus normothermic controls in focal ischemia [Table 1]. Furthermore, this study also had the longest ischemic insult time prior to induction of hypothermia (3 h).
Figure 2.
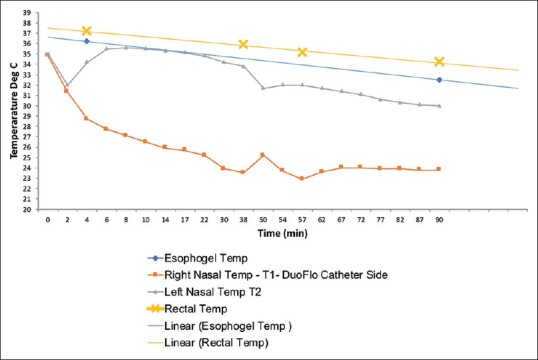
Representative selective hypothermia curve. Note the dramatic difference between core temperatures (esophageal and rectal), and nasopharyngeal ipsilateral and contralateral to the TwinFlo device. The slope of the curves will vary depending on the rate of infusion and the temperature of the infused blood. However, at no point in this 90 min experiment did the core temperatures drop to an unsafe level (e.g. <33°C)
Table 1.
Large animal selective hypothermia experiments
| Author | Method | Species | Ischemia type | Total n | NT | sHT | T to TT (min) | TT(°C) |
|---|---|---|---|---|---|---|---|---|
| Lougheed and Kahn[3] | Bypass | Canine | Global | 6 | 6 | 15-20 | 20 | |
| Schwartz et al.[24] | Bypass | Baboon | 12 | 12 | 26±13 | 18.5 | ||
| Schwartz et al.[25] | Bypass | Baboon | Focal | 8 | 4 | 4 | 12±4 | 27 |
| Mori et al.[12] | Bypass | Swine | Global | 12 | 6 | 6 | 60-120 | 32 |
| Zhang et al.[40] | Epidural | Swine | Focal | 12 | 6 | 6 | 5 | 28 |
| Verdura et al.[10] | Bypass | Canine | Global | 21 | 5 | 16 | 35 | 15 |
| Mattingly et al.[39] | Endovascular Bypass |
Swine | Focal | 25 | 13 | 12 | 25 | <30 |
| Cattaneo et al.[41] | Cooling catheter | Sheep | None | 8 | 180 | 33 | ||
| Wang et al.[42] | ICS | Monkey | None | 4 | 4 | 10 | 33 |
TT: Target temperature, T to TT: Time to TT, NT: Normothermia, sHT: Selective hypothermia, ICS: Ice-cold saline
Techniques in Selective Hypothermia
There is a discrepancy between the evidence of benefit for mild hypothermia (32°C–36°C) and the substantial evidence for significant cerebral metabolism reduction seen with more profound hypothermia.[9] In addition, in an earlier meta-analysis, there appeared to be substantial benefit with very mild hypothermia (35°C) in animal experiments.[15] A later meta-analysis with grouping of target temperatures did not find a difference between less and more aggressive hypothermia.[16] Our group argues that the profound reductions in CMRO2 benefit but that the complications with whole-body hypothermia require a selective approach. With the aid of new catheter technology, we successfully applied cold blood perfusion as a means of achieving moderate to deep hypothermia for neuroprotection without the complications of systemic hypothermia.
Ice-cold saline
No discussion of selective hypothermia is complete without a discussion of ice-cold saline (ICS). There is significant small animal, computational, and some human data to support the safety of this technique. The basic argument, again based mostly on rodent data, is that while deep hypothermia (10°C–24°C) is more effective for global ischemia, whereas whole-body cooling for focal ischemia seems to favor a mild hypothermia (33°C–34°C).[43] A number of techniques are available for a more selective approach to this, but the one that seems most logical is ICS. In a filament occlusion model in rats, saline flushing alone may reduce infarct volume, but the effect is particularly seen with cold saline.[44] Direct measurements of brain temperature indicate a drop to 33°C.[45]
In a series of papers, a theoretical model was developed to evaluate the effect of ICS infusion on human brain temperature. Initial modeling suggested a rate of 30 ml/min × 10 min would produce a brain temperature of 33°C–34°C.[46] The first human study showed only mild changes on jugular bulb temperatures (<1°C) and very minimal core temperature reduction.[29] These were elective patients undergoing angiography for treatment follow-up. Apart from limited shivering, no side effects were seen, and based on TCD, no vasospasm was seen. Subsequently, the mathematical model was updated to predict a 2°C drop in brain temperature with a 10 min infusion of cold saline (the infused saline was measured at 8°C).[47] There is also note of significant differential in inlet versus outlet temperatures due to countercurrent heat transfer expected from flowing blood around the catheter.
In the only large animal experiment available on intracarotid ICS, 4 Rhesus monkeys underwent infusion of 100 ml of ICS over 20 min via a commercially available microcatheter-produced mild hypothermia within 10 min, reaching again 33°C-34°C with minimal systemic cooling.[42] This study specifically did not find evidence for vasospasm, edema, or stroke caused by the procedure. To date, there is no large animal study showing stroke volume reduction utilizing intracarotid infusion of ICS.
A noteworthy variation in ICS used an epidural catheter placed via burrhole to provide selective hypothermia.[40] Using this technique in a permanent MCA occlusion model in swine, temperatures were reduced within 5 min below 30°C in deep brain tissue and maintained for 5 h. Percentage infarct volume was reduced by a statistically significant amount.
Finally, there are two series from the same group applying ICS to ischemic stroke. The first series of 26 patients were treated from 2013 to 14 with mechanical thrombectomy with pre- and postreperfusion intracarotid infusion of ICS.[48] Hemodynamics and basic chemistry and hematocrit appeared stable despite a total infusion of 350 ml of ICS. A subsequent nonrandomized prospective trial enrolled 45 mechanical thrombectomy patients and showed that outcomes and complications appear similar to nonenrolled.[49] When corrected for a number of clinical factors, the final infarct volume appeared smaller in the treated patients. These human series have no direct brain temperature measurements, have selection bias, but do provide further evidence that selective hypothermia via ICS is at least safe even in acute ischemic stroke, although efficacy remains to be demonstrated.
Passive cooling catheters
Another approach for endovascular selective hypothermia is to passively cool the passing blood via a device placed in the carotid. This is a similar idea to the technique for whole-body endovascular cooling used in COOLAID and ICTUS. Instead of placing the device in the inferior vena cava, the catheter is placed in the carotid artery to provide simultaneous access for mechanical thrombectomy and selective hypothermia.[50] In a study of sheep, the effectiveness of this methodology was evaluated.[41] Similar to the TwinFlo system our group described, they introduced their device via an 8F shuttle sheath transfemorally and placed the device into the common carotid artery under fluoroscopy and systemic heparinization. This system uses coolant circulation within a balloon and was maintained for 60 min in the first animal and 180 min in 8 additional animals. Direct cortical brain temperatures were reported as change from baseline. A reduction of 4°C was obtained in just over 2 h, but that degree of reduction was only obtained in 6 out of 8 animals. However, if the target temperature is only 35°C, then this was achievable in 30–40 min. Much like ICS, data regarding efficacy of this technique in ischemic stroke are lacking. However, from a safety standpoint, there did not appear to be thromboembolic complications based on DSA (not histology). Vasospasm was seen at the location of the cooling catheters in the carotid in 6/9 animals but was reported as mild and transient.
Perspectives and Future Directions
Hypothermia is the only neuroprotectant to withstand the test of time and has shown efficacy in both small and large animal models, in addition to selected human clinical scenarios, particularly global ischemia. In focal ischemia, rodent data suggest that mild hypothermia is sufficient. However, large animal evidence supports moderate-to-deep hypothermia. To avoid well-known systemic complications, this requires a selective approach. In the limited number of large animal controlled studies of selective hypothermia, the results have been almost uniformly positive. Of the various methods of selective hypothermia available, only cold blood perfusion has repeatedly been shown to be efficacious for focal ischemia. This is most likely due to the speed of onset and the depth of hypothermia achieved.
With purely percutaneous delivery of selective hypothermia, the speed of setup is shortened such that moderate-to-deep hypothermia is now possible within timeframes appropriate for stroke intervention. Devices such as the TwinFlo described by our group and the balloon cooling catheter system described by Cattaneo have channels for direct mechanical thrombectomy catheters, recognizing the critical role of reperfusion in acute ischemic stroke.[39,41,51] Questions that remain include the timing of reperfusion relative to hypothermia and the duration of hypothermia required for neuroprotective effect. For cold blood perfusion, integration of perfusionists into the stroke intervention pool or a readily available “fool-proof” perfusion setup are required to ensure safe delivery, avoiding issues of hyperperfusion, or insufficient anticoagulation that may impair outcomes.[11] Finally, selective hypothermia experiments including ours have noted contralateral cerebral hemisphere cooling.[3,13,24,39] This should be explored further as it may have implications for global ischemia.
In summary, selective hypothermia produces moderate-to-deep hypothermia without the systemic complications seen in both large animals and humans in temperatures < 32°C–34°C. While there is a tremendous amount of support for mild hypothermia from rodent models, the translation of rodent data to human clinical trials has been poor to date. However, large animal data from several studies show that moderate-to-deep selective hypothermia is effective radiographically, histologically, and even clinically. The same degree of evidence is simply not available for alternative selective cooling methods that produce only mild hypothermia. Thus, we believe that cold blood perfusion provides the most effective and reliable way to induce clinically significant selective hypothermia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bigelow WG, Lindsay WK, Greenwood WF. Hypothermia; its possible role in cardiac surgery: An investigation of factors governing survival in dogs at low body temperatures. Ann Surg. 1950;132:849–66. doi: 10.1097/00000658-195011000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Botterell EH, Lougheed WM, Scott JW, Vandewater SL. Hypothermia, and interruption of carotid, or carotid and vertebral circulation, in the surgical management of intracranial aneurysms. J Neurosurg. 1956;13:1–42. doi: 10.3171/jns.1956.13.1.0001. [DOI] [PubMed] [Google Scholar]
- 3.Lougheed WM, Kahn DS. Circumvention of anoxia during arrest of cerebral circulation for intracranial surgery. J Neurosurg. 1955;12:226–39. doi: 10.3171/jns.1955.12.3.0226. [DOI] [PubMed] [Google Scholar]
- 4.Drake CG, Barr HW, Coles JC, Gergely NF. The use of extracorporeal circulation and profound hypothermia in the treatment of ruptured intracranial aneurysm. J Neurosurg. 1964;21:575–81. doi: 10.3171/jns.1964.21.7.0575. [DOI] [PubMed] [Google Scholar]
- 5.Lawton MT, Raudzens PA, Zabramski JM, Spetzler RF. Hypothermic circulatory arrest in neurovascular surgery: Evolving indications and predictors of patient outcome. Neurosurgery. 1998;43:10–20. doi: 10.1097/00006123-199807000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert M, Busund R, Skagseth A, Nilsen PA, Solbø JP. Resuscitation from accidental hypothermia of 13.7 degrees C with circulatory arrest. Lancet. 2000;355:375–6. doi: 10.1016/S0140-6736(00)01021-7. [DOI] [PubMed] [Google Scholar]
- 7.Walpoth BH, Walpoth-Aslan BN, Mattle HP, Radanov BP, Schroth G, Schaeffler L, et al. Outcome of survivors of accidental deep hypothermia and circulatory arrest treated with extracorporeal blood warming. N Engl J Med. 1997;337:1500–5. doi: 10.1056/NEJM199711203372103. [DOI] [PubMed] [Google Scholar]
- 8.Michenfelder JD, Milde JH. The effect of profound levels of hypothermia (below 14 degrees C) on canine cerebral metabolism. J Cereb Blood Flow Metab. 1992;12:877–80. doi: 10.1038/jcbfm.1992.120. [DOI] [PubMed] [Google Scholar]
- 9.Lanier WL. Cerebral metabolic rate and hypothermia: Their relationship with ischemic neurologic injury. J Neurosurg Anesthesiol. 1995;7:216–21. doi: 10.1097/00008506-199507000-00021. [DOI] [PubMed] [Google Scholar]
- 10.Verdura J, White RJ, Albin MS. Profound selective hypothermia and arrest of arterial circulation to the dog brain. J Neurosurg. 1966;24:1002–6. doi: 10.3171/jns.1966.24.6.1002. [DOI] [PubMed] [Google Scholar]
- 11.Williams BN, Turner EA. Report of 10 operations under local cerebral hypothermia. J Neurol Neurosurg Psychiatry. 1970;33:647–55. doi: 10.1136/jnnp.33.5.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori K, Itoh Y, Saito J, Takeyama Y, Kurata Y, Kaneko M, et al. Post-resuscitative hypothermic bypass reduces ischemic brain injury in swine. Acad Emerg Med. 2001;8:937–45. doi: 10.1111/j.1553-2712.2001.tb01089.x. [DOI] [PubMed] [Google Scholar]
- 13.Mori K, Saito J, Kurata Y, Takeyama Y, Itoh Y, Kaneko M, et al. Rapid development of brain hypothermia using femoral-carotid bypass. Acad Emerg Med. 2001;8:303–8. doi: 10.1111/j.1553-2712.2001.tb02106.x. [DOI] [PubMed] [Google Scholar]
- 14.Ohta T, Kuroiwa T, Sakaguchi I, Sakai N, Moriwaki K. Selective hypothermic perfusion of canine brain. Neurosurgery. 1996;38:1211–5. doi: 10.1097/00006123-199606000-00032. [DOI] [PubMed] [Google Scholar]
- 15.van der Worp HB, Sena ES, Donnan GA, Howells DW, Macleod MR. Hypothermia in animal models of acute ischaemic stroke: A systematic review and meta-analysis. Brain. 2007;130:3063–74. doi: 10.1093/brain/awm083. [DOI] [PubMed] [Google Scholar]
- 16.Dumitrascu OM, Lamb J, Lyden PD. Still cooling after all these years: Meta-analysis of pre-clinical trials of therapeutic hypothermia for acute ischemic stroke. J Cereb Blood Flow Metab. 2016;36:1157–64. doi: 10.1177/0271678X16645112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang ZG, Xue D, Preston E, Karbalai H, Buchan AM. Biphasic opening of the blood-brain barrier following transient focal ischemia: Effects of hypothermia. Can J Neurol Sci. 1999;26:298–304. doi: 10.1017/s0317167100000421. [DOI] [PubMed] [Google Scholar]
- 18.Kozlowski P, Buchan AM, Tuor UI, Xue D, Huang ZG, Chaundy KE, et al. Effect of temperature in focal ischemia of rat brain studied by 31P and 1H spectroscopic imaging. Magn Reson Med. 1997;37:346–54. doi: 10.1002/mrm.1910370307. [DOI] [PubMed] [Google Scholar]
- 19.Xue D, Huang ZG, Smith KE, Buchan AM. Immediate or delayed mild hypothermia prevents focal cerebral infarction. Brain Res. 1992;587:66–72. doi: 10.1016/0006-8993(92)91428-h. [DOI] [PubMed] [Google Scholar]
- 20.Onesti ST, Baker CJ, Sun PP, Solomon RA. Transient hypothermia reduces focal ischemic brain injury in the rat. Neurosurgery. 1991;29:369–73. doi: 10.1097/00006123-199109000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Goto Y, Kassell NF, Hiramatsu K, Soleau SW, Lee KS. Effects of intraischemic hypothermia on cerebral damage in a model of reversible focal ischemia. Neurosurgery. 1993;32:980–4. doi: 10.1227/00006123-199306000-00017. [DOI] [PubMed] [Google Scholar]
- 22.Froehler MT, Ovbiagele B. Therapeutic hypothermia for acute ischemic stroke. Expert Rev Cardiovasc Ther. 2010;8:593–603. doi: 10.1586/erc.09.129. [DOI] [PubMed] [Google Scholar]
- 23.Herrmann AM, Meckel S, Gounis MJ, Kringe L, Motschall E, Mülling C, et al. Large animals in neurointerventional research: A systematic review on models, techniques and their application in endovascular procedures for stroke, aneurysms and vascular malformations. J Cereb Blood Flow Metab. 2019;39:375–94. doi: 10.1177/0271678X19827446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schwartz AE, Stone JG, Finck AD, Sandhu AA, Mongero LB, Adams DC, et al. Isolated cerebral hypothermia by single carotid artery perfusion of extracorporeally cooled blood in baboons. Neurosurgery. 1996;39:577–81. doi: 10.1097/00006123-199609000-00028. [DOI] [PubMed] [Google Scholar]
- 25.Schwartz AE, Finck AD, Stone JG, Connolly ES, Edwards NM, Mongero L. Delayed selective cerebral hypothermia decreases infarct volume after reperfused stroke in baboons. J Neurosurg Anesthesiol. 2011;23:124–30. doi: 10.1097/ANA.0b013e3181fa75ca. [DOI] [PubMed] [Google Scholar]
- 26.Lownie SP, Menkis AH, Craen RA, Mezon B, MacDonald J, Steinman DA. Extracorporeal femoral to carotid artery perfusion in selective brain cooling for a giant aneurysm. Case report. J Neurosurg. 2004;100:343–7. doi: 10.3171/jns.2004.100.2.0343. [DOI] [PubMed] [Google Scholar]
- 27.Holzer M, Bernard SA, Hachimi-Idrissi S, Roine RO, Sterz F, Müllner M, et al. Hypothermia for neuroprotection after cardiac arrest: Systematic review and individual patient data meta-analysis. Crit Care Med. 2005;33:414–8. doi: 10.1097/01.ccm.0000153410.87750.53. [DOI] [PubMed] [Google Scholar]
- 28.Yenari MA, Hemmen TM. Therapeutic hypothermia for brain ischemia: Where have we come and where do we go? Stroke. 2010;41:S72–4. doi: 10.1161/STROKEAHA.110.595371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Choi JH, Marshall RS, Neimark MA, Konstas AA, Lin E, Chiang YT, et al. Selective brain cooling with endovascular intracarotid infusion of cold saline: A pilot feasibility study. AJNR Am J Neuroradiol. 2010;31:928–34. doi: 10.3174/ajnr.A1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.De Georgia MA, Krieger DW, Abou-Chebl A, Devlin TG, Jauss M, Davis SM, et al. Cooling for acute ischemic brain damage (COOL AID): A feasibility trial of endovascular cooling. Neurology. 2004;63:312–7. doi: 10.1212/01.wnl.0000129840.66938.75. [DOI] [PubMed] [Google Scholar]
- 31.Hemmen TM, Raman R, Guluma KZ, Meyer BC, Gomes JA, Cruz-Flores S, et al. Intravenous thrombolysis plus hypothermia for acute treatment of ischemic stroke (ICTuS-L): final results. Stroke. 2010;41:2265–70. doi: 10.1161/STROKEAHA.110.592295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Horn CM, Sun CH, Nogueira RG, Patel VN, Krishnan A, Glenn BA, et al. Endovascular reperfusion and cooling in cerebral acute ischemia (ReCCLAIM I) J Neurointerv Surg. 2014;6:91–5. doi: 10.1136/neurintsurg-2013-010656. [DOI] [PubMed] [Google Scholar]
- 33.Guglielmi G, Ji C, Massoud TF, Kurata A, Lownie SP, Viñuela F, et al. Experimental saccular aneurysms. II. A new model in swine. Neuroradiology. 1994;36:547–50. doi: 10.1007/BF00593518. [DOI] [PubMed] [Google Scholar]
- 34.Massoud TF, Ji C, Vinuela F, Turjman F, Guglielmi G, Duckwiler GR, et al. Laboratory simulations and training in endovascular embolotherapy with a swine arteriovenous malformation model. AJNR Am J Neuroradiol. 1996;17:271–9. [PMC free article] [PubMed] [Google Scholar]
- 35.Lee DH, Wriedt CH, Kaufmann JC, Pelz DM, Fox AJ, Vinuela F. Evaluation of three embolic agents in pig rete. AJNR Am J Neuroradiol. 1989;10:773–6. [PMC free article] [PubMed] [Google Scholar]
- 36.Sakoh M, Ostergaard L, Gjedde A, Røhl L, Vestergaard-Poulsen P, Smith DF, et al. Prediction of tissue survival after middle cerebral artery occlusion based on changes in the apparent diffusion of water. J Neurosurg. 2001;95:450–8. doi: 10.3171/jns.2001.95.3.0450. [DOI] [PubMed] [Google Scholar]
- 37.Imai H, Konno K, Nakamura M, Shimizu T, Kubota C, Seki K, et al. A new model of focal cerebral ischemia in the miniature pig. J Neurosurg. 2006;104:123–32. doi: 10.3171/ped.2006.104.2.123. [DOI] [PubMed] [Google Scholar]
- 38.Cooper JA, Tichauer KM, Boulton M, Elliott J, Diop M, Arango M, et al. Continuous monitoring of absolute cerebral blood flow by near-infrared spectroscopy during global and focal temporary vessel occlusion. J Appl Physiol (1985) 2011;110:1691–8. doi: 10.1152/japplphysiol.01458.2010. [DOI] [PubMed] [Google Scholar]
- 39.Mattingly TK, Denning LM, Siroen KL, Lehrbass B, Lopez-Ojeda P, Stitt L, et al. Catheter based selective hypothermia reduces stroke volume during focal cerebral ischemia in swine. J Neurointerv Surg. 2016;8:418–22. doi: 10.1136/neurintsurg-2014-011562. [DOI] [PubMed] [Google Scholar]
- 40.Zhang L, Cheng H, Shi J, Chen J. Focal epidural cooling reduces the infarction volume of permanent middle cerebral artery occlusion in swine. Surg Neurol. 2007;67:117–21. doi: 10.1016/j.surneu.2006.05.064. [DOI] [PubMed] [Google Scholar]
- 41.Cattaneo G, Schumacher M, Maurer C, Wolfertz J, Jost T, Büchert M, et al. Endovascular cooling catheter for selective brain hypothermia: An animal feasibility study of cooling performance. AJNR Am J Neuroradiol. 2016;37:885–91. doi: 10.3174/ajnr.A4625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang B, Wu D, Dornbos Iii D, Shi J, Ma Y, Zhang M, et al. Local cerebral hypothermia induced by selective infusion of cold lactated ringer's: A feasibility study in rhesus monkeys. Neurol Res. 2016;38:545–52. doi: 10.1080/01616412.2016.1187827. [DOI] [PubMed] [Google Scholar]
- 43.Esposito E, Ebner M, Ziemann U, Poli S. In cold blood: intraarteral cold infusions for selective brain cooling in stroke. J Cereb Blood Flow Metab. 2014;34:743–52. doi: 10.1038/jcbfm.2014.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ji Y, Hu Y, Wu Y, Ji Z, Song W, Wang S, et al. Therapeutic time window of hypothermia is broader than cerebral artery flushing in carotid saline infusion after transient focal ischemic stroke in rats. Neurol Res. 2012;34:657–63. doi: 10.1179/1743132812Y.0000000061. [DOI] [PubMed] [Google Scholar]
- 45.Zhao WH, Ji XM, Ling F, Ding YC, Xing CH, Wu H, et al. Local mild hypothermia induced by intra-arterial cold saline infusion prolongs the time window of onset of reperfusion injury after transient focal ischemia in rats. Neurol Res. 2009;31:43–51. doi: 10.1179/174313208X327982. [DOI] [PubMed] [Google Scholar]
- 46.Konstas AA, Neimark MA, Laine AF, Pile-Spellman J. A theoretical model of selective cooling using intracarotid cold saline infusion in the human brain. J Appl Physiol (1985) 2007;102:1329–40. doi: 10.1152/japplphysiol.00805.2006. [DOI] [PubMed] [Google Scholar]
- 47.Neimark MA, Konstas AA, Lee L, Laine AF, Pile-Spellman J, Choi J. Brain temperature changes during selective cooling with endovascular intracarotid cold saline infusion: Simulation using human data fitted with an integrated mathematical model. J Neurointerv Surg. 2013;5:165–71. doi: 10.1136/neurintsurg-2011-010150. [DOI] [PubMed] [Google Scholar]
- 48.Chen J, Liu L, Zhang H, Geng X, Jiao L, Li G, et al. Endovascular hypothermia in acute ischemic stroke: Pilot study of selective intra-arterial cold saline infusion. Stroke. 2016;47:1933–5. doi: 10.1161/STROKEAHA.116.012727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wu C, Zhao W, An H, Wu L, Chen J, Hussain M, et al. Safety, feasibility, and potential efficacy of intraarterial selective cooling infusion for stroke patients treated with mechanical thrombectomy. J Cereb Blood Flow Metab. 2018;38:2251–60. doi: 10.1177/0271678X18790139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cattaneo G, Schumacher M, Wolfertz J, Jost T, Meckel S. Combined selective cerebral hypothermia and mechanical artery recanalization in acute ischemic stroke:In vitro study of cooling performance. AJNR Am J Neuroradiol. 2015;36:2114–20. doi: 10.3174/ajnr.A4434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goyal M, Menon BK, van Zwam WH, Dippel DW, Mitchell PJ, Demchuk AM, et al. Endovascular thrombectomy after large-vessel ischaemic stroke: A meta-analysis of individual patient data from five randomised trials. Lancet. 2016;387:1723–31. doi: 10.1016/S0140-6736(16)00163-X. [DOI] [PubMed] [Google Scholar]


