Increasing adoption of electronic cigarettes (e-cigarettes) has led to numerous concerns about health effects resulting from long-term use [1,2,3]. While many factors contribute to the popularity of these products [2], the availability of e-cigarettes in mint, fruit, sweet, and other appealing flavors is often cited as a reason for e-cigarette use, especially among youth and young adults [4,5,6,7,8]. Flavoring agents used in e-cigarettes are generally recognized as a safe (GRAS) for ingestion in most consumer products [9]. However, the inhalation toxicity and other potential health effects related to repeated inhalation of many of these flavoring agents remain largely unknown, and may range from acting as contributors to respiratory irritation, up through contributing to the development of systemic diseases [9]. Emerging evidence from in vitro and laboratory studies indicate that one of the most popular classes of flavorings present in e-cigarettes—fruit flavors [6,7,8,10,11]—has been linked to exposure to greater concentrations of known inhalation irritants [12], diminished bronchial epithelial cell metabolic activity and viability, and increased release of pro-inflammatory cytokines [13,14]. Importantly, laboratory findings have also implicated fruit-flavorings in potentially boosting the delivery of nicotine from e-cigarettes to the user relative to other e-cigarette flavorings [15,16], which may contribute to the addictive potential and abuse liability of these products. However, results found in laboratory studies commonly do not translate to observations from naturalistic settings, which merit the examination of this phenomenon using other data sources. Moreover, it is important to examine whether fruit-flavorings may also affect systemic concentrations of other toxicants present in e-cigarettes. Using nationally-representative data, we assessed whether the use of specific e-cigarette flavors was associated with select urinary biomarkers of exposure to nicotine and toxicants in regular users of e-cigarettes.
Using data from Wave 2 of the Population Assessment of Tobacco and Health (PATH) Study Biomarker Restricted Use Files [17,18], we analyzed levels of nicotine (biomarker: cotinine) and three select tobacco-related toxicants among exclusive e-cigarette users who reported using their product within the last 24 h (n = 211). Toxicant exposures examined in this analysis include acrylonitrile (biomarker: CYMA), benzene (biomarker: PMA), and acrolein (biomarker: CEMA), all of which present numerous health hazards (including respiratory irritation and carcinogenic potential), and have been linked to e-cigarette use [19]. Exclusive e-cigarette users reported their use of flavored e-cigarettes within the past 30 days, which were classified into use of (1) fruit-only, (2) tobacco-only, (3) single other flavor (including mint, clove, chocolate, and other reported flavors), and (4) fruit + use of additional flavors.
Due to the lognormal distribution of biomarker data, these outcomes were log-transformed to more readily approximate a normal distribution. Biomarkers with values under the limit of detection (LOD) were imputed by substituting the LOD/√2 [20]. To assess associations between use of flavored e-cigarettes and biomarker concentrations, creatinine-adjusted geometric means were calculated to account for potential differences in urine dilution [21], and differences in biomarker concentrations according to each flavor grouping were compared using simple linear regression models. Pairwise comparisons were conducted to assess between-flavor differences, and p-values were set at 0.05 and were adjusted for multiple comparisons using a Sidak correction. All analyses were weighted in accordance with procedures outlined in the PATH Biomarker Restricted Use File user guide [22], and were conducted using svy procedures in Stata v. 15.0.
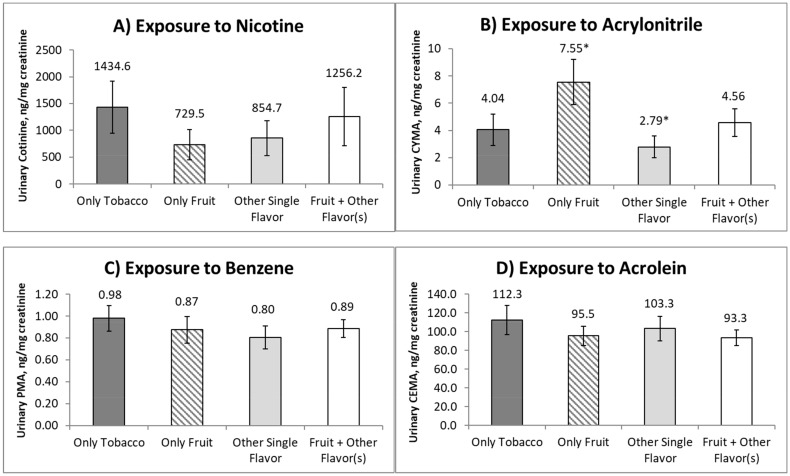
The results of the analysis are displayed in Figure 1. Most exclusive e-cigarette users reported using only mint, clove, chocolate, and other reported flavors (31%), and fruit and additional flavors (31%), followed by tobacco-only (19%), and fruit-only (19%). Users of fruit-only flavored e-cigarettes exhibited significantly higher concentrations of the biomarker for acrylonitrile (CYMA) compared to users of a single other flavor (geometric mean ratio = 2.71, 95% CI: 1.30–5.62, adjusted p-value 0.048). Concentrations of biomarkers of exposure to nicotine (cotinine), benzene (PMA), and acrolein (CEMA) did not significantly differ across flavors.
Figure 1.
Urinary concentrations of biomarkers of exposure to (A) Nicotine, (B) Acrylonitrile, (C) Benzene, and (D) Acrolein, among exclusive users of flavored e-cigarettes, United States, 2015–2016 (n = 211). * indicates a statistically significant difference between flavors (Sidak-adjusted p-value < 0.05).
Using population-based biomarker data, we did not confirm findings from laboratory studies suggesting that fruit-flavored e-cigarettes contribute to significantly elevated concentrations of nicotine among exclusive e-cigarette users. However, we did observe significantly greater concentrations of acrylonitrile among those who used a single e-cigarette flavor other than fruit or menthol. Differences in user behavior, devices, and e-liquids used likely to play a role in this discrepancy, and should be investigated in future studies on this topic. Considering these findings in light of these limitations and the context of existing evidence, future work should aim to further investigate the role that e-cigarette flavors may play in affecting user-health outcomes.
Author Contributions
Conceptualization, M.L.G. and D.M.S.; methodology, D.M.S.; formal analysis, D.M.S.; writing—original draft preparation, D.M.S.; writing—review and editing, M.L.G., R.J.O., L.M.S. and D.M.S.; supervision, M.L.G. and R.J.O.; project administration, M.L.G. and R.J.O.; funding acquisition, M.L.G. and R.J.O.
Funding
This work was supported by Grant No. U54CA228110. This research was supported by NCI and FDA Center for Tobacco Products (CTP). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the Food and Drug Administration.
Conflicts of Interest
M.L.G. receives fees for serving on an advisory board from Johnson & Johnson and grant support from Pfizer. The other authors have no conflicts of interest to declare. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- 1.De Vito E.E., Krishnan-Sarin S. E-cigarettes: Impact of E-Liquid Components and Device Characteristics on Nicotine Exposure. Curr. Neuropharmacol. 2018;16:438–459. doi: 10.2174/1570159X15666171016164430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.National Academies of Sciences Engineering, and Medicine . Public Health Consequences of E-Cigarettes. National Academies Press; Washington, DC, USA: 2018. [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention . Age-Adjusted Percentage of Adults Who Had Ever Used an E-Cigarette, by Age and Ethnicity—National Health Interview Survey, United States, 2014 and 2018. Morbidity and Mortality Weekly Report; Atlanta, GA, USA: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim H., Lim J., Buehler S.S., Brinkman M.C., Johnson N.M., Wilson L., Cross K.S., Clark P.I. Role of sweet and other flavours in liking and disliking of electronic cigarettes. Tob. Control. 2016;25:ii55–ii61. doi: 10.1136/tobaccocontrol-2016-053221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith D.M., Bansal-Travers M., Huang J., Barker D., Hyland A.J., Chaloupka F. Association between use of flavoured tobacco products and quit behaviours: Findings from a cross-sectional survey of US adult tobacco users. Tob. Control. 2016;25:ii73–ii80. doi: 10.1136/tobaccocontrol-2016-053313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Soneji S.S., Knutzen K.E., Villanti A.C. Use of Flavored E-Cigarettes Among Adolescents, Young Adults, and Older Adults: Findings From the Population Assessment for Tobacco and Health Study. Public Health Rep. 2019;134:282–292. doi: 10.1177/0033354919830967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villanti A.C., Johnson A.L., Ambrose B.K., Cummings K.M., Stanton C.A., Rose S.W., Feirman S.P., Tworek C., Glasser A.M., Pearson J.L., et al. Flavored Tobacco Product Use in Youth and Adults: Findings From the First Wave of the PATH Study (2013–2014) Am. J. Prev. Med. 2017;53:139–151. doi: 10.1016/j.amepre.2017.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villanti A.C., Johnson A.L., Glasser A.M., Rose S.W., Ambrose B.K., Conway K.P., Cummings K.M., Stanton C.A., Edwards K.C., Delnevo C.D., et al. Association of Flavored Tobacco Use With Tobacco Initiation and Subsequent Use Among US Youth and Adults, 2013–2015. JAMA Netw. Open. 2019;2:e1913804. doi: 10.1001/jamanetworkopen.2019.13804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tierney P.A., Karpinski C.D., Brown J.E., Luo W., Pankow J.F. Flavour chemicals in electronic cigarette fluids. Tob. Control. 2016;25:e10–e15. doi: 10.1136/tobaccocontrol-2014-052175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schneller L.M., Bansal-Travers M., Goniewicz M.L., McIntosh S., Ossip D., O’Connor R.J. Use of flavored electronic cigarette refill liquids among adults and youth in the US—Results from Wave 2 of the Population Assessment of Tobacco and Health Study (2014–2015) PLoS ONE. 2018;13:e0202744. doi: 10.1371/journal.pone.0202744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schneller L.M., Bansal-Travers M., Goniewicz M.L., McIntosh S., Ossip D., O’Connor R.J. Use of Flavored E-Cigarettes and the Type of E-Cigarette Devices Used among Adults and Youth in the US-Results from Wave 3 of the Population Assessment of Tobacco and Health Study (2015–2016) Int. J. Environ. Res. Public Health. 2019;16:2991. doi: 10.3390/ijerph16162991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kosmider L., Sobczak A., Prokopowicz A., Kurek J., Zaciera M., Knysak J., Smith D., Goniewicz M.L. Cherry-flavoured electronic cigarettes expose users to the inhalation irritant, benzaldehyde. Thorax. 2016;71:376–377. doi: 10.1136/thoraxjnl-2015-207895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaur G., Muthumalage T., Rahman I. Mechanisms of toxicity and biomarkers of flavoring and flavor enhancing chemicals in emerging tobacco and non-tobacco products. Toxicol. Lett. 2018;288:143–155. doi: 10.1016/j.toxlet.2018.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leigh N.J., Lawton R.I., Hershberger P.A., Goniewicz M.L. Flavourings significantly affect inhalation toxicity of aerosol generated from electronic nicotine delivery systems (ENDS) Tob. Control. 2016;25:ii81–ii87. doi: 10.1136/tobaccocontrol-2016-053205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.St Helen G., Dempsey D.A., Havel C.M., Jacob P., III, Benowitz N.L. Impact of e-liquid flavors on nicotine intake and pharmacology of e-cigarettes. Drug Alcohol Depend. 2017;178:391–398. doi: 10.1016/j.drugalcdep.2017.05.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.St Helen G., Shahid M., Chu S., Benowitz N.L. Impact of e-liquid flavors on e-cigarette vaping behavior. Drug Alcohol Depend. 2018;189:42–48. doi: 10.1016/j.drugalcdep.2018.04.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.National Addiction and HIV Data Archive Program (NAHDAP) Population Assessment of Tobacco and Health (PATH) Study Series. NAHDAP; Bethesda, MD, USA: 2019. [Google Scholar]
- 18.Hyland A., Ambrose B.K., Conway K.P., Borek N., Lambert E., Carusi C., Taylor K., Crosse S., Fong G.T., Cummings K.M., et al. Design and methods of the Population Assessment of Tobacco and Health (PATH) Study. Tob. Control. 2017;26:371–378. doi: 10.1136/tobaccocontrol-2016-052934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goniewicz M.L., Smith D.M., Edwards K.C., Blount B.C., Caldwell K.L., Feng J., Wang L., Christensen C., Ambrose B., Borek N., et al. Comparison of Nicotine and Toxicant Exposure in Users of Electronic Cigarettes and Combustible Cigarettes. JAMA Netw. Open. 2018;1:e185937. doi: 10.1001/jamanetworkopen.2018.5937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hornung R.W., Reed L.D. Estimation of Average Concentration in the Presence of Nondetectable Values. Appl. Occup. Environ. Hyg. 1990;5:46–51. doi: 10.1080/1047322X.1990.10389587. [DOI] [Google Scholar]
- 21.Boeniger M.F., Lowry L.K., Rosenberg J. Interpretation of urine results used to assess chemical exposure with emphasis on creatinine adjustments: A review. Am. Ind. Hyg. Assoc. J. 1993;54:615–627. doi: 10.1080/15298669391355134. [DOI] [PubMed] [Google Scholar]
- 22.National Institute on Drug Abuse. Food and Drug Administration. Center for Tobacco Products . Population Assessment of Tobacco and Health (PATH) Study [United States] Biomarker Restricted-Use Files (ICPSR36840) Inter-university Consortium for Political and Social Research; Ann Arbor, MI, USA: 2017. [Google Scholar]