Abstract
Background
There are many types of therapies for cancer. In these days, immunotherapies, especially immune checkpoint inhibitors, are focused on. Though many types of immune checkpoint inhibitors are there, the difference of effect and its mechanism are unclear. Some reports suggest the response rate of anti-PD-1 antibody is superior to that of anti-PD-L1 antibody and could potentially produce different mechanisms of action. On the other hand, Treg also express PD-1; however, their relationship remains unclear.
Methods
In this study, we used osteosarcoma cell lines in vitro and osteosarcoma mouse model in vivo. In vitro, we analyzed the effect of IFNγ for expression of PD-L1 on the surface of cell lines by flowcytometry. In vivo, murine osteosarcoma cell line LM8 was subcutaneously transplanted into the dorsum of mice. Mouse anti-PD-1 antibody was intraperitoneally administered. we analysed the effect for survival of anti-PD-1 antibody and proportion of T cells in the tumour by flowcytometry.
Results
We discovered that IFNγ increased PD-L1 expression on the surface of osteosarcoma cell lines. In assessing the relationship between anti-PD-1 antibody and Treg, we discovered the administration of anti-PD-1 antibody suppresses increases in tumour volume and prolongs overall survival time. In the tumour microenvironment, we found that the administration of anti-PD-1 antibody decreased Treg within the tumour and increased tumour-infiltrating lymphocytes.
Conclusions
Here we clarify for the first time an additional mechanism of anti-tumour effect—as exerted by anti-PD-1 antibody decreasing Treg— we anticipate that our findings will lead to the development of new methods for cancer treatment.
Keywords: PD-1, Treg, Osteosarcoma, Anti-PD-1 antibody
Backgrounds
In the microenvironment of cancer, the role of innate immunity is inhibited in a process known as immune tolerance. One of the mechanisms of immune tolerance is the immune checkpoint mechanism, whereby T cells are suppressed to prevent excessive immune responses. Several types of immune checkpoint molecules are known, namely the cytotoxic T-lymphocyte antigen 4 (CTLA-4) and lymphocyte activation gene 3 (LAG-3), in addition to programmed cell death 1 (PD-1) and its ligand 1 (PD-L1) [1, 2]. PD-1 is expressed on the surface of cytotoxic T cells and transmits suppressive signals to T cells by binding to PD-L1.
Normal cells are believed to express PD-L1 in an inflammatory environment, suppress T cells, and prevent excessive tissue damage from long-term persistence and spread of inflammation [3]. However, in some types of cancers, PD-L1 is reported to be expressed on the surface of cancer cells by means of stimulation from interferon gamma (IFNγ), a proinflammatory cytokine [1, 4–6]. Cancer has been implicated to prevent attacks from the immune system by suppressing T cell activation by binding the PD-L1 that are expressed on cancer cells to the PD-1 on cytotoxic T cells [7]. Therefore, when anti-PD-1 or anti-PD-L1 antibodies are allowed to react to a specified antigen, PD-1 is unable to bind to PD-L1, and an anti-tumour effect is exerted by disabling their immunotolerance [8]. To date, two reports have analysed a study population of over 3000 patients. Although the reported effect of anti-PD-1 and anti-PD-L1 antibodies were equivalent in a 2017 study [9], a 2018 study showed that the response rate of anti-PD-1 was superior to that of anti-PD-L1 antibody [10]. These results indicate that different mechanisms of action may exist as the anti-PD-1 antibody suppresses tumours.
Immune checkpoint molecules also play an important role in Treg that are involved in suppressing the function of cytotoxic T cells. Treg expresses CTLA-4, which is an immunity checkpoint molecule on the cell surface that suppresses the activity of antigen-presenting cell (APC), resulting in the suppression of T cell activation [11]. The anti-tumour effect of anti-CTLA-4 antibody is obtained by the inhibition of CTLA-4 on Treg and thus reversing the suppression of T cell activation [12, 13]. Some reports have noted the expression of PD-1 on the surface of Treg [14–17], and the importance of PD-1 on Treg have been pointed [16, 18]. Although there are few comprehensive studies that describe the relationship between anti-PD-1 antibody and Treg [19], the effect of anti-PD-1 antibody on Treg is not clear. In regards to the therapeutic effect of anti-PD-1 antibody against osteosarcoma, there are only three interim reports on clinical trials [20–22] and one basic research report [23]. Moreover, although PD-L1 is reportedly expressed in osteosarcoma [24], its expression mechanism is unknown.
This study used osteosarcoma as a tumour model to elucidate its relationship to the anti-tumour effect of anti-PD-1 antibody and Treg. Osteosarcoma is reported as a tumour that is susceptible to immunotherapy [25] with greater infiltration of CD8 + cells than other sarcomas [26], and prognosis is considered better when there is more infiltration of CD8 + cells [27]. Because osteosarcoma is a solid tumour, it is suitable for evaluating the relationship between anti-PD-1 antibody and Treg in animal experiments.
In this study, we first evaluated the mechanism of PD-L1 expression in osteosarcoma cells in vitro to establish osteosarcoma as a tumour model. The anti-tumour effect was also confirmed in vivo from the changes in tumour volume and overall survival time of anti-PD-1 antibody administration using a subcutaneously implanted mouse model of osteosarcoma. Furthermore, changes in the tumour microenvironments were evaluated in detail, and the relationship between anti-PD-1 antibody and Treg was examined. From these experiments, we report that anti-PD-1 antibody suppresses tumour-infiltrating Treg and exerts an antitumour effect.
Methods
Cell lines
Murrin osteosarcoma cell line (LM8, Riken cell bank, Tokyo, Japan, RBC Cat# RCB1450), human osteosarcoma cell lines HOS (Riken cell bank, RBC Cat# RCB0428, RRID:CVCL_0312) and SaOS-2 (Riken cell bank, RCB Cat# RCB0992, RRID:CVCL_0548) were cultured in α-MEM containing 5% fetal bovine serum (FBS). 143B human osteosarcoma cells (Riken cell bank, RCB Cat# RCB0701, RRID:CVCL_2270) were cultured in Dulbecco’s Modified Eagle Medium (DMEM) containing 5% FBS. All cell cultures were maintained in 5% CO2 at 37 °C. For all cell lines, frozen aliquots of 10^6 cells were stored at a passage below 5 and a fresh aliquot used after 5 consecutive passages. All of cell lines were additionally tested by Riken cell bank and found to be negative for mycoplasma.
Mice
Mice were housed and maintained at the Committee for Animal Experiments of Shinshu University. Mice were housed with an inverse 12 h day-night cycle with lights on at 9 am in a temperature-controlled room. All mice were allowed free access to water and a maintenance diet. Based on the national regulations and guidelines, all experimental procedures were reviewed by the Committee for Animal Experiments and finally approved by the president of Shinshu University. The animal protocol was approved by the Committee for Animal Experiments of Shinshu University (Approval Number 280112).
Male C3H/HeSlc mice (3 weeks of age, 13.59 ± 1.13(Body weight ± S.D.)) were bought by Japan SLC (Shizuoka, Japan). The established protocol [28] is used for the standardized protocol for euthanasia.
IFNγ stimulation and PD-L1 expression in osteosarcoma cell lines
Recombinant IFNγ (R & D systems, Minneapolis, MN, USA) species-matched to human osteosarcoma cell lines (HOS, SaOS-2, 143B) and murine osteosarcoma cell line (LM8) was administered and incubated for 24 h (n = 3). Cells were detached with trypsin, and surface markers were detected by flow cytometry.
Administration of 4H2 on osteosarcoma mouse model
Murine osteosarcoma cell line LM8 was subcutaneously transplanted into the dorsum of 4-week old mice (1 × 106 cells per mouse, n = 5). Mouse anti-PD-1 antibody (4H2, Ono Pharmaceutical Co., Osaka, Japan) was intraperitoneally administered three times per week at 20 mg/kg of body weight per dose for a total of five doses. 200 μl of PBS was intraperitoneally administered to the control group. Mice were followed up until reaching the humane endpoint or until natural death. Macroscopic tumour size was calculated using the established method [29] three times a week. Body weight, and μCT were observed over time.
Tumour microenvironment after administration of 4H2
Subcutaneously transplanted mouse models of osteosarcoma were prepared (n = 3). To obtain a sufficient amount of tumour at 2 weeks after the initial administration of 4H2, the initial administration was withheld until day 7. 4H2 was intraperitoneally administered three times per week at 20 mg/kg of body weight per dose for a total of five doses. 200 μl of PBS was intraperitoneally administered to the control group. All mice were euthanized at day 21, and the tumour and spleen were excised.
Long-term administration of 4H2 on osteosarcoma mouse model
Subcutaneously transplanted mouse models of osteosarcoma were prepared (n = 5). In the short-term administration group, 4H2 was intraperitoneally administered twice a week at 20 mg/kg per dose for a total of four doses. In the long-term administration group, administration of anti-PD-1 antibody was continued twice a week until the endpoint. For the control group, PBS was administered twice a week until the endpoint. Mice were followed up until reaching our euthanasia criteria or until natural death. Changes in tumour size, body weight, and μCT were observed over time.
Antibodies, reagents, and flow cytometry
Fluorochrome-conjugated antibodies and reagents used in this research are described in Additional file 1: Table S1. Foxp3 Transcription Factor Staining Buffer Set (eBioscience, San Diego, CA, USA) was used for intracellular staining of Foxp3 and Ki-67. Stained cells were analysed with FACS Canto II flow cytometer or FACS Cellesta flow cytometer (BD Bioscience, Haryana, India). To collect and analyse the data, well established method of multicolor flow cytometry [30] was used. Flow cytometry data were analysed with Kaluza software ver.1.5a (Beckman Coulter, Brea, CA, USA).
Cell isolation
Mice were euthanized by inhaled isoflurane overdose. The tumour of the dorsal subcutaneous and spleen were excised in a clean environment. The excised spleen was physically triturated and suspended in RPMI 1640 medium (with 10% FBS, 25 mM HEPES). Cells from tumours were isolated using a previously reported [31] method whereby tumours were minced and incubated with digestion buffer (RPMI 1640 medium, 10% FBS, 25 mM HEPES, 300 Unit Type2 collagenase [Worthington Biochemical, Lakewood, NJ, USA]) in a shaker for 1 h at 37 °C. Dispersed cells were filtered through a 70-μm cell strainer to eliminate clumps and debris. After centrifugation for 3 min (500 g), cells from the spleen and tumour were resuspended in red blood cell lysis buffer (G-Biosciences, Louis, MO, USA) and incubated at room temperature for 1 min to remove erythrocytes. Cells were filtered again through a 40 μm cell strainer. Finally, the cells were re-pelleted and re-suspended in RPMI 1640 medium.
Statistical analysis
The mice were separated to experimental and control group at random. Statistical analysis was performed with SPSS software ver. 25 (IBM, Armonk, NY, USA) using the unpaired two-tailed t-test or Bonferroni’s multiple comparison test. Kaplan-Meier curves were created by the same software and log rank analysis was used for comparison between groups. P values less than 0.05 was considered statistically significant.
Results
IFNγ increases PD-L1 expression in osteosarcoma cell lines
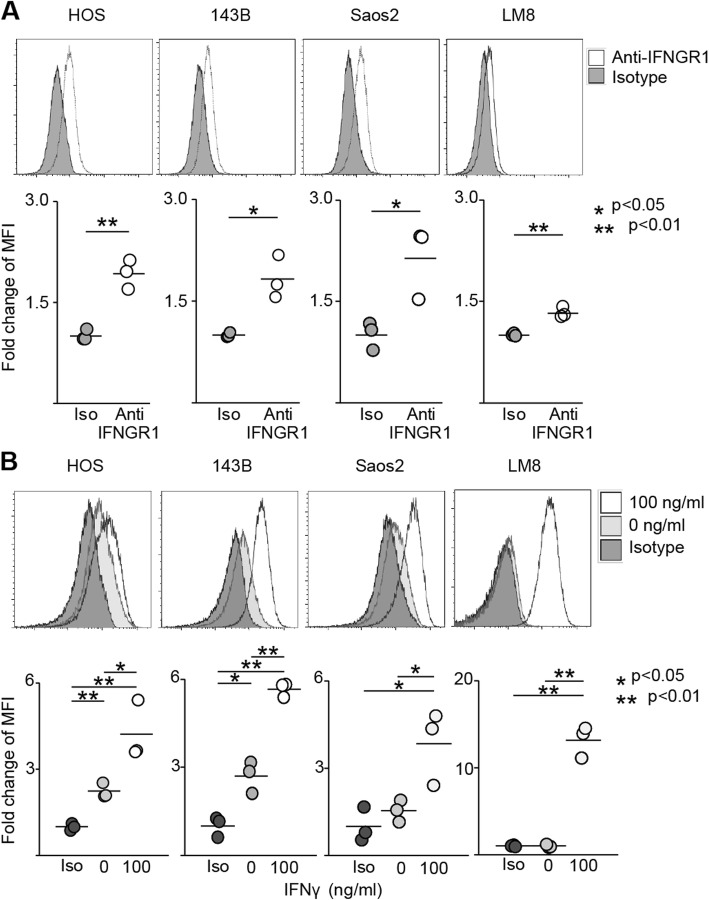
Osteosarcoma cell lines were used to verify the mechanism of PD-L1 expression in osteosarcoma. Analysis of human (HOS, SaOS-2, 143B) and mouse (LM8) osteosarcoma cell lines by flow cytometry revealed that IFNGR1 was expressed in all cell lines (HOS; 1.92, p = 0.0022, 143B; 1.83, p = 0.011, SaOS-2; 2.14, p = 0.027, and LM8; 1.3, p = 0.0017. Figure 1a). When IFNγ was applied, PD-L1 expression increased in all cell lines (HOS; 0 group 2.24, 100 group 4.23, p value: iso vs 0 = 0.0019, iso vs 100 = 0.0056, 0 vs 100 = 0.031. 143B; 0 group 2.70, 100 group 5.65, p value: iso vs 0 = 0.010, iso vs 100 < 0.0001, 0 vs 100 = 0.0010. SaOS-2; 0 group 1.54, 100 group 3.85, p value: iso vs 0 = 0.25, iso vs 100 = 0.025, 0 vs 100 = 0.039. LM8; 0 group 0.99, 100 group 13.19, p value: iso vs 0 = 0.93, iso vs 100 = 0.00034, 0 vs 100 = 0.00034. Figure 1b). In SaOS-2 and LM8, no significant expression of PD-L1 was observed unless stimulated by IFNγ; in contrast, PD-L1 was expressed in HOS and 143B without IFNγ stimulation.
Fig. 1.
IFNγ increases PD-L1 expression in osteosarcoma cell lines. Surface markers of human and murine osteosarcoma cell line were evaluated by flow cytometry. The upper row shows representative specimen. In the lower row, each specimen is plotted (n = 3), and the average value is indicated by a horizontal bar. a Expression of IFNGR1 in each cell line. b Expression of PD-L1 in each cell line. Iso-type control, anti-PD-L1 staining with/without IFNγ were evaluated
Anti-PD-1 antibody improves survival curve in vivo
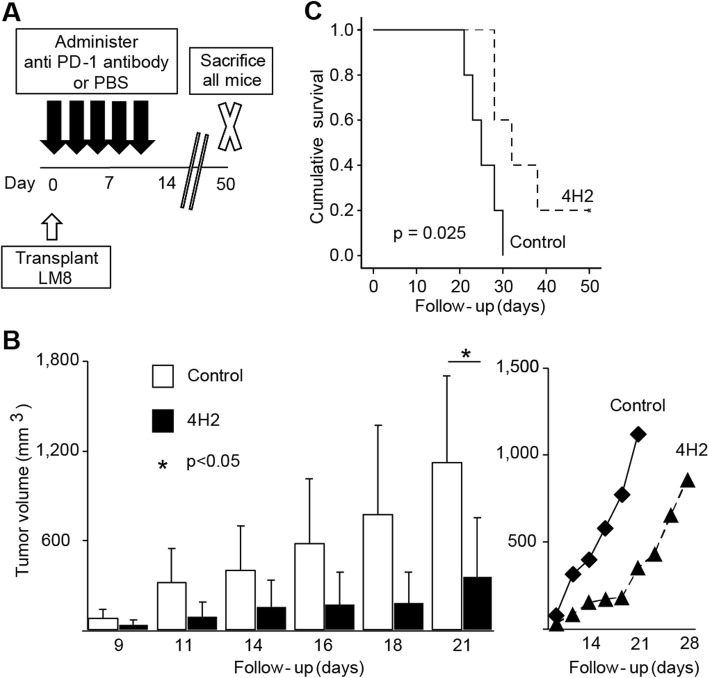
Anti-PD-1 antibody (4H2) was administered to subcutaneously implanted models of murine osteosarcoma cell line LM8 and evaluated in vivo. First, anti-PD-1 antibody was administered three times per week for a total of five times and compared to the control (Fig. 2a). An increase in tumour volume was suppressed in the 4H2 group, and a significant difference was found at 3 weeks after transplantation (Day 9; control 79.6 ± 59.6 mm3, 4H2 33.8 ± 30.5 mm3, p = 0.16. Day 11; control 317.7 ± 228.9 mm3, 4H2 86.0 ± 102.2 mm3, p = 0.073. Day 14; control 400.2 ± 298.1 mm3, 4H2 153.6 ± 177.8 mm3, p = 0.15. Day 16; control 578.4 ± 435.7 mm3, 4H2 172.6 ± 212.9 mm3, p = 0.098. Day 18; control 771.1 ± 600.9 mm3, 4H2 183.3 ± 201.9 mm3, p = 0.072. Day 21; control 1122.1 ± 579.6 mm3, 4H2 352.8 ± 399.9 mm3, p = 0.040. Figure 2b). The tumour diameter rapidly increased in the 4H2 administration group after day 18 (Fig. 2b, right). Survival curves were significantly improved (p = 0.025, Fig. 2c), and mean survival significantly increased from 25.4 ± 1.6 days in the control group to 35.2 ± 3.7 days in the 4H2 group (p = 0.047, Fig. 2d).
Fig. 2.
Anti-PD-1 antibody improves survival curve in vivo. The LM8 murine osteosarcoma cell line was subcutaneously transplanted to the dorsal region of the C3H mouse, and 4H2 was administered (n = 5). Error bars indicate S.D. a Schema of experimental overview. b Changes in tumour volume with time to first death. The right line graph plots the mean value of the tumour volume. c Evaluation of overall survival rate by Kaplan-Meier curve. P value of log rank method was 0.025
Anti-PD-1 antibody changes the tumour microenvironment
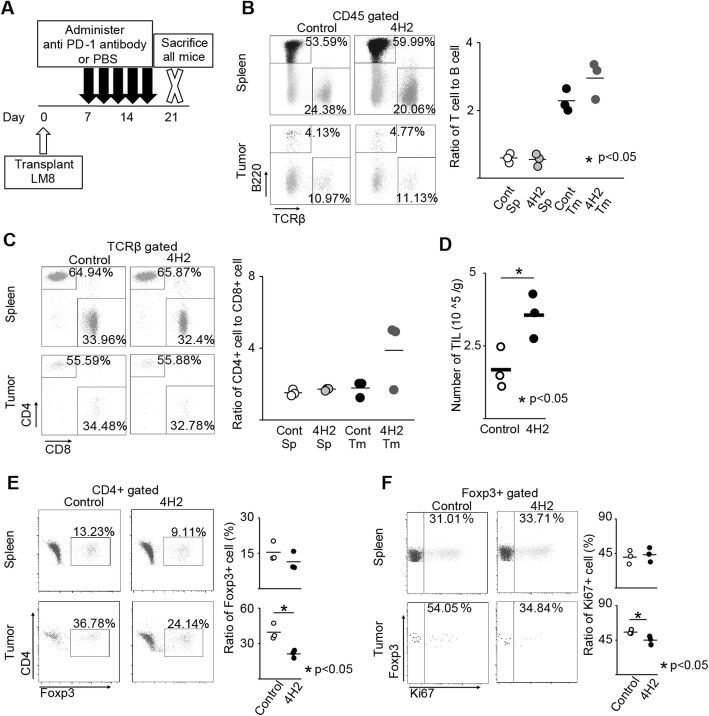
To investigate changes in the tumour microenvironment following the administration of anti-PD-1 antibody, all mice were euthanized 2 weeks after the initial administration of anti-PD-1 antibody, spleen and tumour were collected, and cells were isolated (Fig. 3a). Expression of surface and intracellular antigens were evaluated by flow cytometry, and the proportion of immune cells in the spleen and tumour was examined. In addition, focusing on the expression of PD-1 molecule on the surface of Treg that suppresses T cells, we also examined the change in the proportion of Treg in CD4 + cells after the administration of anti-PD-1 antibody.
Fig. 3.
Anti-PD-1 antibody changes the tumour microenvironment. The proportion of spleen and tumour immune cells were evaluated (n = 3). The representative specimen is shown, left. Each specimen is plotted, and the average value is indicated by a horizontal bar, right. a Schema of experimental overview. b Ratio of T cell to B cell. c Ratio of CD4 + cell to CD8 + cell in TCRβ + cells. d Number of TILs recovered from the tumour per unit weight. e Percentage of Foxp3 + cells in CD4 + cells. f Percentage of Ki-67 + cells in Foxp3 + cells
In the spleen, the administration of anti-PD-1 antibody showed no changes in the ratio of T cell to B cell (Control; 0.53 ± 0.16, 4H2; 0.54 ± 0.19. p = 0.73, Fig. 3b), ratio of CD4 + T cell to CD8 + T cell (Control; 1.6 ± 0.29, 4H2; 1.7 ± 0.07. p = 0.15, Fig. 3c), and percentage of Foxp3 + Treg in CD4 + cells (Control; 16.5 ± 4.0%; 4H2; 11.5 ± 3.9%. p = 0.15, Fig. 3e). The number of tumour-infiltrating lymphocytes increased per tumour weight after anti-PD-1 antibody administration (Control; 1.7 ± 0.7 × 105 /g, 4H2; 3.6 ± 0.8 × 105 /g. p = 0.047, Fig. 3d). Within the tumour, the ratio of T cells in CD45 + cells were higher compared to the spleen (Fig. 3b, right). However, the administration of anti-PD-1 antibody showed no changes in the ratio of B cell to T cell within the tumour (Control; 2.2 ± 0.29, 4H2; 3.0 ± 0.55. p = 0.14, Fig. 3b) and the ratio of CD4 + T cell and CD8 + T cell (Control; 1.8 ± 0.37, 4H2; 3.9 ± 1.9. p = 0.13, Fig. 3c). On the other hand, the percentage of Foxp3 + Treg in CD4 + T cells within the tumour was significantly decreased with the administration of anti-PD-1 antibody (Control; 39.5 ± 6.7%, 4H2; 21.2 ± 3.2%. p = 0.013Fig. 3e). Moreover, Ki67, which is highly expressed in effector Treg that exhibits strong immunosuppressive activity, decreased in the group treated with anti-PD-1 antibody (Control; 55.5 ± 2.9%, 4H2; 45.0 ± 5.7%. p = 0.046, Fig. 3f).
Long-term administration enhances the effect of anti-PD-1 antibody
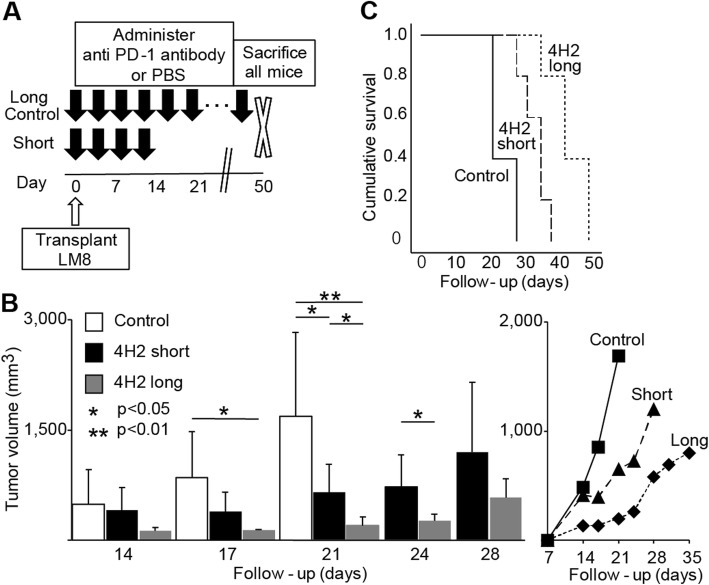
We found that the long-term administration of anti-PD-1 antibody suppressed tumour volume and prolonged overall survival. Even in the group administered with 4H2 for 2 weeks, the speed of tumour growth increased at approximately 1 week after final administration (Fig. 2b, right). We investigated whether long-term anti-tumour effect can be obtained by continuously administering 4H2 over an extended period of time.
For the long-term administration group, 4H2 was administered twice a week from day 0 until death or having reached our euthanasia criteria. The results were compared with a control group in addition to a short-term administration group that received only four administrations in total (Fig. 4a). In both the long-term and short-term groups, the increase in tumour volume was suppressed compared to the control group (Day 14; control 487.3 ± 478.6 mm3, short-term 412.8 ± 302.8 mm3, long-term 132.3 ± 41.2 mm3, p value: control vs short-term = 0.72, control vs long-term = 0.093, short-term vs long-term = 0.074. Day 17; control 904.8 ± 549.8 mm3, short-term 392.9 ± 260.9 mm3, long-term 132.8 ± 16.3 mm3. p value: control vs short-term = 0.097, control vs long-term = 0.014, short-term vs long-term = 0.057. Day 21; control 1844.1 ± 1041.5 mm3, short-term 649.7 ± 384.5 mm3, long-term 198.3 ± 124.0 mm3. p value: control vs short-term = 0.043, control vs long-term = 0.0080, short-term vs long-term = 0.037. Day 24; short-term 726.5 ± 430.8 mm3, long-term 260.1 ± 92.3 mm3. p = 0.045. Day 28; short-term 1200.4 ± 952.8 mm3, long-term 586.1 ± 253.2 mm3. p = 0.20. Day 31; long-term 694.4 ± 231.2 mm3. Day 35; long-term 800.5 ± 202.9 mm3. Figure 4b), the survival curve was significantly improved (p = 0.0002. Figure 4c), and the mean days of survival were significantly extended (control; 23.8 ± 3.8 days, short-term; 33.4 ± 3.9 days, long-term; 43.4 ± 5.9 days. p value: control vs short-term = 0.0044, control-term vs long-term = 0.00024, short-term vs long-term = 0.013. Figure 4d). Moreover, in a comparison between the long-term and short-term administration groups, the tumour volume in the long-term administration group suppressed further as the survival curve and mean survival period also increased further (Fig. 4b).
Fig. 4.
Long-term administration enhances the effect of anti-PD-1 antibody. The control group, the short-term group that was limited to four doses and the long-term group that continued administration until natural death or euthanasia criteria were compared (n = 5). Error bars indicate S.D. a Schema of experimental overview. b Changes in tumour volume with time until first death. The right line graph plots the mean value of tumour volume. c Evaluation of overall survival rate by Kaplan-Meier curve. P value of log rank method was 0.0002
Discussion
In this study, we selected osteosarcoma as a tumour model due to its susceptibility to immunotherapy. First, the pattern of expression for PD-L1 in osteosarcoma cells was confirmed. Next, we verified the effectiveness of anti-PD-1 antibody on osteosarcoma in animal experiments and clarified the mechanism of action of anti-PD-1 antibody by evaluating the tumour microenvironment. The above results demonstrate that osteosarcoma is suitable as a model for evaluating the action of anti-PD-1 antibody on tumour.
Two types of PD-L1 expression in the tumour are known: an innate mechanism induced by genetic mutation and an adaptive mechanism induced by the stimulation of inflammatory cytokine, the latter of which is primarily caused by IFNγ [1]. Constitutive PD-L1 expression, known as innate immune resistance, is created by the deletion or silencing of PTEN found in glioblastoma [32] or by a signal transducer and activator of transcription 3 (STAT 3) signalling found in lymphoma and occasionally in lung cancer [33]. PD-L1 expression by immune response, known as adaptive immune resistance, mainly causes IFNγ stimulation to resolve inflammation in normal tissue at an appropriate time and to avoid excessive tissue damage. Though it is still not clear how much concentration of IFN-γ is appropriate to reproduce tumour microenvironment in vitro sturdy, there are some studies about relationship between tumour and IFN-γ. For example, in ovarian cancer and angiosarcoma, IFNγ induces PD-L1 expression and is known to be involved in immune tolerance [4, 5]. In this study, we found that IFNγ enhances the expression of PD-L1 in osteosarcoma and the result is compatible with other reports. It is known that IFNγ is produced by inflammatory cells such as T cells and NK cells, and this in vitro result shows the possibility that adaptive PD-L1 expression is occurring in response to inflammation of osteosarcoma. In addition, the existence of a negative feedback loop has been reported, whereby PD-L1 is expressed on the tumour surface by T cell activation and IFNγ production, and T cell activity is suppressed via PD-L1 [1]. In osteosarcoma, T-cells produce IFNγ that express PD-L1, and immune tolerance may be caused by a negative feedback loop through the PD-L1. In other words, the inhibition of PD-1/PD-L1 interaction with anti-PD-1 antibody may potentially cause T cells to produce an anti-tumour effect without undergoing negative feedback.
Furthermore, we confirmed the inhibition of tumour growth by anti-PD-1 antibody and the prolonged overall survival for subcutaneously implanted mouse models of osteosarcoma in vivo. These results demonstrate that osteosarcoma is a suitable model for evaluating the effect of anti-PD-1 antibody on tumours.
Many studies have been conducted on changes in the tumour microenvironment during the use of immune checkpoint inhibitors in various carcinomas [34–37]. It has been reported that the infiltration of CD8 + T cells into the tumour is increased by the anti-PD-1 antibody [35]. We showed that the number of tumours infiltrating lymphocytes per unit weight increases in osteosarcoma by the administration of anti-PD-1 antibody, and this result is consistent with previous reports. On the other hand, the relationship between anti-PD-1 antibody and CD4+ is still unclear. Recently, Zuazo et al. reported anti-PD-1 antibody increase CD4+ cells and some reports also showed increasing of CD4+ cells in their data [19, 38]. In our data, though the mechanism is not disclosed, CD4+ cells in tumour tended to increase.
It is widely known that Treg suppresses immunity against tumours in the tumour microenvironment [39]. Treg is characterized by Foxp3 +, and Miyara et al. have described a classification of Foxp3 + T cells and reported that there are many effector Treg fractions in tumour tissue [14]. Effector Treg is considered a fraction with strong immunosuppressive function amongst Foxp3 + T cells and induces high expressions of PD-1 and Ki-67 [15]. Other fractions of Foxp3 + T cells have a weak suppressive function, and expressions of PD-1 and Ki-67 are not observed. The results of this study show that by using anti-PD-1 antibody, the proportion of Foxp3 + T cells in CD4 + cells and Ki-67 + cells in Foxp3 + T cells decreases. This suggests that the administration of anti-PD-1 antibody eliminates effector Treg expressing PD-1 among Foxp3 + T cells. Like PD-1, CTLA-4 is a molecule that expresses a high level of effector Treg. Reduction of Treg by administration of anti-CTLA-4 antibody is well known [12, 13, 37, 40, 41], and the cause is reported to be due to the antibody-dependent cell cytotoxicity (ADCC) of the anti-CTLA-4 antibody [11, 41]. ADCC is the activation of macrophages and NK cells that recognize the Fc region of the antibody to migrate and kill the cells when the antibody binds to cells. Because PD-1 molecule is expressed on Treg surface like that of CTLA-4 [24] and ADCC is similarly caused by binding to anti-PD-1 antibody, the administration of anti-PD-1 antibody to decrease Treg has been hypothesized for a long time [42], but have not yet to be proven. Recently some reports mentioned about the relationship between anti-PD-1 antibody and Treg, but it is still controversial. Kamada et al. reported the tendency of decrease of Treg after anti-PD-1 therapy for gastric cancer patients. Asano et al. reported the importance of PD-1 on the proliferation of Treg by using anti-PD-1 antibody. Zhang et al. reported PD-1-dificient Treg showed increased their suppressive activity in Foxp3 decreased mouse and concluded to affect the expression of both PD-1 and FoxP3 can disrupt the T-cell homeostasis [17]. Here, we showed for the first time that Treg decreases by administration of anti-PD-1 antibody in osteosarcoma. Moreover, as there are no other reports that clearly describe the decrease in Treg as a result of administrating anti-PD-1 antibody in other types of tumours, this is the first discovery of its kind in the world.
In this study, we showed that the survival time of subcutaneously transplanted mouse models of osteosarcoma was prolonged by the administration of anti-PD-1 antibody. Furthermore, the survival time is likely to extend further with a long-term administration of anti-PD-1 antibody. As expected, long-term administration of anti-PD-1 antibody was found to prolong survival time compared to short-term administration in this study.
We revealed for the first time that anti-PD-1 antibody suppresses the infiltration of Treg into tumour and exerts an antitumour effect. Although osteosarcoma was used as a tumour model in this study, future studies should investigate these results further in the context of other tumours. If the mechanism of action in tumour suppression of anti-PD-1 antibody is established in many types of cancer, it will enable us to provide an explain regarding the higher response rate of anti-PD-1 antibody compared to anti-PD-L1 antibody in clinical practice [10]. In terms of clinical application, these findings might also change the effect prediction of anti-PD-1 antibody based on the expression of PD-L1 on the tumour surface because of its antitumour ability via decreasing Treg.
We believe that this study not only affects the use of anti-PD-1 antibody in clinical practice, but also serves to facilitate the development of new treatments for tumours with a focus on the relationship between anti-PD-1 antibody and Treg.
It is limitation of this study that the most results are from mouse transplantation model of osteosarcoma. Because it is difficult to be proven this model behaves the same as the human disease, further studies like using humanized mice or collecting the data from clinical specimen of osteosarcoma used anti-PD-1 antibody are needed.
Conclusions
The suppression of Treg by the administration of anti-PD-1 antibody is a new finding and may offer new insights into the mechanism of action of the anti-PD-1 antibody. The results of this study could be a useful asset in designing a combination therapy of anti-PD-1 antibody with other drugs. Moreover, because an antitumour effect can be anticipated through the suppression of Treg, clinical importance can also be placed in the possible therapeutic effects for tumours with no expression of PD-L1.
Supplementary information
Additional file 1: Table S1. List of antibodies.
Acknowledgments
We thank our laboratory assistant and the staff at the Department of Molecular and Cellular Immunology, Division of Instrumental Analysis, and Division of Animal Research at Shinshu University for their assistance.
Abbreviations
- ADCC
Antibody-dependent cell cytotoxicity
- APC
Antigen-presenting cell
- CTLA-4
Cytotoxic T-lymphocyte antigen 4
- DMEM
Dulbecco’s Modified Eagle Medium
- FBS
Fetal bovine serum
- IFN
Interferon
- LAG-3
Lymphocyte activation gene 3
- PD-1
Programmed cell death 1
- PD-L1
Programmed cell death ligand 1
- STAT3
Signal transducer and activator of transcription 3
Authors’ contributions
KY, MO, JS, HK and NS contributed to the study design. CK, HIshida, MT, and KU performed validation experiments in cell lines and TK, HIdeta, AS, TT, MT, KA, and TU performed validation experiments with live animals. KY and HH conducted flow cytometry experiments and its analysis. KY, MO and NS wrote the manuscript. All authors have read and approved to publish this manuscript.
Funding
This research was supported by the JSPS KAKENHI (grant no. 16 K20045 and 18 K16652). Mouse anti-PD-1 antibody (4H2) was provided from Ono Pharmaceutical Co., Ltd. (Osaka, Japan). They did not intervene to any step of the design of the study, collection, analysis, and interpretation of data or in writing the manuscript.
Availability of data and materials
Data presented in this manuscript are available from the corresponding author upon reasonable request.
Ethics approval and consent to participate
Mice were housed and maintained at the Division of Animal Research of Shinshu University. Based on the national regulations and guidelines, all experimental procedures were reviewed by the Committee for Animal Experiments and finally approved by the president of Shinshu University (Approval Number 280112). The applications of all cell lines for academic research are permitted.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Masanori Okamoto, Email: ryouyuma@shinshu-u.ac.jp.
Naoto Saito, Email: saitoko@shinshu-u.ac.jp.
Supplementary information
Supplementary information accompanies this paper at 10.1186/s12885-019-6499-y.
References
- 1.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi: 10.1038/nrc3239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–151. doi: 10.1016/S1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 3.Pardoll DM. Immunology beats cancer: a blueprint for successful translation. Nat Immunol. 2012;13(12):1129–1132. doi: 10.1038/ni.2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Honda Y, Otsuka A, Ono S, Yamamoto Y, Seidel JA, Morita S, et al. Infiltration of PD-1-positive cells in combination with tumor site PD-L1 expression is a positive prognostic factor in cutaneous angiosarcoma. Oncoimmunology. 2017;6(1):e1253657. doi: 10.1080/2162402X.2016.1253657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abiko K, Mandai M, Hamanishi J, Yoshioka Y, Matsumura N, Baba T, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19(6):1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 6.Mandai M, Hamanishi J, Abiko K, Matsumura N, Baba T, Konishi I. Dual faces of IFNgamma in cancer progression: a role of PD-L1 induction in the determination of pro- and antitumor immunity. Clin Cancer Res. 2016;22(10):2329–2334. doi: 10.1158/1078-0432.CCR-16-0224. [DOI] [PubMed] [Google Scholar]
- 7.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 8.Malas S, Harrasser M, Lacy KE, Karagiannis SN. Antibody therapies for melanoma: new and emerging opportunities to activate immunity (review) Oncol Rep. 2014;32(3):875–886. doi: 10.3892/or.2014.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pillai RN, Behera M, Owonikoko TK, Kamphorst AO, Pakkala S, Belani CP, et al. Comparison of the toxicity profile of PD-1 versus PD-L1 inhibitors in non-small cell lung cancer: a systematic analysis of the literature. Cancer. 2018;124(2):271–277. doi: 10.1002/cncr.31043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.You W, Liu M, Miao JD, Liao YQ, Song YB, Cai DK, et al. A network meta-analysis comparing the efficacy and safety of anti-PD-1 with anti-PD-L1 in non-small cell lung cancer. J Cancer. 2018;9(7):1200–1206. doi: 10.7150/jca.22361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zheng Y, Rudensky AY. Foxp3 in control of the regulatory T cell lineage. Nat Immunol. 2007;8(5):457–462. doi: 10.1038/ni1455. [DOI] [PubMed] [Google Scholar]
- 12.Quezada SA, Simpson TR, Peggs KS, Merghoub T, Vider J, Fan X, et al. Tumor-reactive CD4(+) T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J Exp Med. 2010;207(3):637–650. doi: 10.1084/jem.20091918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Selby MJ, Engelhardt JJ, Quigley M, Henning KA, Chen T, Srinivasan M, et al. Anti-CTLA-4 antibodies of IgG2a isotype enhance antitumor activity through reduction of intratumoral regulatory T cells. Cancer Immunol Res. 2013;1(1):32–42. doi: 10.1158/2326-6066.CIR-13-0013. [DOI] [PubMed] [Google Scholar]
- 14.Miyara M, Yoshioka Y, Kitoh A, Shima T, Wing K, Niwa A, et al. Functional delineation and differentiation dynamics of human CD4+ T cells expressing the FoxP3 transcription factor. Immunity. 2009;30(6):899–911. doi: 10.1016/j.immuni.2009.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Park HJ, Park JS, Jeong YH, Son J, Ban YH, Lee BH, et al. PD-1 upregulated on regulatory T cells during chronic virus infection enhances the suppression of CD8+ T cell immune response via the interaction with PD-L1 expressed on CD8+ T cells. J Immunol. 2015;194(12):5801–5811. doi: 10.4049/jimmunol.1401936. [DOI] [PubMed] [Google Scholar]
- 16.Asano Takeru, Meguri Yusuke, Yoshioka Takanori, Kishi Yuriko, Iwamoto Miki, Nakamura Makoto, Sando Yasuhisa, Yagita Hideo, Koreth John, Kim Haesook T., Alyea Edwin P., Armand Philippe, Cutler Corey S., Ho Vincent T., Antin Joseph H., Soiffer Robert J., Maeda Yoshinobu, Tanimoto Mitsune, Ritz Jerome, Matsuoka Ken-ichi. PD-1 modulates regulatory T-cell homeostasis during low-dose interleukin-2 therapy. Blood. 2017;129(15):2186–2197. doi: 10.1182/blood-2016-09-741629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang B, Chikuma S, Hori S, Fagarasan S, Honjo T. Nonoverlapping roles of PD-1 and FoxP3 in maintaining immune tolerance in a novel autoimmune pancreatitis mouse model. Proc Natl Acad Sci U S A. 2016;113(30):8490–8495. doi: 10.1073/pnas.1608873113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gianchecchi E, Fierabracci A. Inhibitory receptors and pathways of lymphocytes: the role of PD-1 in treg development and their involvement in autoimmunity onset and cancer progression. Front Immunol. 2018;9:2374. doi: 10.3389/fimmu.2018.02374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamada T, Togashi Y, Tay C, Ha D, Sasaki A, Nakamura Y, et al. PD-1<sup>+</sup> regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer. Proc Natl Acad Sci. 2019;116(20):9999–10008. doi: 10.1073/pnas.1822001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tawbi HA, Burgess M, Bolejack V, Van Tine BA, Schuetze SM, Hu J, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. doi: 10.1016/S1470-2045(17)30624-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess MA, Crowley J, Reinke DK, Riedel RF, George S, Movva S, et al. SARC 028: a phase II study of the anti-PD1 antibody pembrolizumab (P) in patients (Pts) with advanced sarcomas. J Clin Oncol. 2015;33(15_suppl):TPS10578-TPS. doi: 10.1200/jco.2015.33.15_suppl.tps10578. [DOI] [Google Scholar]
- 22.Paoluzzi L, Cacavio A, Ghesani M, Karambelkar A, Rapkiewicz A, Weber J, et al. Response to anti-PD1 therapy with nivolumab in metastatic sarcomas. Clin Sarcoma Res. 2016;6:24. doi: 10.1186/s13569-016-0064-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zheng B, Ren T, Huang Y, Sun K, Wang S, Bao X, et al. PD-1 axis expression in musculoskeletal tumors and antitumor effect of nivolumab in osteosarcoma model of humanized mouse. J Hematol Oncol. 2018;11(1):16. doi: 10.1186/s13045-018-0560-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen JK, Cote GM, Choy E, Yang P, Harmon D, Schwab J, et al. Programmed cell death ligand 1 expression in osteosarcoma. Cancer Immunol Res. 2014;2(7):690–698. doi: 10.1158/2326-6066.CIR-13-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wedekind MF, Wagner LM, Cripe TP. Immunotherapy for osteosarcoma: where do we go from here? Pediatr Blood Cancer. 2018;65(9):e27227. doi: 10.1002/pbc.27227. [DOI] [PubMed] [Google Scholar]
- 26.van Erp AEM, Versleijen-Jonkers YMH, Hillebrandt-Roeffen MHS, van Houdt L, Gorris MAJ, van Dam LS, et al. Expression and clinical association of programmed cell death-1, programmed death-ligand-1 and CD8(+) lymphocytes in primary sarcomas is subtype dependent. Oncotarget. 2017;8(41):71371–71384. doi: 10.18632/oncotarget.19071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Brouchet A, Illac C, Gilhodes J, Bouvier C, Aubert S, Guinebretiere JM, et al. CD163-positive tumor-associated macrophages and CD8-positive cytotoxic lymphocytes are powerful diagnostic markers for the therapeutic stratification of osteosarcoma patients: an immunohistochemical analysis of the biopsies fromthe French OS2006 phase 3 trial. Oncoimmunology. 2017;6(9):e1331193. doi: 10.1080/2162402X.2017.1331193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Workman P, Aboagye EO, Balkwill F, Balmain A, Bruder G, Chaplin DJ, et al. Guidelines for the welfare and use of animals in cancer research. Br J Cancer. 2010;102:1555. doi: 10.1038/sj.bjc.6605642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratti C, Botti L, Cancila V, Galvan S, Torselli I, Garofalo C, et al. Trabectedin overrides osteosarcoma differentiative block and reprograms the tumor immune environment enabling effective combination with immune checkpoint inhibitors. Clin Cancer Res. 2017;23(17):5149–5161. doi: 10.1158/1078-0432.CCR-16-3186. [DOI] [PubMed] [Google Scholar]
- 30.Maciorowski Z, Chattopadhyay PK, Jain P. Basic multicolor flow cytometry. Curr Protoc Immunol. 2017;117:5.4.1–5.4.38. doi: 10.1002/cpim.26. [DOI] [PubMed] [Google Scholar]
- 31.Xiao Q, Wu J, Wang WJ, Chen S, Zheng Y, Yu X, et al. DKK2 imparts tumor immunity evasion through beta-catenin-independent suppression of cytotoxic immune-cell activation. Nat Med. 2018;24(3):262–270. doi: 10.1038/nm.4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parsa AT, Waldron JS, Panner A, Crane CA, Parney IF, Barry JJ, et al. Loss of tumor suppressor PTEN function increases B7-H1 expression and immunoresistance in glioma. Nat Med. 2007;13(1):84–88. doi: 10.1038/nm1517. [DOI] [PubMed] [Google Scholar]
- 33.Marzec M, Zhang Q, Goradia A, Raghunath PN, Liu X, Paessler M, et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1) Proc Natl Acad Sci U S A. 2008;105(52):20852–20857. doi: 10.1073/pnas.0810958105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alsaab HO, Sau S, Alzhrani R, Tatiparti K, Bhise K, Kashaw SK, et al. PD-1 and PD-L1 checkpoint signaling inhibition for cancer immunotherapy: mechanism, combinations, and clinical outcome. Front Pharmacol. 2017;8:561. doi: 10.3389/fphar.2017.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tumeh PC, Harview CL, Yearley JH, Shintaku IP, Taylor EJ, Robert L, et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature. 2014;515(7528):568–571. doi: 10.1038/nature13954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sun C, Mezzadra R, Schumacher TN. Regulation and function of the PD-L1 checkpoint. Immunity. 2018;48(3):434–452. doi: 10.1016/j.immuni.2018.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liakou CI, Kamat A, Tang DN, Chen H, Sun J, Troncoso P, et al. CTLA-4 blockade increases IFNgamma-producing CD4+ICOShi cells to shift the ratio of effector to regulatory T cells in cancer patients. Proc Natl Acad Sci U S A. 2008;105(39):14987–14992. doi: 10.1073/pnas.0806075105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zuazo M, Arasanz H, Fernández-Hinojal G, García-Granda MJ, Gato M, Bocanegra A, et al. Functional systemic CD4 immunity is required for clinical responses to PD-L1/PD-1 blockade therapy. EMBO Mol Med. 2019;11(7):e10293. doi: 10.15252/emmm.201910293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Josefowicz SZ, Lu L-F, Rudensky AY. Regulatory T cells: mechanisms of differentiation and function. Annu Rev Immunol. 2012;30(1):531–564. doi: 10.1146/annurev.immunol.25.022106.141623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Callahan MK, Wolchok JD, Allison JP. Anti-CTLA-4 antibody therapy: immune monitoring during clinical development of a novel immunotherapy. Semin Oncol. 2010;37(5):473–484. doi: 10.1053/j.seminoncol.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Simpson TR, Li F, Montalvo-Ortiz W, Sepulveda MA, Bergerhoff K, Arce F, et al. Fc-dependent depletion of tumor-infiltrating regulatory T cells co-defines the efficacy of anti-CTLA-4 therapy against melanoma. J Exp Med. 2013;210(9):1695–1710. doi: 10.1084/jem.20130579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Takeuchi Y, Nishikawa H. Roles of regulatory T cells in cancer immunity. Int Immunol. 2016;28(8):401–409. doi: 10.1093/intimm/dxw025. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Table S1. List of antibodies.
Data Availability Statement
Data presented in this manuscript are available from the corresponding author upon reasonable request.