Abstract
Background:
Alemtuzumab is a highly effective therapy for relapsing-remitting multiple sclerosis (RRMS), and immune thrombocytopenia (ITP) has been identified as a risk.
Objective:
To examine ITP incidence, treatment, and outcomes during the clinical development of alemtuzumab for RRMS and discuss postmarketing experience outside clinical trials.
Methods:
CAMMS223 and Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis (CARE-MS) I and II investigated two annual courses of alemtuzumab 12 mg (or 24 mg in CAMMS223/CARE-MS II) versus subcutaneous interferon beta-1a three times per week. Patients completing core studies could enroll in an extension. Monthly monitoring for ITP continued until 48 months after the last alemtuzumab infusion.
Results:
Of 1485 alemtuzumab-treated MS patients in the clinical development program, 33 (2.2%) developed ITP (alemtuzumab 12 mg, 24 [2.0%]; alemtuzumab 24 mg, 9 [3.3%]) over median 6.1 years of follow-up after the first infusion; most had a sustained response to first-line ITP therapy with corticosteroids, platelets, and/or intravenous immunoglobulin. All cases occurred within 48 months of the last alemtuzumab infusion. Postmarketing surveillance data suggest that the ITP incidence is not higher in clinical practice than in clinical trials.
Conclusion:
Alemtuzumab-associated ITP occurs in approximately 2% of patients and is responsive to therapy. Careful monitoring is key for detection and favorable outcomes.
Keywords: Alemtuzumab, disease-modifying therapy, immune thrombocytopenia, relapsing-remitting multiple sclerosis, safety
Introduction
Alemtuzumab (LEMTRADA®, Sanofi Genzyme) is a humanized anti-CD52 monoclonal antibody approved for the treatment of relapsing forms of multiple sclerosis (MS). The alemtuzumab clinical development program included the phase 2 CAMMS223 study, phase 3 Comparison of Alemtuzumab and Rebif® Efficacy in Multiple Sclerosis (CARE-MS) I and II studies, CAMMS03409 extension study, and the ongoing TOPAZ extension study.1–7 Compared with subcutaneous interferon beta-1a (SC IFNB-1a; Rebif, EMD Serono, Inc.), patients with active relapsing-remitting MS (RRMS) treated with alemtuzumab had significantly reduced relapses and slowed brain volume loss in phase 2 and 3 studies, and significantly reduced 6-month confirmed disability worsening in CAMMS223 and CARE-MS II.2,4,5 Efficacy was maintained over 6 years (CARE-MS)3,6,7 or 10 years (CAMMS223),8 with a majority of patients receiving no additional alemtuzumab or other disease-modifying therapies (DMTs). Risk for autoimmune adverse events was increased, most commonly affecting the thyroid, and less frequently manifesting as immune thrombocytopenia (ITP) or nephropathy.
Non-alemtuzumab-related ITP is characterized by antibody- and cell-mediated platelet destruction and decreased platelet production, and may predispose patients to bleeding.9,10 ITP may occur as a primary condition or secondary to infections (e.g. HIV, hepatitis C, Helicobacter pylori), immunodeficiencies, broader autoimmune diseases (e.g. systemic lupus erythematosus, antiphospholipid syndrome, Evans syndrome), and lymphoproliferative disorders.11–13 Primary ITP in adults usually assumes a chronic course (>12 months),14 requiring ongoing surveillance and treatment.15 Many patients (50%–75%) do not respond to first-line treatment, or will have loss of response after tapering, requiring further treatment.15 In contrast, pediatric ITP is self-limited in approximately two-thirds of cases and often does not require treatment.14,16,17
In the CAMMS223 study, ITP occurred in six alemtuzumab-treated patients (3%) and was distinct from typical primary ITP in adults in that it was characterized by responsiveness to conventional ITP therapies with prolonged remission.18 These cases developed months or years after alemtuzumab exposure and thus were also distinct from typical drug-induced ITP, which occurs during drug exposure and resolves within days of withdrawal.18
The index ITP case for alemtuzumab, observed during CAMMS223, involved unreported cutaneous signs of ITP and a fatal brain hemorrhage prior to diagnosis.4 A monitoring program for ITP was therefore instituted during CAMMS223 and all subsequent alemtuzumab MS clinical trials, and in the postmarketing setting in the form of a Risk Management Plan or Risk Evaluation and Mitigation Strategy (RMP/REMS).19,20 Here, we report ITP incidence, detection, management, and outcomes throughout the alemtuzumab clinical development program, the postmarketing ITP incidence, and implications for ITP management.
Methods
Study design
This analysis is based on pooled data from the phase 2 and 3 studies and the extension study, from December 2002 until the end of the extension study in February 2016.
Core study methods were described previously.2,4,5 Briefly, the 3-year CAMMS223 study (NCT00050778) and the 2-year CARE-MS studies (NCT00530348; NCT00548405) were rater-blinded, head-to-head, active-controlled studies in which patients were randomized to two annual courses of alemtuzumab or to SC IFNB-1a 44 μg thrice weekly. Alemtuzumab was administered as intravenous infusions of 12 or 24 mg/day on five consecutive days at baseline and on three consecutive days 12 months later. The 24-mg dose was only administered in CAMMS223 and CARE-MS II. Patients from CAMMS223 could participate in an extended follow-up period up to 2 years (5 years in total) and then enroll in the same extension study as patients from the CARE-MS studies.8,21
In the CAMMS03409 extension study (NCT00930553), patients initially randomized to alemtuzumab could receive as-needed retreatment (12 mg/day on three consecutive days, at least 12 months after the prior treatment course) based on relapse or radiographic criteria, or other DMT at the investigator’s discretion.3,6 Patients who received SC IFNB-1a in the core studies were offered alemtuzumab 12 mg/day at the extension study baseline (five consecutive days) and 12 months later (three consecutive days), and thereafter on an as-needed basis at least 12 months after the prior treatment course.
ITP monitoring and definition
The ITP monitoring program included education of health care providers and patients on signs and symptoms of ITP, monthly complete blood count (CBC) monitoring, and monthly patient symptom questionnaires offset from CBCs by 2 weeks. Monitoring continued until 48 months following the last alemtuzumab infusion; the 48-month monitoring period restarted if patients were re-treated with alemtuzumab. Percentage compliance with monthly CBC monitoring was calculated as follows: (number of monthly CBCs carried out divided by number of expected monthly CBCs) × 100.
ITP was defined according to diagnostic criteria outlined by an international working group22 as either a confirmed platelet count ⩾ 50 × 109/L and <100 × 109/L on ⩾2 consecutive occasions over a period of at least 1 month, or a confirmed platelet count < 50 × 109/L without clumping documented on ⩾2 consecutive occasions over any period of time; and normal hemoglobin, normal white blood cell count, absence of splenomegaly, normal peripheral smear except for a decrease in platelets without clumping, and no evidence of an alternative non-autoimmune etiology of thrombocytopenia. Each case was evaluated and deemed “medically confirmed” by the sponsor if it met this definition.
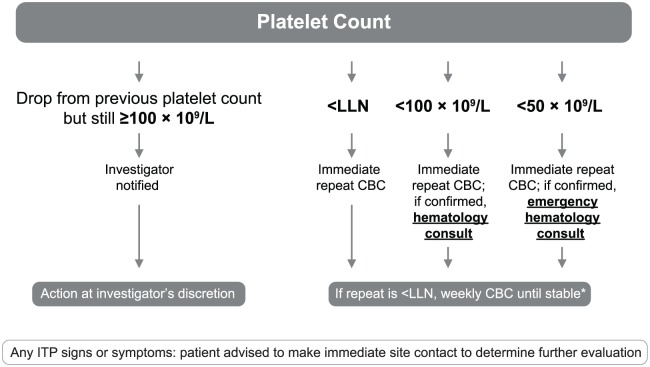
Platelet counts below the lower limit of normal (LLN) triggered weekly CBCs until counts stabilized (Figure 1). A hematology consultation was indicated for platelet counts < 100 × 109/L. After ITP diagnosis, alemtuzumab was to be discontinued permanently, and patients continued follow-up at least every 3 months for at least 48 months from ITP diagnosis. ITP treatment was at the discretion of the treating physician. Complete response to ITP treatment was defined as a platelet count of ⩾100 × 109/L on two separate occasions ⩾7 days apart;22 complete remission required the additional criterion of an absence of ongoing ITP therapy.
Figure 1.
Platelet count monitoring to facilitate detection of ITP. *Weekly CBC protocol: weekly platelet counts for ⩾8 weeks. Monthly monitoring could be resumed when platelet counts were either within the normal range for eight readings, or were stabilized (i.e. eight consecutive readings ⩾ 100 × 109/L and average of last four counts ⩾ average of four prior counts).
CBC: complete blood count; ITP: immune thrombocytopenia; LLN: lower limit of normal.
Assessments in patients with ITP
Patients with ITP were evaluated for the number of alemtuzumab courses received, timing of ITP onset, treatments, timing of complete response, baseline characteristics, and development of other autoimmune adverse events. Alemtuzumab MS efficacy assessments included annualized relapse rate (ARR) and mean Expanded Disability Status Scale (EDSS) score, and were evaluated in patients with ITP 12 months before and 12 months after their ITP event.
Results
Patients
In the clinical development program, 1216 and 269 patients with MS were exposed to alemtuzumab 12 mg (the now-approved dosage) or 24 mg, respectively, with their initial course in either CAMMS223 (n = 215), CARE-MS I (n = 376), CARE-MS II (n = 596), or the extension (n = 298; for patients initially randomized to SC IFNB-1a). At the data cutoff in February 2016, there were 8632 patient-years of follow-up since alemtuzumab initiation, with a median follow-up time of 6.1 years (range, 0.7–12.7 years) per patient.
ITP incidence, timing, and alemtuzumab exposure
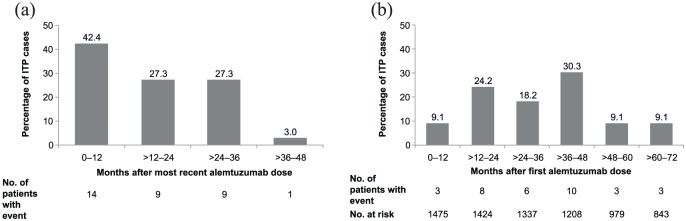
A total of 33 patients in the clinical development program had ITP, giving an overall incidence of 2.2% (alemtuzumab 12 mg, 2.0%; n = 24; Table 1) and corresponding to a rate of 0.4 (95% confidence interval [CI], 0.3–0.5) patients per 100 patient-years (alemtuzumab 12 mg, 0.4 [0.3–0.6]). All ITP cases began within 48 months of the most recent alemtuzumab infusion (median, 15 months; range, 1–44 months; Figure 2[a]), within the monitoring window of the postmarketing RMP/REMS. Median follow-up time since the last alemtuzumab dose for all patients was 3.5 years (range, 0–11.3 years). The median time of ITP onset from alemtuzumab initiation was 31 months (range, 4–71 months; Figure 2[b]). Of patients who did not develop ITP, 611 had ⩾4 years’ follow-up after the last dose and 841 had <4 years’ follow-up after the last dose.
Table 1.
Incidence of medically confirmed, protocol-defined ITP in the Alemtuzumab Clinical Development Program.
| Study | Alemtuzumab 12 mg, n/N (%) |
Alemtuzumab 24 mg, n/N (%) |
Alemtuzumab Pooled, n/N (%) |
|---|---|---|---|
| Core studies (total) | 9/918 (1.0) | 7/269 (2.6) | 16/1187a (1.3) |
| CAMMS223 | 2/107 (1.9) | 4/108 (3.7) | 6/215 (2.8) |
| CARE-MS I | 3/376 (0.8) | – | 3/376 (0.8) |
| CARE-MS II | 4/435 (0.9) | 3/161 (1.9) | 7/596 (1.2) |
| Extension studyb | 17/1100 (1.5)c | 2/214 (0.9) | 19/1314a (1.4) |
| Totald | 24/1216 (2.0) | 9/269 (3.3) | 33/1485a (2.2) |
CARE-MS: Comparison of Alemtuzumab and Rebif Efficacy in Multiple Sclerosis; ITP: immune thrombocytopenia.
Notes on total patient numbers: number with MS who received alemtuzumab in core studies = 1187; number ever treated with alemtuzumab (including those who received subcutaneous interferon beta-1a [SC IFNB-1a] in the core studies) who enrolled in the extension = 1314; number with MS ever treated with alemtuzumab (including those who did not enroll in the extension) = 1485.
Numerators include two patients who received SC IFNB-1a in the core studies and developed ITP after receiving alemtuzumab in the extension.
One ITP patient was randomized to alemtuzumab 24 mg but received the 12-mg dose; this patient is counted in the 12-mg column.
One patient with an ITP event in CAMMS223 had an ITP relapse during the extension, and one patient had an ITP event that began during CARE-MS I and was ongoing at the time of enrollment into the extension; these patients are counted once in the “Total” row.
Figure 2.
Timing of ITP onset relative to alemtuzumab administration: (a) time from the most recent course to ITP onset and (b) time from the first course to ITP onset.
Analysis of baseline demographic, clinical, and magnetic resonance imaging (MRI) characteristics, history of prior MS therapies, and history of other autoimmune diseases did not yield any characteristics predictive of ITP (data not shown). Furthermore, alemtuzumab-treated patients who developed ITP did not have a higher incidence of on-study autoimmune thyroid disorders compared with those who did not develop ITP (30% vs 41%; p = 0.3). Of the 33 patients who developed ITP, 3 (9%) received only one alemtuzumab course, 21 (64%) received two courses, and 8 (24%) and 1 (3%) received three and four courses, respectively. Among patients in the overall study population, ITP incidence did not increase in patients who received three and four courses of alemtuzumab compared with patients who received two courses, with no patients receiving five or six courses developing ITP (Table 2).
Table 2.
Incidence of ITP by number of courses received among all patients treated with alemtuzumab in the Clinical Development Program (N = 1485).
| Number of courses received | Incidence, n/N (%) |
|---|---|
| 1 | 3/50 (6.0) |
| 2 | 21/873 (2.4) |
| 3 | 8/367 (2.2) |
| 4 | 1/133 (0.8) |
| 5 | 0/50 (0) |
| 6 | 0/10 (0) |
| 7 | 0/2 (0) |
ITP: immune thrombocytopenia.
One patient received further alemtuzumab treatment after ITP diagnosis (a protocol deviation). In this patient, alemtuzumab retreatment (i.e. Course 3) occurred 4 years after the onset of ITP, with no subsequent events related to low platelet counts. All other patients permanently discontinued alemtuzumab after ITP diagnosis.
ITP detection and monitoring compliance
The RMP/REMS facilitated the timely detection of ITP events to allow for prompt treatment. Approximately 80% of cases (both asymptomatic and symptomatic) were detected through monthly CBC monitoring and 20% through patient recognition of signs and symptoms. Mean nadir platelet count was 11 × 109/L (median, 5 × 109/L; range, 1 × 109/L to 41 × 109/L). Petechiae, easy bruising, and menorrhagia were the most common signs and symptoms. Seven patients (21%) were asymptomatic. Compliance with monthly CBC monitoring was 95.3%.
Response to ITP therapy
Of the 33 alemtuzumab-treated patients with ITP, 3 (9%) were untreated, 21 (64%) received first-line therapy only, and the remaining 9 (27%) received second-line therapy (Table 3). Untreated patients included the index patient who died before diagnosis and 2 patients for whom ITP resolved spontaneously. First-line therapy consisted of steroids with or without intravenous immunoglobulins and/or platelet transfusion; second-line therapy was mostly rituximab. Decisions to treat with rituximab primarily resulted from platelet counts not being maintained once steroids were tapered. Other reasons for rituximab use were no immediate response to first-line therapy in one patient and poor tolerance to steroids in another. After treatment with rituximab, platelet counts for these patients remained above the LLN. Two patients underwent splenectomy as second-line therapy, according to the local protocol (see Supplementary material for case details, Patients A and B).
Table 3.
ITP therapies used and patient response in the Alemtuzumab Clinical Development Program.
| ITP treatment | n (%) | Median (range) months to first complete responsea | Median (range) months of complete remission at last follow-upb |
|---|---|---|---|
| Untreatedc | 3 (9.1) | 0.48 (0.46–0.49) | 34.1 (10.8–57.4) |
| Patients receiving only first-line treatmentd | 21 (63.6) | 0.4 (0.1–2.7) | 33.5 (5.7–110.0) |
| Steroids | 12 | 0.4 (0.2–2.3) | 33.3 (15.0–110.0) |
| Steroids + IVIg/Rho(D) Ig | 4 | 0.3 (0.2–0.6) | 38.3 (5.7–82.2) |
| Steroids + platelets | 3 | 0.4 (0.1–0.5) | 33.7 (27.8–83.4) |
| Steroids + platelets + IVIg/Rho(D) Ig | 2 | 1.4 (0.1–2.7) | 36.3 (20.9–51.7) |
| Patients receiving first- and second-line treatment | 9 (27.3) | 0.5 (0.1–1.7) | 25.6 (11.5–49.6) |
| Steroids + rituximab | 1 | 0.1 | 29.6 |
| Steroids + platelets + rituximab | 1 | 1.7 | 33.8 |
| Steroids + IVIg + rituximab | 2 | 0.4 (0.2–0.5) | 11.6 (11.5–11.8) |
| Steroids + platelets + IVIg + rituximab | 3 | 0.5 (0.4–1.0) | 48.9 (21.1–49.6) |
| Steroids + splenectomy | 2 | 0.7 (0.3–1.0) | 21.6 (21.6) |
ITP: immune thrombocytopenia; Ig: immunoglobulin; IV: intravenous: IVIg: intravenous immunoglobulin.
Measured from the time of ITP onset.
Includes only patients who were in complete remission at the last follow-up. Of 33 patients with ITP, 1 died of ITP and 4 were not in complete remission at the last follow-up.
Includes the index case who did not receive treatment as the patient died prior to diagnosis of ITP.
Includes patient with initial spontaneous recovery, then subsequent ITP relapse, which required first-line treatment.
For patients receiving first- or second-line therapy, median time to first complete response was less than 1 month. Complete remission was achieved by 28 of 32 surviving ITP patients by the time of the last follow-up or at the end of the extension study. Median duration of ongoing complete remission at the time of analysis was 33 months (range, 6–110 months). Four patients had not yet achieved complete remission at the time of the last on-study platelet count. Of these four, three had platelet counts > 100 × 109/L but had ongoing steroid treatment; one of these later went on to have a splenectomy after the study ended followed by a normal platelet count (Supplementary material, Patient B). In the fourth patient, ITP recurred in a delayed manner several years after the initial event. The initial onset was 16 months after the last alemtuzumab course. At 8 years after the last alemtuzumab course and 18 months after starting fingolimod, the patient had an ITP recurrence for which she was treated. Platelet count was <100 × 109/L at the time of study discontinuation (Supplementary material, Patient C).
ITP does not affect the clinical efficacy of alemtuzumab in MS patients
Analyses of the small population of alemtuzumab-treated patients with ITP suggest that the ITP event did not adversely affect MS clinical outcomes. ARR remained low during the 12-month period before (0.06 [95% CI, 0.017–0.241]) and after (0.03 [95% CI, 0.005–0.216]) ITP onset. Mean EDSS score was also stable 12 months before (2.64 [95% CI, 2.03–3.26]) and 12 months after (2.83 [95% CI, 2.20–3.45]) ITP onset.
Postmarketing experience
Based on the exposure of 18,561 patients treated worldwide with alemtuzumab for RRMS through 31 December 2017, the postmarketing frequency of ITP was 0.72%.23 This is lower than expected based on clinical trial evidence, although postmarketing frequency and clinical trial incidence rates are not directly comparable due to limitations of postmarketing reporting. In the authors’ clinical experience, ITP incidence among alemtuzumab-treated patients (0.4%) is consistent with this postmarketing estimate.
Two ITP-related deaths have been reported in the postmarketing setting. In the first case, ITP developed in a patient 6 months after the second alemtuzumab course. After initial treatment, the patient suffered a non-complicated intracranial hemorrhage and then underwent splenectomy, after which abdominal bleeding was reported. The patient died 2 weeks later of cerebral/cerebellar hemorrhage. In the second case, a patient developed ITP 10 months after alemtuzumab initiation, followed by aplastic marrow and autoimmune myelofibrosis, and was treated. The patient died 6 months later after refusing medical care; the suspected cause was bacterial sepsis (Supplementary material, Patients D and E).
Discussion
ITP in alemtuzumab-treated RRMS patients occurred at a greater frequency (0.4 patients per 100 patient-years) than primary ITP in the general population (0.0016–0.0039 cases per 100 patient-years).16 Risk was not further increased in the subset of patients who received additional alemtuzumab beyond the initial two courses. ITP was detectable with platelet count monitoring and patient education, and most patients had sustained resolution of ITP after therapy. ITP cases were generally identified through monthly blood monitoring; however, most patients also presented with signs and symptoms, which they had been trained to identify. The risk for ITP should be considered within the context of the overall benefit:risk profile of alemtuzumab. Treatment with alemtuzumab reduces 6-month confirmed disability worsening, relapses, and MRI lesion activity, and slows brain volume loss compared with SC IFNB-1a.2,4,5 Furthermore, a subset of alemtuzumab-treated patients experience 6-month confirmed disability improvement.5,24 These effects are maintained over 5–10 years in the absence of regular, continuous dosing.3,6–8,21 For the few patients who did develop ITP, it was not associated with an increase in MS disease activity.
The broader experience of the alemtuzumab clinical development program confirmed the nature of ITP observed in the phase 2 study.18 In contrast to adult primary ITP, which usually assumes a chronic course,9 ITP in alemtuzumab-treated patients usually has a more limited course and is responsive to conventional therapies. First complete response to ITP therapy was typically achieved in less than a month. A majority of patients achieved durable remission of ITP after first-line therapy alone; in all cases in which second-line therapy was used, it induced sustained remission.
These results are also aligned with results from a long-term follow-up of 87 patients from the initial investigator-led studies of alemtuzumab in MS.25 Over a median 7-year follow-up, three patients developed ITP: one with spontaneous recovery, one with sustained remission after first-line therapy, and one with persistent thrombocytopenia over 6 years, despite one rituximab treatment and periodic pulse steroids. All three cases occurred within 48 months of the most recent alemtuzumab course.
The postmarketing data collected by the sponsor identified a lower incidence of alemtuzumab-related ITP in practice than in clinical trials. Reasons for this difference may include variations in patient populations, duration of follow-up, or reporting methods. Postmarketing data are more susceptible to under-reporting, unavailability of clinical details, or inaccurate diagnoses. However, the incidence of ITP in the authors’ experience (0.4%) is consistent with the sponsor-collected frequency. At this point, the real-world experience does not suggest that ITP incidence is greater than observed in clinical trials.
Mechanisms of autoimmune adverse events in alemtuzumab-treated patients are poorly understood. Given the delayed onset of ITP after alemtuzumab exposure, it may be driven by factors including lymphocyte repopulation patterns18; reduced thymopoiesis, homeostatic proliferation of T cells that have escaped depletion, resulting reduced T-cell clonality26; and deletion of an immune tolerance–promoting CD8+ T-cell population.27 These hypotheses are yet to be tested rigorously.
Interestingly, although some alemtuzumab-treated patients may develop ITP, alemtuzumab has also been used to treat severe steroid-refractory adult-onset ITP. Jung et al.28 reported two cases of ITP that responded to alemtuzumab treatment, resulting in a complete response 24 weeks after treatment, in patients for whom other therapies failed to resolve low platelet counts.
Several groups have reported that during the week of alemtuzumab infusion, a mild-to-moderate decrease in platelet count may occur (but remaining above 50 × 109/L), which recovers spontaneously over 1–2 weeks.29–31 This transient thrombocytopenia is considered an infusion-associated reaction and should not be confused with ITP. The authors of the current report have also observed this phenomenon.
In the absence of reliable predictors of ITP development among prospective alemtuzumab patients, vigilant monitoring is mandated and is critical for early detection, adequate management, and morbidity minimization. The success of the monitoring protocol in the clinical trials was demonstrated by the absence of fatalities or serious morbidity from ITP after its implementation. The high rate of compliance with blood monitoring demonstrates the feasibility of long-term monitoring, at least among clinical trial patients. The time frame for monitoring (until 48 months after the last alemtuzumab infusion) is supported by data showing that 97% of ITP cases occurred within 36 months of the most recent alemtuzumab course; only one patient developed ITP between 36 and 48 months. The REMS for alemtuzumab in the United States20 and the RMP in the European Union (EU) and other countries around the world19 guide monitoring in the postmarketing setting. These programs are based on the monitoring protocol implemented during the MS clinical development program and similarly require monitoring until 48 months after the last alemtuzumab infusion. Patients selected for alemtuzumab treatment in the real-world setting must understand the importance of, and agree to, adherence to the REMS/RMP; furthermore, patient education and involvement are essential for the effectiveness of the monitoring program, as mucocutaneous manifestations may provide the first warning of severe thrombocytopenia. The success of the clinical trial monitoring protocol, and its close alignment with the REMS/RMP, suggests that the REMS/RMP is well designed to enable early detection of ITP, thereby allowing timely and appropriate management for optimal outcomes.
Vigilant surveillance by the clinician, thorough patient education, and early referral to a hematologist are key to patient safety. In our experience, both laboratory monitoring and patient recognition of signs and symptoms were critical to the diagnosis of ITP. Obtaining a hematology consult upon any abnormal monitoring criteria or bleeding signs or symptoms ensures the best care for patients. Other etiologies of thrombocytopenia must be considered and ruled out.32 Referral to hematologists also allows for careful selection of individual treatment, considering factors including severity of thrombocytopenia, extent of bleeding, comorbidities, activity and lifestyle, tolerance to side effects, and the need for non-ITP medications that may create a bleeding risk.33
Supplemental Material
Supplemental material, MSJ816612_supplementary_info for Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management by Adam Cuker, Ann D Bass, Congor Nadj, Mark A Agius, Brian Steingo, Krzysztof W Selmaj, Timothy Thoits, Alexandre Guerreiro, Bart Van Wijmeersch, Tjalf Ziemssen, Sven G Meuth, Christopher C LaGanke, Karthinathan Thangavelu, Claudio E Rodriguez, Darren P Baker, David H Margolin and Ann Jannsens in Multiple Sclerosis Journal
Acknowledgments
The authors and Sanofi would like to thank the patients for their participation in the CAMMS223, CARE-MS I, CARE-MS II, and CAMMS03409 studies, as well as the Steering Committees and the investigators. All authors had full access to the study data and take responsibility for the integrity of the data and the accuracy of the data analysis. Colin Mitchell, PhD, and Jonathan Valenzano, PharmD, of Sanofi reviewed the manuscript. Catherine Pennella of Sanofi provided assistance with gathering material for individual cases, and Nadia Daizadeh, PhD, of Sanofi provided additional statistical support. Editorial support was provided by Valerie P Zediak, PhD, and Richard J Hogan, PhD, Envision Scientific Solutions, and was funded by Sanofi. The ClinicalTrials.gov identifiers are CAMMS223 (NCT00050778), CARE-MS I (NCT00530348), CARE-MS II (NCT00548405), and CAMMS03409 (NCT00930553).
Footnotes
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: A.C. has received consulting fees from Kedrion, Sanofi, and Synergy and institutional grant support from Alexion, Bayer, Bioverativ, Novo Nordisk, Pfizer, Shire, Spark Therapeutics, and Syntimmune. A.D.B. has received consulting fees, fees for non-CME services, and research support from Biogen, Mallinckrodt, Novartis, Roche-Genentech, Sanofi, and Teva Neuroscience. C.N. has received consulting fees and speaking fees from Bayer, Bayhill, Biogen, Merck, Novartis, Roche, Sanofi, and Teva. M.A.A. has received consulting and lecture fees and institutional grant support from Biogen, Celgene, MedImmune, Novartis, Roche/Genentech, Sanofi, and Serono. B.S. has received consulting, speaking fees, and/or grant/research support from Acorda, Biogen, EMD Serono, Mallinckrodt, Novartis, Sanofi, and Teva. K.W.S. has received fees for consulting and/or speaking from Biogen, Merck, Novartis, Roche, Sanofi, and Synthon. T.T. has nothing to disclose. A.G. has nothing to disclose. B.V.W. has received research and travel grants, honoraria for MS expert advice, and speaking fees from Bayer-Schering, Biogen, Merck Serono, Novartis, Roche, Sanofi, and Teva. T.Z. has received consulting or speaking fees from Almirall, Bayer, Biogen, Merck, Novartis, Roche, Sanofi, and Teva and grant/research support from Biogen, Novartis, Sanofi, and Teva. S.G.M. has received honoraria for lecturing, travel expenses for attending meetings, and financial research support from Almirall, Bayer HealthCare, Biogen, Diamed, Merck Serono, Novartis, Novo Nordisk, ONO Pharma, Roche, Sanofi, and Teva. C.C.L. has received compensation for consulting from Acorda Therapeutics, Bayer, Biogen, Cephalon, EMD Serono, Novartis, Pfizer, Questcor, Sanofi, Strativa, Teva, and UCB. K.T. and D.P.B. are employees of Sanofi. C.E.R. was an employee of Sanofi at the time of the analysis and is currently employed by Sunovion Pharmaceuticals. D.H.M. was an employee of Sanofi at the time of the analysis and is currently employed by Cerevance, Inc. A.J. has received educational grant support from Janssen and consultancy fees from Abbvie, Janssen, Roche, and Sanofi.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The CAMMS223, CARE-MS I, CARE-MS II, and CAMMS03409 studies were funded by Sanofi and Bayer HealthCare Pharmaceuticals. Both Sanofi and Bayer Schering Pharma approved the design of the CAMMS223, CARE-MS I, CARE-MS II, and CAMMS03409 studies. Only Sanofi contributed to the conduct of the studies; collection, management, analysis, and interpretation of the data; preparation, review, and approval of the manuscript; and the decision to submit the manuscript for publication.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Adam Cuker, Departments of Medicine and Pathology & Laboratory Medicine, Hospital of the University of Pennsylvania, Philadelphia, PA, USA.
Ann D Bass, Neurology Center of San Antonio, San Antonio, TX, USA.
Congor Nadj, Institute of Neurology, Novi Sad, Serbia.
Mark A Agius, Woodland Clinic, Dignity Health, Woodland, CA, USA.
Brian Steingo, Fort Lauderdale MS Center, Pompano Beach, FL, USA.
Krzysztof W Selmaj, University of Warmia and Mazury in Olsztyn, Olsztyn, Poland.
Timothy Thoits, College of Human Medicine, MSU Spectrum Health, Grand Rapids, MI, USA.
Alexandre Guerreiro, Instituto de Doenças Neurológicas do Hospital Mãe de Deus, Porto Alegre, Brazil.
Bart Van Wijmeersch, Rehabilitation and MS Centre Overpelt, BIOMED, University of Hasselt, Hasselt, Belgium.
Tjalf Ziemssen, Center of Clinical Neuroscience, University Hospital Carl Gustav Carus, Dresden, Germany.
Sven G Meuth, Department of Neurology, University of Münster, Münster, Germany.
Christopher C LaGanke, North Central Neurology Associates, Cullman, AL, USA.
Karthinathan Thangavelu, Sanofi, Cambridge, MA, USA.
Claudio E Rodriguez, Sanofi, Cambridge, MA, USA; aCurrent affiliation: Sunovion Pharmaceuticals, Marlborough, MA, USA.
Darren P Baker, Sanofi, Cambridge, MA, USA.
David H Margolin, Sanofi, Cambridge, MA, USA; bCurrent affiliation: Cerevance, Inc., Boston, MA, USA.
Ann Jannsens, Department of Haematology, University Hospitals Leuven, Leuven, Belgium.
References
- 1. Brinar V, Giovannoni G, Havrdova E, et al. A phase 3b/4 long-term study of alemtuzumab in patients with relapsing-remitting multiple sclerosis: TOPAZ study design. Neurology 2015; 84: P7.219. [Google Scholar]
- 2. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380: 1819–1828. [DOI] [PubMed] [Google Scholar]
- 3. Coles AJ, Cohen JA, Fox EJ, et al. Alemtuzumab CARE-MS II 5-year follow-up: Efficacy and safety findings. Neurology 2017; 89: 1117–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coles AJ, Compston DA, Selmaj KW, et al. Alemtuzumab vs. interferon beta-1a in early multiple sclerosis. N Engl J Med 2008; 359: 1786–1801. [DOI] [PubMed] [Google Scholar]
- 5. Coles AJ, Twyman CL, Arnold DL, et al. Alemtuzumab for patients with relapsing multiple sclerosis after disease-modifying therapy: A randomised controlled phase 3 trial. Lancet 2012; 380: 1829–1839. [DOI] [PubMed] [Google Scholar]
- 6. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89: 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ziemssen T, Thomas K. Alemtuzumab in the long-term treatment of relapsing-remitting multiple sclerosis: An update on the clinical trial evidence and data from the real world. Ther Adv Neurol Disord 2017; 10: 343–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Coles AJ, Habek M, Bass AD, et al. Durable efficacy of alemtuzumab over 10 years: Long-term follow-up of patients with RRMS from the CAMMS223 study. Neurology 2016; 86(Suppl. 16): P3053. [Google Scholar]
- 9. Lakshmanan S, Cuker A. Contemporary management of primary immune thrombocytopenia in adults. J Thromb Haemost 2012; 10: 1988–1998. [DOI] [PubMed] [Google Scholar]
- 10. Stasi R. Immune thrombocytopenia: Pathophysiologic and clinical update. Semin Thromb Hemost 2012; 38: 454–462. [DOI] [PubMed] [Google Scholar]
- 11. Arnold DM, Curtis BR, Bakchoul T. Recommendations for standardization of laboratory testing for drug-induced immune thrombocytopenia: Communication from the SSC of the ISTH. J Thromb Haemost 2015; 13: 676–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bussel JB. Therapeutic approaches to secondary immune thrombocytopenic purpura. Semin Hematol 2009; 46(Suppl. 2): S44–S58. [DOI] [PubMed] [Google Scholar]
- 13. George JN, Aster RH. Drug-induced thrombocytopenia: Pathogenesis, evaluation, and management. Hematology Am Soc Hematol Educ Program 2009; 153–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. D’Orazio JA, Neely J, Farhoudi N. ITP in children: Pathophysiology and current treatment approaches. J Pediatr Hematol Oncol 2013; 35: 1–13. [DOI] [PubMed] [Google Scholar]
- 15. Sailer T, Lechner K, Panzer S, et al. The course of severe autoimmune thrombocytopenia in patients not undergoing splenectomy. Haematologica 2006; 91: 1041–1045. [PubMed] [Google Scholar]
- 16. Cuker A, Cines DB. Immune thrombocytopenia. Hematology Am Soc Hematol Educ Program 2010; 2010: 377–384. [DOI] [PubMed] [Google Scholar]
- 17. Neunert CE, Buchanan GR, Imbach P, et al. Bleeding manifestations and management of children with persistent and chronic immune thrombocytopenia: Data from the Intercontinental Cooperative ITP Study Group (ICIS). Blood 2013; 121: 4457–4462. [DOI] [PubMed] [Google Scholar]
- 18. Cuker A, Coles AJ, Sullivan H, et al. A distinctive form of immune thrombocytopenia in a phase 2 study of alemtuzumab for the treatment of relapsing-remitting multiple sclerosis. Blood 2011; 118: 6299–6305. [DOI] [PubMed] [Google Scholar]
- 19. Genzyme Therapeutics Ltd. LEMTRADA™ (alemtuzumab 12 mg concentrate for solution for infusion). EU summary of product characteristics, http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/003718/WC500150521.pdf (2018, accessed 12 February 2018).
- 20. Sanofi Genzyme. LEMTRADA REMS (Risk Evaluation and Mitigation Strategy) Program, https://www.lemtradarems.com (2017, accessed 28 September 2017).
- 21. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab more effective than interferon beta-1a at 5-year follow-up of CAMMS223 clinical trial. Neurology 2012; 78: 1069–1078. [DOI] [PubMed] [Google Scholar]
- 22. Rodeghiero F, Stasi R, Gernsheimer T, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood 2009; 113: 2386–2393. [DOI] [PubMed] [Google Scholar]
- 23. Singer BA, Alroughani R, Brassat D, et al. Durable clinical outcomes with alemtuzumab in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS II patients (TOPAZ study). Neurology 2018; 90(Suppl. 15): P3.369. [Google Scholar]
- 24. Coles AJ, Fox E, Vladic A, et al. Alemtuzumab versus interferon beta-1a in early relapsing-remitting multiple sclerosis: Post-hoc and subset analyses of clinical efficacy outcomes. Lancet Neurol 2011; 10: 338–348. [DOI] [PubMed] [Google Scholar]
- 25. Tuohy O, Costelloe L, Hill-Cawthorne G, et al. Alemtuzumab treatment of multiple sclerosis: Long-term safety and efficacy. J Neurol Neurosurg Psychiatry 2015; 86: 208–215. [DOI] [PubMed] [Google Scholar]
- 26. Jones JL, Thompson SA, Loh P, et al. Human autoimmunity after lymphocyte depletion is caused by homeostatic T-cell proliferation. Proc Natl Acad Sci U S A 2013; 110: 20200–20205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. von Kutzleben S, Pryce G, Giovannoni G, et al. Depletion of CD52-positive cells inhibits the development of central nervous system autoimmune disease, but deletes an immune-tolerance promoting CD8 T-cell population. Implications for secondary autoimmunity of alemtuzumab in multiple sclerosis. Immunology 2017; 150: 444–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jung CW, Cho SH, Park S, et al. Successful treatment of steroid-refractory immune thrombocytopenia with alemtuzumab. Blood Res 2016; 51: 297–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Derlet A, Agius M, Apperson M. Symptomatic thrombocytopenia after one dose of alemtuzumab with successful rechallenge. Neurology 2017; 88:P5.400. [Google Scholar]
- 30. Ranganathan U, Kaunzner U, Foster S, et al. Immediate transient thrombocytopenia at the time of alemtuzumab infusion in multiple sclerosis. Mult Scler 2017; 24: 540–542. [DOI] [PubMed] [Google Scholar]
- 31. Thomas K, Eisele J, Rodriguez-Leal FA, et al. Acute effects of alemtuzumab infusion in patients with active relapsing-remitting MS. Neurol Neuroimmunol Neuroinflamm 2016; 3: e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cines DB, Bussel JB. How I treat idiopathic thrombocytopenic purpura (ITP). Blood 2005; 106: 2244–2251. [DOI] [PubMed] [Google Scholar]
- 33. Provan D, Stasi R, Newland AC, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. Blood 2010; 115: 168–186. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, MSJ816612_supplementary_info for Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management by Adam Cuker, Ann D Bass, Congor Nadj, Mark A Agius, Brian Steingo, Krzysztof W Selmaj, Timothy Thoits, Alexandre Guerreiro, Bart Van Wijmeersch, Tjalf Ziemssen, Sven G Meuth, Christopher C LaGanke, Karthinathan Thangavelu, Claudio E Rodriguez, Darren P Baker, David H Margolin and Ann Jannsens in Multiple Sclerosis Journal