Abstract
BACKGROUND AND PURPOSE:
Cofilin-actin rods are covalently linked aggregates of cofilin-1 and actin. Under ischemic conditions, these rods have been observed in neuronal dendrites and axons and may contribute to the loss of these processes. Hypothermia (Hypo) and the 70 kD inducible heat shock protein (Hsp70) are both known to improve outcomes after stroke, but the mechanisms are uncertain. Here, we evaluated the effect of these factors on cofilin-actin rod formation in a mouse model of stroke.
MATERIALS AND METHODS:
Mice were subjected to distal middle cerebral artery occlusion (dMCAO) and treated with Hypo using a paradigm previously shown to be neuroprotective. We similarly studied mice that overexpressed transgenic (Tg) or were deficient knockout (Ko) in the inducible 70 kDa heat shock protein (Hsp70), also previously shown to be protective by our group and others. Cofilin-actin rod formation was assessed by histological analysis at 4 and 24 h after dMCAO. Its expression was analyzed in three different regions, namely, infarct core (the center of the infarct), middle cerebral artery (MCA) borderzone (the edge of the brain regions supplied by the MCA), and the ischemic borderzone (border of ischemic lesion). Ischemic lesion size and neurological deficits were also assessed.
RESULTS:
Both Hypo-treated and Hsp70 Tg mice had smaller lesion sizes and improved neurological outcomes, whereas Hsp70 Ko mice had larger lesion sizes and worsened neurological outcomes. Cofilin-actin rods were increased after stroke, but were reduced by therapeutic Hypo and in Hsp70 Tg mice. In contrast, cofilin-actin rods were increased in ischemic brains of Hsp70 Ko mice.
CONCLUSIONS:
Cofilin-actin rod formation was suppressed under the conditions of neuroprotection and increased under circumstances where outcome was worsened. This suggests that cofilin-actin rods may act to participate in or exacerbate ischemic pathology and warrants further study as a potential therapeutic target.
Keywords: Cofilin-actin rod, heat shock protein 70, hypothermia, neuroprotection, stroke
Introduction
Cofilin-actin rods, or cofilin rods, are linear intracellular aggregates comprised of a 1:1 ratio of actin and cofilin that form selectively in neurons, and have been observed largely in neurites. These rods have been characterized in human Alzheimer's disease and in models of neurodegenerative disorders, where they have been suggested to contribute to neurodegeneration.[1] Cofilin rods are induced by adenosine triphosphate (ATP) depletion or oxidative stress, both of which have been well documented in brain ischemia. The biochemical factors driving rod formation are now well established. One key step is dephosphorylation of cofilin-1, through reduced activity of LIM kinase or increased activity of slingshot (SL) phosphatase SL-1, which promotes cofilin-1 binding to actin. A second key step is the formation of intermolecular disulfide bonds between cofilin-1 molecules.[1,2] The rods form macromolecular aggregates that interrupt the microtubule skeleton within affected neurites and, as such, may affect organelle transport and lead to synapse disruption and loss of dendritic spines. A causal link between rod formation and distal neurite demise has been demonstrated by simple overexpression of cofilin-1, which is sufficient to induce rod formation and associated neurite degeneration.[3] It has also been demonstrated by neurite preservation after targeted suppression of rod formation.[4,5] The mechanisms by which rod formation causes neurite demise remain to be established, but impaired axonal and dendritic transport, mitochondrial fission, prolonged mitochondrial depolarization, and ATP depletion have been observed in neurite-containing rods, and may be contributory.[3,6,7,8] In neuron cultures, rods can be induced by peroxide, ATP depletion, glutamate, and endothelin 1.[3,5,6]
Studies on Alzheimer's disease indicate that rod formation may be triggered by Aβ protein and prion protein-dependent generation of reactive oxygen species. Further, loss of dendrites and spines has been correlated to cognitive impairment.[9] Dendritic injury has been less studied in brain ischemia, but it has been established that ischemia also leads to loss of dendrites and spines in neurons, even with survival of the parent cell body.[10,11,12,13,14] A recent article from our group showed cofilin rod formation after both transient and permanent brain ischemia.[15] Rod formation was observed within 4 h after reperfusion, and where infarction occurred, rods persisted for at least 24 h and extended into the peri-infarct tissue. Rods were observed only in neurons, and within cell bodies as well as processes, particularly dendrites. In another recent study, suppression of rod formation using viral-mediated overexpression of LIM kinase showed a protective effect on synaptic function and dendritic structure in both cultured neurons and a rat transient ischemia model.[4]
To further clarify the significance of rod formation, we undertook this study to compare rod formation in well-established models of neuroprotection in experimental stroke. We anticipate that rod formation under the conditions of ischemic neuroprotection may provide insight into the significance of these rods. In this study, we chose therapeutic hypothermia (Hypo) and the 70 kD inducible heat shock protein (Hsp70) as models of neuroprotection. We and others have previously shown that therapeutic cooling and Hsp70 overexpression or induction led to consistent reduction in lesion size and improvement in neurological deficits.[16,17] We also compared this in a model of Hsp70 deficiency, where ischemic brain injury led to worsened outcomes.[18] We predicted, based on past literature, that cofilin rods should increase with ischemic brain injury and decrease under neuroprotective conditions.
Materials and Methods
Animals
All experimental procedures were approved by the San Francisco Veterans Affairs Medical Center Institutional Animal Care and Use Committee, and were in accordance with the National Institutes of Health and Animal Research: Reporting in vivo Experiments guidelines.
For the genetic mouse models, male Hsp70 transgenic (Hsp70Tg) and Hsp70 knockout (Hsp70Ko) mice were produced from breeder mice originally generated by the Dillmann (University of California, San Diego)[19] and Pandita (Southwestern University) laboratories,[20] as described in our previous report.[18] Hemizygotic (Hsp70 Tg) and homozygotic (Hsp70 Ko) mice were compared with wild-type (Wt) littermates. All other experiments were carried out on male C57/BL6 mice.
Stroke model and hypothermia treatment
All surgical techniques were performed under aseptic conditions. Stroke surgeries were performed under inhalational anesthesia. Anesthesia was induced by inhalation of 3% isoflurane in medical air: O2 (80%:20%). Surgical planes of anesthesia were assessed by the absence of hindleg withdrawal in response to a pinch. A 1-cm skin incision was created between the left margin of the orbit and the tragus, and the temporalis muscle was incised. A focal cerebral infarct was induced by permanent occlusion of the left distal middle cerebral artery (dMCAO) as previously described.[21,22] Briefly, a small craniotomy was made above the proximal segment of the middle cerebral artery (MCA), and the MCA was exposed after the dura was opened and retracted. The MCA was occluded by coagulation at the MCA segment just proximal to the olfactory branch. Rectal temperature was maintained between 36.5°C and 37.5°C during the procedures by using a thermometer connected to a heating pad. At the end of the surgery, the craniotomy and incision sites were closed, and the animals were allowed to recover. Mice were returned to their cages, were allowed free access to food and water, and were housed in a climate-controlled environment (25°C). The mice were included in the study if they showed neurological deficits as defined by a Bederson score of at least 3 (circling behavior) at 24 h post-dMCAO.
For Hypo studies, Wt mice were randomly divided into a Hypo treatment group and a normothermic (nontreatment, control) group. In the Hypo group, cooling began at the time of dMCAO and maintained for 2 h with a rectal temperature of 31°C, followed by rewarming. This paradigm was chosen as it was previously shown to consistently lead to neuroprotection in our hands.[23,24] In the normothermic group, rectal temperature was maintained in the normal range (36.5°C–37.5°C) throughout the experiment.
Animals with no observable neurological deficits at the time of recovery from anesthesia were removed from the experiment. The animals were sacrificed at 4, 24, and 48 h after ischemia for the following studies.
Neurobehavioral assessment
Neurological assessments using a modified Bederson's score were performed using a neurological scoring system before surgery and 2 and 24 h afterward.[25,26] This score was modified for use in mice and applied as follows: 0, no observable neurological deficit; 1, unable to extend the contralateral forelimb; 2, flexion of the contralateral forelimb; 3, mild circling to the contralateral side; 4, severe circling; and 5, falling to the contralateral side.
Histology
At the end of the observational period, the mice were euthanized with an isoflurane overdose followed by brain removal. The mice were perfused transcardially with cold normal saline. Brains were sunk in 20% sucrose overnight and frozen at − 80°C. Frozen sections (40 μm thickness) were cryosectioned in the coronal plane. Sections were permeabilized with 95% methanol and 5% 0.01 M phosphate-buffered saline for 15 minutes at -20°C.[15]
Infarct volume assessment
To evaluate infarct volume, the brain sections were stained with cresyl violet, as previously described.[18,27] Coronal sections at the level of the third ventricle, 1.2 mm posterior to the bregma, were stained with cresyl violet. We identified areas of damage depending on microscopic evidence of cell death. The infarct areas were measured using an image analysis system (ImageJ, NIH, Bethesda, MD, USA) and calculated by subtracting the normal ipsilateral area from that of the contralateral hemisphere to correct for brain edema.[28]
Immunohistochemistry and image analysis
To assess the temporal change of cofilin rod formation after stroke, tissue sections were harvested 4 and 24 h after stroke and were stained by rabbit anti-cofilin antibody (1:500, ACFL02, Cytoskeleton, Denver, CO, USA). After overnight (4°C) incubation in primary antibodies, the sections were reacted with secondary antibodies (Alexa Fluor 594 conjugated goat anti-rabbit IgG (1:1000, Life Technologies, Carlsbad, CA, USA) at room temperature for 1 h. The stained sections were mounted on glass slides in 4',6'-diadino-2-phenylindole (DAPI)-containing mounting medium (Vector Laboratories, Burlingame, CA, USA). Antibody to cofilin-1 was used to identify the formation of cofilin-actin rods, utilizing the fact that the aggregated cofilin-1 in the rods generates a signal far greater than the background cofilin-1 signal.[15]
All images were prepared for confocal microscopy under identical illumination and data capture conditions. Investigators photographing and analyzing the immunostains were blinded to the experimental conditions.
For the quantitative analysis, a coronal section 0.2 mm posterior to the bregma was used. For each section, quantitative analysis of cofilin-actin rod expression was carried out in predefined regions: the “MCA border, the “ischemic core,” and the “ischemic borderzone.” This approach was used to permit comparisons at different time points, during which the anatomical location of borderzone evolves. As the MCA border is assumed to be the farthest margin occupied by an infarction due to MCAO, the MCA border may be considered the area where the ischemic region may be spared with a suitable neuroprotective treatment; the MCA border was anatomically defined as constant regions at 1.8 and 3.6 mm lateral from the midline, within the cortex corresponding to the edge of the brain supplied by the MCA.[21,29] The “ischemic core” was defined as the center of the ischemic lesion, well within the boundaries of the infarct. The “ischemic borderzone” was defined as the region immediately adjacent to the ischemic lesion,[15] as visually delineated by NeuN and DAPI staining. These regions of interest (ROI) were defined as shown in Figures 1a and 3a. Brains were harvested 4 and 24 h after stroke. In each area, ROI (nonoverlapping high-power fields; × 630) were selected and photographed for quantitative analysis. Analysis of cofilin rod expression was performed using ImageJ software (NIH, Bethesda, MD, USA). The immuno-positive area was automatically measured with binary images processed by Image J software in the selected ROIs and expressed as percent of the area of ROI area, as reported previously.[15]
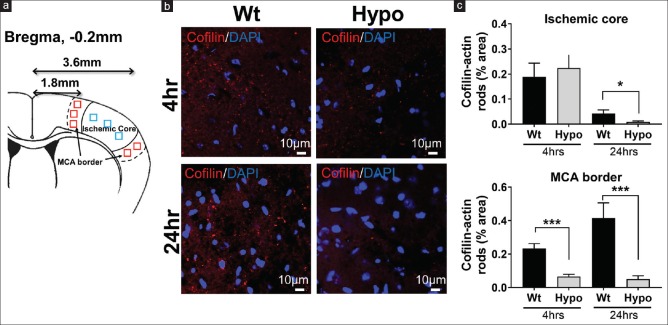
Figure 1.
Cofilin-actin rod formation was reduced by therapeutic hypothermia. (a) Schematic showing where regions of interests were sampled as they represented the ischemic core (3.6 mm from Bregma, blue squares) and the brain margins supplied by the middle cerebral artery (MCA border 1.8 mm from Bregma, red squares). (b) Representative images of cofilin (red) and DAPI (blue) staining taken from the MCA border at 4 and 24 h postmiddle cerebral artery occlusion among normothermic and hypothermic animals. Fewer rods were observed in the hypothermia brain. (c) Quantification of the brain regions shows the percentage area occupied by cofilin-actin rods within the ischemic core and middle cerebral artery (MCA) border at 4 and 24 h. Within the core, rods increased in both groups at 4 h and markedly reduced at 24 h, with significant reduction in the hypothermia group at 24 h. Within the MCA border, rods were more marked at 24 h compared to 4 h, but at both time points, rods were reduced in the hypothermia group (*P < 0.05, ***P < 0.01)
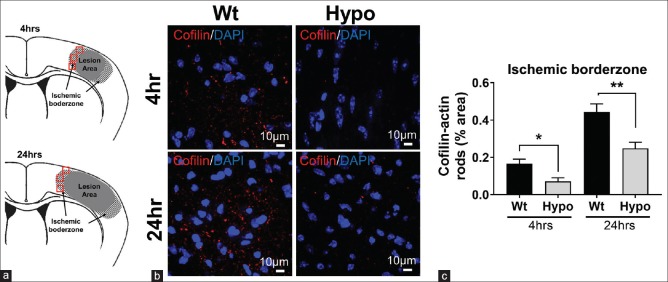
Figure 3.
Cofilin-actin rod formation was reduced by hypothermia in the ischemic borderzone. (a) Schematic showing where regions of interest were sampled from the ischemic borderzone. In contrast to the middle cerebral artery border (MCA border), this area was defined as the region adjacent to the infarct and where DAPI and NeuN staining were present (red squares). (b) Representative images taken from the ischemic borderzone regions of normothermic (Wt, wild type) and hypothermic (Hypo) brains showing cofilin rods (red). Nuclei are shown by DAPI staining (blue). (c) Quantification of cofilin staining from the borderzone region. Cofilin staining was higher at 24 h compared to 4 h, but the extent of staining was reduced in hypothermia brains at both time points (*P < 0.05, **P < 0.01)
Statistical analysis
The animals were randomized to the different experimental groups, and all ratings and analyses were performed by investigators blinded to the experimental conditions. Sample size calculations were performed based on our prior studies.[22,27] All statistical analyses were performed using GraphPad Prism version 7.0 (GraphPad Software, San Diego, CA, USA). All data were presented as the mean ± standard error. The differences between the two groups were compared using an unpaired t-test, and multiple comparisons were performed using one-way ANOVA followed by Bonferroni's post hoc test where data were normally distributed. Nonparametric tests of Mann–Whitney or Wilcoxon rank-sum were applied where data were not normally distributed. P < 0.05 was considered statistically significant.
Results
All animals survived the stroke surgeries. There were no mortalities and no exclusions.
Therapeutic hypothermia is neuroprotective against experimental stroke
Consistent with previously published reports, therapeutic Hypo provided neuroprotection against experimental stroke in mice.[23] Neurological deficits after dMCAO surgery were significantly better in the hypothermia-treated mice (Hypo) compared to that of control mice (Wt) [Figure 2a]. At the time of recovery from anesthesia and Hypo treatment (2 h after dMCAO), no significant difference in neurological deficit was observed, whereas at the time of 24 h after dMCAO surgery, statistically significantly (P < 0.05) better score of modified Bederson's scoring system was observed in Hypo-treated mice (1.0 ± 0.0), compared to that of control mice (2.5 ± 0.39).
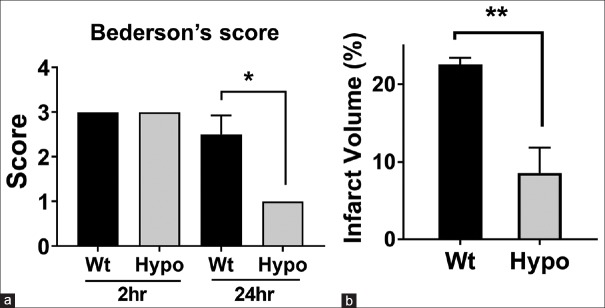
Figure 2.
Hypothermia protects against experimental stroke. (a) Bederson scores, to estimate neurological deficits, were improved under the conditions of therapeutic hypothermia (Hypo) 24 h post middle cerebral artery occlusion compared to animals exposed to middle cerebral artery occlusion under normothermic conditions (Wt, wild type). (b) Infarct volumes at 24 h were also smaller among hypothermia animals. (*P < 0.05, **P < 0.01)
In line with neurological assessments, Hypo decreased infarct volume after dMCAO [Figure 2b]. Hypothermic mice (Hypo; 8.6% ± 2.7%) showed statistically significantly (P < 0.01) smaller infarct volumes compared to that of control mice that remained normothermic (Wt; 22.6% ± 0.7%) from cresyl violet stains 48 h after dMCAO. These findings are in line with prior observations that Hypo treatment can provide a significant neuroprotective effect in experimental stroke.[17,23,30]
Hypothermia-reduced cofilin-actin rod formation
Cofilin-actin rod formation was assessed 4 and 24 h after dMCAO surgery in the MCA border and ischemic core (defined in the methods section). A representative image from the area of the MCA border [Figure 1b] shows that cofilin-actin rods are visible 4 h after dMCAO in the ischemic core and, to a lesser extent, the MCA borderzone. By 24 h, rod formation increased in the MCA borderzone among normothermic mice, whereas fewer rods were seen within the ischemic core. In contrast, rod formation reduced in mice treated with Hypo at both 4 and 24 h. Figure 1c shows quantitative results from the “ischemic core” and “penumbra.” In the “ischemic core,” the % area occupied by cofilin-actin rods statistically significantly (P < 0.05) decreased in Hypo mice at 24 h after stroke, compared to that of Wt. At the MCA border, Hypo mice had statistically significantly less rod formation compared to Wt mice, at both 4 (P < 0.05) and 24 h (P < 0.01) after stroke.
The reduction of cofilin rod formation by therapeutic Hypo was not simply due to reduced infarct volume, as it was also reduced in the ischemic borderzone regions of the smaller infarcts seen in brains of the Hypo mice [Figure 3]. When the ischemic borderzone area of the lesions was assessed, ROIs were set as shown in Figure 3a. The ischemic borderzone region varied from animal to animal, depending on where the edge of the NeuN staining occurred. Representative images obtained from borderzone regions [Figure 3b] show that in the Wt group, cofilin-actin rod formation increased at both 4 and 24 h after stroke, but markedly suppressed in Hypo mice. Quantitative analysis revealed that cofilin-actin rod formation statistically significantly reduced in mice with Hypo treatment (Hypo) compared to control mice (Wt), at both 4 (P < 0.05) and 24 h (P < 0.01) after stroke [Figure 3c]. These results suggest that under the conditions of therapeutic Hypo, cofilin-actin rod formation is reduced.
Hsp70 and neuroprotection after experimental stroke
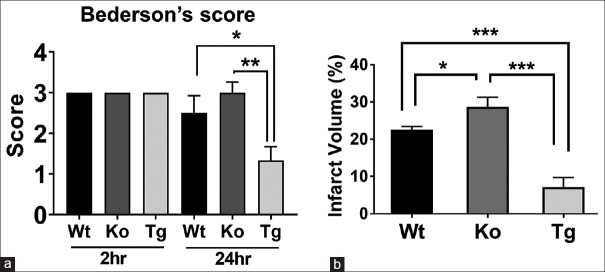
We then turned to a second model of neuroprotection and studied Tg mice that overexpress Hsp70 (Hsp70 Tg) and compared them to mice which lack Hsp70 (Hsp70Ko). We had previously shown that Hsp70 Tg mice have smaller infarcts and better neurological scores compared to Wt,[26] whereas Hsp70Ko mice have the opposite.[18] Consistent with our previously published reports,[18,26] Hsp70 overexpression led to improved neurological outcomes, whereas Hsp70 deficiency led to worsened outcomes compared to Wt mice. Neurological deficits assessed by the modified Bederson's scoring system improved among Hsp70 Tg mice (1.3 ± 0.3) and worsened in Hsp70Ko mice (3.0 ± 0.2), compared to that of Wt mice (2.5 ± 0.4), at 24 h after dMCAO surgery [Figure 4a]. Similarly, infarct volumes [Figure 4b] measured from cresyl violet-stained brain sections were significantly smaller in the Hsp70 Tg mice (7.2% ± 2.2%) and significantly larger in the Hsp70Ko mice (28.7% ± 2.2%), compared to that of Wt mice (22.5% ± 0.7%).
Figure 4.
Hsp70 overexpression improves outcome from experimental stroke, whereas Hsp70 deficiency worsens it. (a) Neurological deficits as estimated by the Bederson score were similar 2 h postmiddle cerebral artery occlusion among wild type (Wt), Hsp70-deficient (Ko, knockout) and Hsp70-overexpressing (Tg, transgenic) mice, but by 24 h, deficits were the most severe among the knockout and least severe among the transgenic groups. (b) Lesion volumes at 24 h increased in the knockout brains and decreased in the transgenic brains compared to wild type. *P < 0.05, **P < 0.01, ***P < 0.001
Hsp70 overexpression decreases and Hsp70 deficiency increases cofilin-actin rod expression
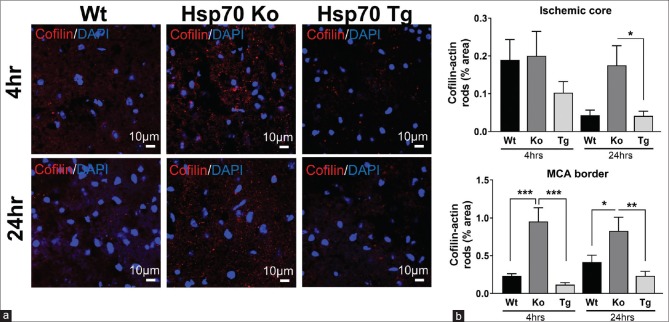
Similar to assessments carried out in the Hypo studies, formation of cofilin-actin rods was measured in the MCA border, ischemic core, and ischemic borderzone at 4 and 24 h after stroke. Representative images obtained from the MCA border are shown in Figure 5a. Compared to Wt mice, Hsp70Ko mice showed increased expression of cofilin-actin rod at both 4 and 24 h after stroke. In contrast, Hsp70Tg mice showed decreased expression of cofilin-actin rods at both 4 and 24 h after stroke. Results of the quantitative analysis in the ischemic core and MCA border are shown in Figure 5b. In the ischemic core, Hsp70Tg mice showed decreased cofilin-actin rod formation. In the area of the MCA borderzone, quantitative analysis revealed that cofilin-actin rod expression at both 4 and 24 h after stroke significantly increased in Hsp70Ko mice (4 h, P < 0.001; 24 h, P < 0.05) and significantly decreased in Hsp70Tg mice (4 h, P < 0.001; 24 h, P < 0.01), compared to Wt mice.
Figure 5.
Cofilin-actin rod formation was decreased by Hsp70 overexpression and increased by Hsp70 deficiency. (a) Representative images from the middle cerebral artery border of wild type (Wt), Hsp70 knockout (Ko), and Hsp70 transgenic (Tg) brains show that cofilin staining (red) is the highest among the Hsp70 knockout brain compared to wild type and Hsp70 transgenic. (b) Quantification of cofilin staining shows that Hsp70-deficient (knockout) brains had the most cofilin staining, whereas the least was seen in brains of mice where Hsp70 was overexpressed (transgenic). Wild type brains had an intermediate amount of cofilin staining (*P < 0.05, **P < 0.01, ***P < 0.001)
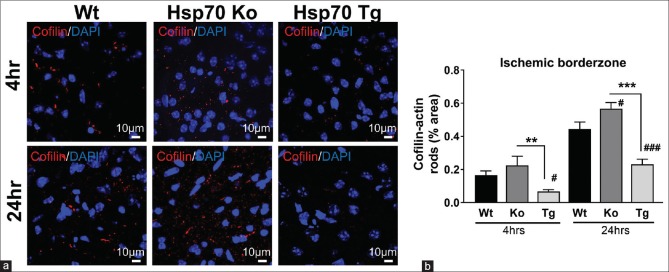
Similar to observations from the Hypo studies, cofilin rod formation was not simply altered because of the differences in infarct size. Representative images obtained from MCA borderzone regions are shown in Figure 6a. Hsp70Ko mice have increased cofilin-actin rod formation, whereas Hsp70Tg mice have fewer cofilin-actin rods at both 4 and 24 h after stroke, compared to that of Wt mice. Quantitative analysis revealed similar patterns comparing Hsp70Tg and Wt groups at both 4 (P < 0.05) and 24 h (P < 0.001) after stroke. In contrast, Hsp70Ko mice showed more cofilin-actin rods compared to Wt mice, at 24 h (P < 0.05) after stroke [Figure 6b].
Figure 6.
Cofilin-actin rod formation within the ischemic borderzone was decreased by Hsp70 overexpression and increased by Hsp70 deficiency. (a) Representative images from the ischemic borderzone regions of wild type (Wt), Hsp70 knockout (Ko), and Hsp70 transgenic (Tg) brains show that cofilin staining (red) is the highest among the Hsp70 knockout brain and lowest in the Hsp70 transgenic brain, with intermediate staining in the wild-type brain. (b) Quantification of cofilin staining shows that Hsp70 deficient (knockout) brains had the most cofilin staining, while the least was seen in Hsp70 overexpression (transgenic). Wild-type brains had an intermediate amount of cofilin staining (#P < 0.05 vs. wild type, ###P < 0.001 vs. wild type; **P < 0.01, ***P < 0.001)
Discussion
In line with prior reports of neuroprotection by therapeutic Hypo and Hsp70 overexpression, our studies also showed that these interventions led to improved neurological outcome, whereas mice deficient in Hsp70 (Hsp70Ko) had worsened outcomes. In parallel, cofilin-actin rod formation decreased under the conditions of improved outcomes and increased when neurological outcomes are worse. Together, these observations are consistent with the idea that cofilin-actin rod formation may contribute to ischemic brain injury, particularly injury to neuronal processes.
As both experiments led to changes in infarct volume due to the interventions themselves, this imposed a technical challenge for quantifying peri-infarct rod formation. Our first approach evaluated rod formation in all brains at the same anatomical site that corresponded to a penumbral region at the very edge of the territory supplied by the MCA (MCA border), but also where we observed rod formation in the normothermic Wt mice. However, that approach could be biased because that the same anatomical location in the Hypo and Hsp70 Tg mice would have been further from the infarct margins because their infarcts are generally smaller. Conversely, the anatomic location among Hsp70-deficient mice would be closer to the ischemic core due to the generally larger infarcts observed in these mice. For these reasons, we also assessed rod formation in regions defined by the infarct margin (ischemic borderzone), independent of the infarct size. As we show, using either criteria, both Hypo and Hsp70 overexpression suppressed rod formation at both 4 and 24 h, in addition to reducing infarct volume, whereas Hsp70 deficiency did the opposite.
Hypothermia has previously been shown to reduce oxidative stress in ischemic brain,[17] and oxidative stress has also been shown to drive cofilin-actin rod formation. Cofilin rod formation results from actin binding and requires dephosphorylation of cofilin-1. Rod formation also requires the formation of intermolecular disulphide bonds between cofilin-1 molecules. Both of these steps are promoted by oxidative stress in the following manner. First, SL phosphatase (SSH-1 L) dephosphorylates cofilin-1 and is itself activated by release from the scaffolding protein 14-3-3ζ under oxidant stress conditions. Second, the oxidation of cysteines 39 and 147 of cofilin-1 to sulfenic or sulfinic acids facilitates subsequent formation of intermolecular disulfide bonds between cofilin-1 molecules that stabilize rod formation.[1,2] These mechanisms thus provide a potential pathway by which Hypo suppresses rod formation, but given the pleiotropic effects of Hypo on cell physiology, other mechanisms are also likely possible.
How Hsp70 overexpression might suppress rod formation is not immediately apparent, as to our knowledge, no direct interactions between Hsp70 and cofilin-1 or actin have been described. However, Hsp70 has previously been shown to protect the hypothalamus against oxidative stress in models of heat stroke[31] and in cultured hippocampal neurons. Hsp70 has also been shown to attenuate inflammation after brain ischemia,[26,32] which, in turn, would also lead to reductions in oxidative stress. Hsp70 may affect rod formation simply by its chaperone functions, where it has been shown to prevent protein aggregation in several models of neurological disease.[33,34] Whether either property is a significant factor mediating the neuroprotection observed with Hypo remains to be established.
Conclusions
The observations presented here establish correlations between Hypo and reduced rod formation and between Hsp70 and reduced rod formation after brain ischemia, but they do not prove that reduced rod formation is a major factor mediating either beneficial effect. However, our observations that rod formation is reduced under the conditions of neuroprotection indicate that their presence is likely to be more harmful than beneficial in brain ischemia. Further, mechanisms of injury to neuronal processes (dendrites and axons) are poorly understood, and cofilin rod formation is becoming increasingly recognized as both a marker and participant in their pathology. Ongoing studies using genetic and pharmacological means of suppressing cofilin rod formation may be able to resolve this contribution of suppressed rod formation to preservation of neuronal processes by Hypo and Hsp70.
Financial support and sponsorship
This study was financially supported by the NIH NINDS (R01 NS106441 and R03 NS101246 to MY), the Veterans Affairs Merit Program (I01 BX00589 to MY), the Uehara Foundation (2016 Postdoctoral Fellowship to KK), and the National Research Foundation of Korea (NRF-2018R1C1B6006159 to JSY).
Conflicts of interest
There are no conflicts of interest.
References
- 1.Bamburg JR, Bernstein BW. Actin dynamics and cofilin-actin rods in Alzheimer disease. Cytoskeleton (Hoboken) 2016;73:477–97. doi: 10.1002/cm.21282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernstein BW, Bamburg JR. ADF/cofilin: A functional node in cell biology. Trends Cell Biol. 2010;20:187–95. doi: 10.1016/j.tcb.2010.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Minamide LS, Striegl AM, Boyle JA, Meberg PJ, Bamburg JR. Neurodegenerative stimuli induce persistent ADF/cofilin-actin rods that disrupt distal neurite function. Nat Cell Biol. 2000;2:628–36. doi: 10.1038/35023579. [DOI] [PubMed] [Google Scholar]
- 4.Shu L, Chen B, Chen B, Xu H, Wang G, Huang Y, et al. Brain ischemic insult induces cofilin rod formation leading to synaptic dysfunction in neurons. J Cereb Blood Flow Metab. 2019;39:2181–95. doi: 10.1177/0271678X18785567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tam SW, Feng R, Lau WK, Law AC, Yeung PK, Chung SK. Endothelin type B receptor promotes cofilin rod formation and dendritic loss in neurons by inducing oxidative stress and cofilin activation. J Biol Chem. 2019;294:12495–506. doi: 10.1074/jbc.RA118.005155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bernstein BW, Shaw AE, Minamide LS, Pak CW, Bamburg JR. Incorporation of cofilin into rods depends on disulfide intermolecular bonds: Implications for actin regulation and neurodegenerative disease. J Neurosci. 2012;32:6670–81. doi: 10.1523/JNEUROSCI.6020-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cichon J, Sun C, Chen B, Jiang M, Chen XA, Sun Y, et al. Cofilin aggregation blocks intracellular trafficking and induces synaptic loss in hippocampal neurons. J Biol Chem. 2012;287:3919–29. doi: 10.1074/jbc.M111.301911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Madineni A, Alhadidi Q, Shah ZA. Cofilin inhibition restores neuronal cell death in oxygen-glucose deprivation model of ischemia. Mol Neurobiol. 2016;53:867–78. doi: 10.1007/s12035-014-9056-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheff SW, Price DA. Synaptic pathology in Alzheimer's disease: A review of ultrastructural studies. Neurobiol Aging. 2003;24:1029–46. doi: 10.1016/j.neurobiolaging.2003.08.002. [DOI] [PubMed] [Google Scholar]
- 10.Brown CE, Li P, Boyd JD, Delaney KR, Murphy TH. Extensive turnover of dendritic spines and vascular remodeling in cortical tissues recovering from stroke. J Neurosci. 2007;27:4101–9. doi: 10.1523/JNEUROSCI.4295-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brown CE, Murphy TH. Livin' on the edge: Imaging dendritic spine turnover in the peri-infarct zone during ischemic stroke and recovery. Neuroscientist. 2008;14:139–46. doi: 10.1177/1073858407309854. [DOI] [PubMed] [Google Scholar]
- 12.Brown CE, Wong C, Murphy TH. Rapid morphologic plasticity of peri-infarct dendritic spines after focal ischemic stroke. Stroke. 2008;39:1286–91. doi: 10.1161/STROKEAHA.107.498238. [DOI] [PubMed] [Google Scholar]
- 13.Iyirhiaro GO, Brust TB, Rashidian J, Galehdar Z, Osman A, Phillips M, et al. Delayed combinatorial treatment with flavopiridol and minocycline provides longer term protection for neuronal soma but not dendrites following global ischemia. J Neurochem. 2008;105:703–13. doi: 10.1111/j.1471-4159.2007.05166.x. [DOI] [PubMed] [Google Scholar]
- 14.Tanay E, Mundel P, Sommer C. Short-term ischemia usually used for ischemic preconditioning causes loss of dendritic integrity after long-term survival in the gerbil hippocampus. Brain Res. 2006;1112:222–6. doi: 10.1016/j.brainres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Won SJ, Minnella AM, Wu L, Eun CH, Rome E, Herson PS, et al. Cofilin-actin rod formation in neuronal processes after brain ischemia. PLoS One. 2018;13:e0198709. doi: 10.1371/journal.pone.0198709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JY, Han Y, Lee JE, Yenari MA. The 70-kDa heat shock protein (Hsp70) as a therapeutic target for stroke. Expert Opin Ther Targets. 2018;22:191–9. doi: 10.1080/14728222.2018.1439477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13:267–78. doi: 10.1038/nrn3174. [DOI] [PubMed] [Google Scholar]
- 18.Kim JY, Kim N, Zheng Z, Lee JE, Yenari MA. 70-kDa heat shock protein downregulates dynamin in experimental stroke: A new therapeutic target? Stroke. 2016;47:2103–11. doi: 10.1161/STROKEAHA.116.012763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marber MS, Mestril R, Chi SH, Sayen MR, Yellon DM, Dillmann WH. Overexpression of the rat inducible 70-kD heat stress protein in a transgenic mouse increases the resistance of the heart to ischemic injury. J Clin Invest. 1995;95:1446–56. doi: 10.1172/JCI117815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hunt CR, Dix DJ, Sharma GG, Pandita RK, Gupta A, Funk M, et al. Genomic instability and enhanced radiosensitivity in Hsp701-and Hsp703-deficient mice. Mol Cell Biol. 2004;24:899–911. doi: 10.1128/MCB.24.2.899-911.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kurisu K, Abumiya T, Ito M, Gekka M, Osanai T, Shichinohe H, et al. Transarterial regional hypothermia provides robust neuroprotection in a rat model of permanent middle cerebral artery occlusion with transient collateral hypoperfusion. Brain Res. 2016;1651:95–103. doi: 10.1016/j.brainres.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 22.Kurisu K, Zheng Z, Kim JY, Shi J, Kanoke A, Liu J, et al. Triggering receptor expressed on myeloid cells-2 expression in the brain is required for maximal phagocytic activity and improved neurological outcomes following experimental stroke. J Cereb Blood Flow Metab. 2019;39:1906–18. doi: 10.1177/0271678X18817282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kawabori M, Hokari M, Zheng Z, Kim JY, Calosing C, Hsieh CL, et al. Triggering receptor expressed on myeloid cells-2 correlates to hypothermic neuroprotection in ischemic stroke. Ther Hypothermia Temp Manag. 2013;3:189–98. doi: 10.1089/ther.2013.0020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kim JY, Kim N, Lee JE, Yenari MA. Hypothermia identifies dynamin as a potential therapeutic target in experimental stroke. Ther Hypothermia Temp Manag. 2017;7:171–7. doi: 10.1089/ther.2017.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yenari MA, Xu L, Tang XN, Qiao Y, Giffard RG. Microglia potentiate damage to blood-brain barrier constituents: Improvement by minocycline in vivo and in vitro. Stroke. 2006;37:1087–93. doi: 10.1161/01.STR.0000206281.77178.ac. [DOI] [PubMed] [Google Scholar]
- 26.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 27.Kawabori M, Kacimi R, Kauppinen T, Calosing C, Kim JY, Hsieh CL, et al. Triggering receptor expressed on myeloid cells 2 (TREM2) deficiency attenuates phagocytic activities of microglia and exacerbates ischemic damage in experimental stroke. J Neurosci. 2015;35:3384–96. doi: 10.1523/JNEUROSCI.2620-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Swanson RA, Morton MT, Tsao-Wu G, Savalos RA, Sharp FR. A semiautomated method for measuring brain infarct volume. JCBFM. 1990;10:290–3. doi: 10.1038/jcbfm.1990.47. [DOI] [PubMed] [Google Scholar]
- 29.Shichinohe H, Tan C, Abumiya T, Nakayama N, Kazumata K, Hokari M, et al. Neuroprotective effects of cilostazol are mediated by multiple mechanisms in a mouse model of permanent focal ischemia. Brain Res. 2015;1602:53–61. doi: 10.1016/j.brainres.2015.01.022. [DOI] [PubMed] [Google Scholar]
- 30.Kurisu K, Kim JY, You J, Yenari MA. Therapeutic hypothermia and neuroprotection in acute neurological disease. Curr Med Chem. 2019;26:5430–55. doi: 10.2174/0929867326666190506124836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen ZC, Wu WS, Lin MT, Hsu CC. Protective effect of transgenic expression of porcine heat shock protein 70 on hypothalamic ischemic and oxidative damage in a mouse model of heatstroke. BMC Neurosci. 2009;10:111. doi: 10.1186/1471-2202-10-111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim JY, Yenari MA. The immune modulating properties of the heat shock proteins after brain injury. Anat Cell Biol. 2013;46:1–7. doi: 10.5115/acb.2013.46.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giffard RG, Xu L, Zhao H, Carrico W, Ouyang Y, Qiao Y, et al. Chaperones, protein aggregation, and brain protection from hypoxic/ischemic injury. J Exp Biol. 2004;207:3213–20. doi: 10.1242/jeb.01034. [DOI] [PubMed] [Google Scholar]
- 34.Lackie RE, Maciejewski A, Ostapchenko VG, Marques-Lopes J, Choy WY, Duennwald ML, et al. The Hsp70/Hsp90 chaperone machinery in neurodegenerative diseases. Front Neurosci. 2017;11:254. doi: 10.3389/fnins.2017.00254. [DOI] [PMC free article] [PubMed] [Google Scholar]