Abstract
Primary congenital glaucoma (PCG) is a rare disease affecting children early in life. PCG was considered untreatable with inevitable blindness. However, recent advances in biochemical and genetic studies, the introduction of new diagnostic tools, intraocular pressure (IOP) lowering medications and improvement of surgical techniques have led to a better understanding of this devastating disease and preserving the vision of affected children. This paper presents an updated and broad overview of PCG in terms of the epidemiology and genetic aspects, particularly in Saudi Arabia, the clinical presentation and diagnostic approach to PCG with major emphasis on the treatment options.
Keywords: Congenital, Glaucoma, Cornea, Genetics, Goniotomy, Trabeculotomy, Trabeculectomy
Introduction and historical background
Congenital glaucoma is a developmental glaucoma occurring before the age of three years due to an obstruction that prevents adequate drainage of aqueous humor caused by abnormal development of the trabecular meshwork (TM) and anterior chamber angle.1 This arbitrary age has been estimated since it corresponds to the age at which the eye grows in response to high intraocular pressure IOP.1 Children with congenital glaucoma typically present with globe enlargement (buphthalmos), edema and opacification of the cornea with rupture of Descemet's membrane (Haab’s striae). Additional clinical features include thinning of the anterior sclera and iris atrophy, anomalously deep anterior chamber and structurally normal posterior segment except for progressive glaucomatous optic atrophy.2 Moreover, visual acuity may be reduced and/or visual fields may be restricted. In untreated or treated late cases, blindness occurs invariably.1, 2.
Primary congenital glaucoma (PCG) was first described by Hippocrates (460–377 BC) when he noticed abnormal enlargement of the eyes in infants but did not relate the condition to elevated IOP. PCG was not well understood until the early 18th century when Berger first linked the elevated IOP to enlargement of the globe. In 1869, von Muralt established the classical form of buphthalmos as a form of glaucoma. In the late 1800s, the Manual of Human and Comparative Histology published in 1873 described the angle drainage system, including the anatomical structure of Schlemm’s canal and Descemet’s membrane, referring to them as an anterior lymphatic drainage system.3 Thereafter, the Atlas of the Pathological Anatomy of the Eyeball translated to English from German by William Gowers in 1875, established that angle structure malformation as the culprit for PCG.4 In the early 1900s, congenital glaucoma seemed untreatable and Anderson commented: “The future of patients with hydrophthalmia is dark” and “one seeks in vain for a best operation in the treatment of hydrophthalmia”.5. However, the introduction of goniotomy in 1938 by Barkan has dramatically changed the poor prognosis of congenital glaucoma.6 In addition, the introduction of new diagnostic tools, IOP-lowering medications, and improvement of surgical techniques has improved the prognosis of this devastating disease and preserved the vision of affected children. The present article aims to provide an updated overview of PCG.
Terminology and classification
Buphthalmos and hydrophthalmos are merely descriptive terms and do not imply etiology or appropriate therapy, hence it should not be used diagnostically. According to the World Glaucoma Association, childhood glaucoma is also classified as primary and secondary. Congenital and infantile glaucoma, together with juvenile open-angle glaucoma, constitute primary childhood glaucoma. PCG can be further subcategorized by its age of onset. PCG with onset at birth to less than 1 month old is referred to as the neonatal/ newborn onset primary glaucoma. Late-onset PCG is defined as PCG with its onset after 2 years of age. Children will be called to have infantile-onset PCG if the age of onset is between neonatal/newborn and late-onset glaucoma (i.e. between 1–24 months old).7 Hoskins and Shaffer classification system used a more anatomical approach to classification based on the area of dysgenesis. PCG refers to glaucoma due to isolated trabeculodysgenesis. The angle is maldeveloped, with an absence of angle recess and iris often inserted directly onto the trabecular meshwork.8
A simple and practical classification has been developed based on the mechanisms of anterior segment formation and their abnormalities which led to different clinical forms of congenital glaucoma.9 Trabeculodysgenesis is an isolated anomaly of the irido-corneal angle leading to isolated congenital glaucoma. The only anatomical anomaly, visible in gonioscopy, is Barkan's “pseudo-membrane” with an anterior insertion of the iris masking the trabeculum. Irido- trabeculodysgenesis refers to an anomaly of the angle and iris, often accompanied by glaucoma.
Epidemiology and genetic aspects
PCG is a rare disease with variable incidence across countries and ethnic groups. The incidence of PCG in western countries, such as Ireland, Britain, and the USA, lies within 1 per 10–20,000 live births.10, 11, 12, 13, 14 However, the incidence of PCG is higher in the Middle East, including Saudi Arabia, where consanguineous marriages are more prevalent. The estimated incidence of PCG in Saudi Arabia is 1 per 2500 live births.15, 16 According to the congenital glaucoma registry at King Khaled Eye Specialist Hospital, the Southern region of Saudi Arabia has the highest prevalence rate of PCG (27.8%), followed by the Western province (23.6%) and the Central region (22.2%). However, the lowest prevalence was recorded in the Eastern province (11.1%) and the Northern province (9%).17
The relationship between consanguinity and a higher incidence of PCG is further supported by the significantly higher rate of consanguinity in the parents of children with PCG than that of the parents of secondary congenital glaucoma patients.18 In races with a higher incidence, the presentation of PCG occurs at an earlier age compared to other races with lower incidence. The mean age of presentation ranges from 3 to 4 months among Asians, Saudi Arabians, and Indians to 11 months in Western countries.17, 19 The incidence of PCG showed a slight male predominance in both Western and Asian countries, accounting for 65% of the cases.17, 19
The initial step in the establishment of the pathophysiology of PCG is to understand the link between PCG and gene abnormalities. PCG caused by CYP1B1 or LTBP2 pathogenic variants is inherited in an autosomal recessive manner.20 Loci of recessively inherited PCG (gene GLC3) have been identified by genetic linkage analysis.21, 22 Two other loci, GLC3B on 1p36 and GLC3C on 14q24.3, have also been linked to PCG. However, the related genes and pathogenic variants are not known. The majority of congenital glaucoma cases map to GLC3A locus on chromosome 2 (2p21). Families linked to these loci display severe phenotypes with an autosomal recessive inheritance pattern. Some types of juvenile-onset glaucoma with autosomal dominant inheritance patterns have been mapped to chromosome 1q23-q25 (TIGR/MYOC gene).22
Mutations in the CYP1B1 gene (encoding cytochrome P450 enzyme 1B1) in the GLC3A locus are associated with the PCG phenotype. There are more than 70 distinct mutations in the CYP1B1 gene that have been described in PCG; indicating the genetic heterogeneity of the condition. CYP1B1 gene mutation is the predominant genetic pattern of PCG in the Middle East (Turkey and Saudi Arabia). It has been reported that 87% of familial and 27% of sporadic cases are due to mutations in this gene.23 Generally, the probability of identifying biallelic pathogenic variants in CYP1B1 increases with the presence of bilateral and severe disease, a positive family history for the disease and parental consanguinity.
CYB1B1 and MYOC mutations were also detected in early-onset glaucoma in humans, whereas mutations in the CYP1B1 and FOXC1 were identified in mice with early-onset glaucoma.24, 25 Because angle structures are mainly derived from neural crest cells, it is possible that defects in genes expressed in neural crest cells could also contribute to PCG.
In Saudi Arabia, several studies were conducted to identify the abnormal genes associated with PCG.26, 27, 28 So far, CYP1B1 was the only gene found to be associated with bilateral PCG in consanguineous Saudi Arabian families. CYP1B1 mutations were identified in 96% of cases in one study reported by Bejjani et al.26 and in 75.9% of cases of the study described by Abu-Amero et al.27 A recent study conducted at King Abdulaziz University Hospital in Jeddah city, which included 23 PCG Saudi patients, has confirmed that CYP1B1 mutations are the most frequent cause of PCG in the Saudi Arabian population. The study showed that 21 of the studied patients (91%) were positive for CYP1B1 mutations, and 2 (9%) were negative, with p.Gly61Glu being the major disease-associated allele.28 Genetic studies may help in family counseling as well as in serving as a baseline for the future development of gene therapy.
Clinical and diagnostic approach
The diagnosis of PCG can be relatively easy when the child presents with the classical features. However, the diagnosis can be difficult when the clinical features of glaucoma are not obviously manifested especially if the glaucoma is bilateral and the parents lend less attention due to the lack of marked asymmetry between both eyes in the early stages of PCG.
Nevertheless, a rapid diagnosis is essential to start providing urgent care and preserve the final functional outcome. Congenital glaucoma can be uni- or bilateral, symmetrical or asymmetrical. The condition is generally discovered at birth or in the first months of life. The classic triad of epiphora, photophobia, and blepharospasm is the most common presentation of PCG. Moreover, a history of congenital glaucoma among the child’s siblings plays a crucial role in the diagnosis. Characteristic signs of PCG include corneal opacity, increased corneal diameter (megalocornea), buphthalmos, elevated IOP and optic nerve head changes (Fig. 1, Fig. 2, Fig. 3, Fig. 4, Fig. 5). In cases where PCG is suspected, it is mandatory to perform an emergency ophthalmic examination under sedation or general anesthesia to measure the IOP and corneal diameter.29, 30
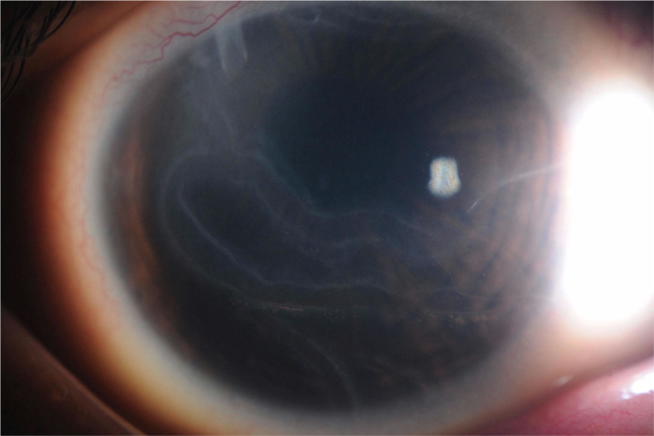
Fig. 1.
External photo of Haab's striae which represent a break in the Descemet layer.
Fig. 2.
Gonioscopy photo showing anterior insertion of the iris masking the trabecular meshwork as seen in congenital glaucoma.
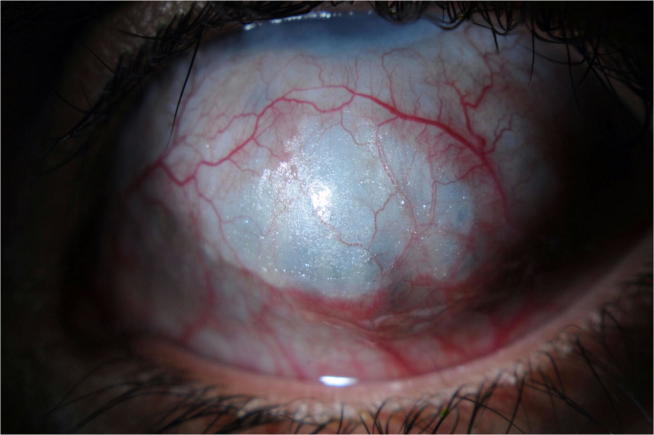
Fig. 3.
External photo showing anterior scleral thinning and staphyloma.
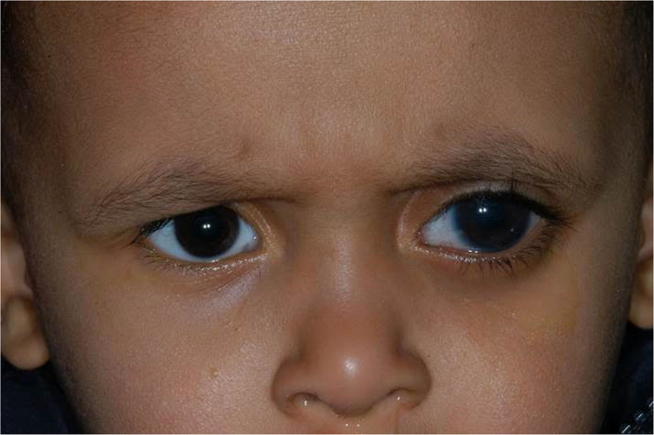
Fig. 4.
External photo showing buphthalmos of the left eye.
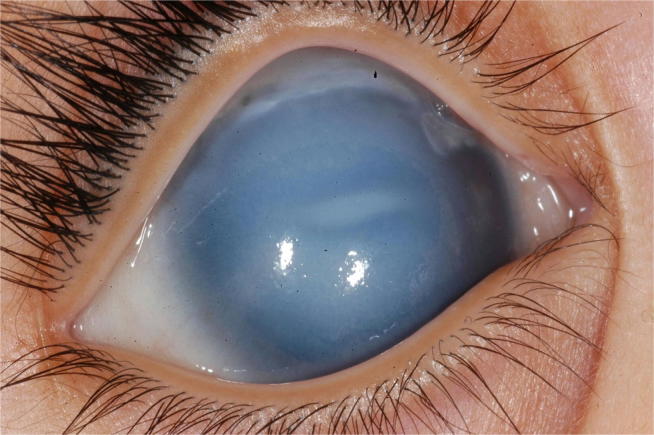
Fig. 5.
External photo showing corneal enlargement and edema in a neonate with primary congenital glaucoma.
In PCG, the corneal diameter, which normally does not exceed 10 mm at birth, is increased considerably. The transparency of the cornea is altered by stromal edema, which can worsen very quickly; explaining the urgency of management. Horizontal lines of Descemet membrane rupture (Haab’s striae), (Fig. 1) are often present and may persist even after pressure reduction.30 These horizontal lines are classically distinguished from those observed after corneal trauma (by forceps delivery in particular) that appear as vertical splits on slit-lamp biomicroscopy. When the corneal transparency permits, the examination of the angle shows Barkan's “pseudo-membrane” and an anterior insertion of the iris masking the trabeculum over all or part of the periphery (Fig. 2). PCG is also manifested by thinning of the anterior sclera (Fig. 3) and iris atrophy, anomalously deep anterior chamber, and structurally normal posterior segment except for progressive glaucomatous optic atrophy.31 The papilla, when it is analyzable, is more excavated than normal. It is important to know that the physiological papillary excavation in infants is less than that of adults, usually less than 0.3.32 In contrast to adult glaucoma and due to the elasticity of the scleral canal, papillary excavation is reversible in patients with congenital glaucoma.32, 33, 34
Given that the standard Goldmann applanation tonometer cannot be used in children under general anesthesia placed in the supine position, the Perkins tonometer, a hand-held variant of Goldmann’s tonometer, is preferred. IOP readings measured with other instruments should be interpreted with caution, for example, it has been shown that Tono-pen measurements tend to overestimate IOP in PCG patients with a recording above 16 mmHg.35 An IOP greater than 21 mm Hg in one or both eyes on at least two occasions is considered abnormally elevated. In general, normal eye pressures in children are 12.02 ± 3.74 mm Hg.36 However, several factors must be considered with regard to measuring the IOP in neonates and infants. First, physiological IOP in children is lower than adults and increases with age (it is possible to establish a curve analogous to that used for size).36, 37 For example, physiological pressure recordings soon after birth, at 6 months, and at 1 year are 5 mmHg, 7 mmHg, and 8 mmHg respectively. Secondly, general anesthesia decreases IOP by about 30% and the measurement only balances after a few minutes of intubation (less if a laryngeal mask is used).38
The visual function in children with congenital glaucoma can be impaired by a variety of mechanisms: severe myopia due to globe expansion, corneal disorders, irregular astigmatism due to Descemet membrane breaks and optical nerve fiber damage.39
In some cases, the cornea remains transparent despite buphthalmos (Fig. 4) and the presence of corneal edema (Fig. 5). In these cases, fundus examination and optic nerve head evaluation can establish the diagnosis since any excavation more than 0.1 Cup/Disc ratio in children less than one-year-old is abnormal.38
In addition to the above-mentioned examination findings, ultrasound examination is useful in PCG. Ultrasound A is used to measure the axial length of the eye which typically increases in cases of PCG. At birth, the axial length of the eye does not exceed 18 mm and it gradually increases to the adult size of 22 mm around the age of 2 years. Moreover, in cases of total corneal opacity, the anterior chamber can still be examined but with a limited visualization to the posterior segment. In such conditions, the use of ultrasound B is helpful to rule out any posterior segment pathology.38, 39
The discovery of genes associated with the occurrence of PCG in humans increasingly allowed the use of molecular diagnosis within families segregating such genes. The molecular diagnosis applied to glaucoma can aid in the prevention, treatment, and prognosis of children with PCG.40 Molecular diagnosis allows the identification of children at a high risk of developing glaucoma even before pathological processes have been able to initiate an irreversible loss of visual field. Since glaucoma is an insidious disease, molecular screening is becoming a preferred prevention tool in asymptomatic individuals with a high risk. Thus, close ophthalmologic monitoring can focus on family members identified as carriers of glaucoma-associated mutations which will save the social resources and costs associated with close follow-up of all members of a glaucomatous family.
The use of an adequate molecular screening program within a population requires the identification of the genes involved in the targeted disease. When the genes are identified, it is necessary to determine which gene variations are actually responsible for the disease as compared to benign polymorphisms encountered in the general population. Once these mutations are identified, it is crucial to estimate their distribution among the affected population and establish the genotype/phenotype correlations of each of these mutations. Based on the above-mentioned measurements, an adequate molecular screening service can be offered to individuals at risk of PCG.23, 41, 42
Treatment of PCG
Surgical treatment is the mainstay of management in cases of PCG, however, medical therapy has a significant role in different clinical scenarios such as: to lower the IOP initially as the surgical intervention is arranged, in cases of qualified surgical success, and in between surgeries in cases requiring repeat interventions to allow some time for visual maturation. In general, the purpose of treatment is to lower the elevated IOP to a level that preserves visual function without causing extensive damage to the optic nerve and/or the retina. In cases where the eye still has good potential for visual recovery, it is very important to prevent further visual deterioration. On the other hand, unfortunately, in cases of late presentation a significant amount of irreversible damage had already occurred and treatment in such instances aims to preserve the current visual status and prevent the fellow eye from subsequent deterioration.
Medical treatment
IOP-lowering medications work either by decreasing aqueous humor secretion or increasing its elimination. They are, in almost all cases, used topically in the form of eye drops. Medications that work by decreasing aqueous humor secretion include alpha-agonists, beta-blockers, and carbonic anhydrase inhibitors, whereas medications that work by increasing elimination of aqueous humor include adrenaline derivatives, parasympathomimetics, and prostaglandin analogues. All of the topical medications are roughly equivalent in terms of efficacy but some studies encourage the use of beta-blockers and carbonic anhydrase inhibitors as both of these medications have limited side effects.43, 50
The side effects of these medications should be kept in mind, due to possible significant systemic consequences, nevertheless most of these adverse events can be avoided by knowing the patient’s history, adjusting the medication dosage, and adequately following the patient to identify the onset of new symptoms or signs that may develop.43, 44
The most common complication caused by beta-blockers is respiratory distress which can be minimized by choosing a 0.25% dosage instead of the routinely used 0.5% and avoiding their usage in patients with bronchial asthma. Another important consideration is to choose the short-acting alpha-agonist, apraclonidine, instead of brimonidine to decrease the risk of respiratory depression.44 Carbonic anhydrase inhibitors should only be given after ruling out sickle cell disease and renal impairment as they might result in acute occlusive systemic episodes and renal dysfunction respectively.50
Novel drug delivery systems, such as the bimatoprost sustained-release implant, are now being tested in clinical trials for the management of adult glaucomas. This approach can serve as an alternative to traditional topical therapy in children in the future.44
Surgical treatment
Early surgical intervention is of prime importance in the management of patients with PCG. The primary goal of all surgical procedures is to eliminate the resistance to aqueous outflow caused by the anatomical anomaly in the anterior chamber angle.43 To accomplish this goal, resistance is either eliminated via an internal approach (goniotomy) or an external approach (trabeculotomy or trabeculectomy). All these procedures are based on the work of Barkan6, 45 deLuise and Anderson,46 Ho and Walton,47 Bowman et al.48 and Sharaawy & Bhartiya.49
The choice of surgical procedure depends primarily upon the corneal status. In countries where patients present with mild or moderate corneal edema, goniotomy is usually associated with a high success rate. In countries, such as the Middle East, where nearly all patients present with corneal clouding, goniotomy is technically impossible. In these instances, an external approach is utilized.53, 54 Glaucoma drainage implants and cyclodestructive procedures are usually reserved for later in the course of management after failure of the primary surgical procedure.
Goniotomy, introduced by Barkan in 1938,6 consists of an incision of the trabeculum (using a 30 Gauge needle) under direct visualization with a gonioscopic lens. An adequate visualization of the anterior chamber angle is essential, hence, the cornea must be sufficiently clear. The main complication of this procedure is the appearance of a hyphema that can elevate the postoperative IOP.44, 49 The success rate after goniotomy varies in different reports from 50% to 90% depending on the number of goniotomies required, the initial severity of the disease and the presence of corneal cloudiness at presentation.50 With proper case selection, the outcomes of goniotomy are satisfactory. It has been reported that one goniotomy was sufficient to reach a normal IOP in 72% of PCG patients and the success rate after 2 goniotomies increases to 94%.51, 52 The main predictors of success are the timing of diagnosis, where the higher success rate was observed among those who present between 1 and 24 months after birth, and a lower degree of refractive error.50, 51, 52
In patients with a cloudy cornea, however, the use of goniotomy is difficult as corneal cloudiness prevents direct visualization of the anterior chamber angle.60 In such cases, endoscopic goniotomy allows direct visualization of angle structure and allows angle opening to 300° with a success rate of up to 50%.54, 55, 56
Trabeculotomy, on the other hand, can be performed even in the presence of a cloudy cornea. It was first described in 1960 by Smith.57 Under the protection of a conjunctival flap and a scleral flap, the procedure starts by inserting a probe into the Schlemm canal, then by rotating the probe towards the anterior chamber collapsing the trabeculum and making it possible to re-establish communication between the anterior chamber and the Schlemm canal. The repetition of the gesture to the right and to the left of the opening of the Schlemm canal at 12o'clock makes it possible to open 120–180° of trabeculum.57 The data from a Chinese retrospective study reported that lowering the IOP to less than or equal to 21 mm Hg after trabeculotomy was achieved in 91% and 87% among children aged one and three years, respectively.58 These results were comparable to the data from Western countries in the 1980s which reported a 75–90% reduction of IOP after trabeculotomy.59, 60 However, hyphema is frequent and there is a risk of hypotony, cyclodialysis, or detachment of the Descemet membrane.61
The 360° trabeculotomy is one modification of the surgery which permits the entire angle to be opened in a single session instead of opening approximately one-third of the chamber angle. This could be achieved by threading a 6–0 prolene suture or a lighted canaloplasty catheter through the Schlemm’s canal. Mendicino et al. have reported that 92% of PCG patients that underwent 360° trabeculotomy had an IOP level that is below 22 mm Hg with a single procedure compared to only 57.5% of patients undergoing goniotomy.62
Another modification to traditional trabeculotomy is the utilization of a modified probe tailored to the Schlemm’s canal curvature. The underlying principle is that patients with varying corneal diameter would have accordingly varying canal curvature. In a retrospective study, Filous et al.63 utilized one of three different probes, classifying patients based on their corneal diameters. The authors reported a mean decrease of 47% in IOP with a surgical success in 87% of the eyes.63, 78 A literature review shows a shortage of prospective data comparing traditional trabeculotomy with these modified trabeculotomy procedures.
Trabeculectomy was described for the first time by Cairns in 1968.64 It has been proposed since the 1980s as a first intervention by some authors in the treatment of PCG with a long-term success rate varying from 54% to 90% in PCG patients.65, 66 The risk of postoperative complications of trabeculectomy was comparable to other techniques.66, 67
Deep sclerectomy was popularized in the early 1990s for the treatment of adult open-angle glaucoma68, 69 with the advantage of offering a success rate that is similar to that of trabeculectomy with a lower risk of complications, that is why some surgeons in current practice prefer to attempt deep sclerectomy over trabeculectomy.70 With regards to PCG, some may doubt the utility of deep sclerectomy in congenital glaucoma given that the obstruction to aqueous humor flow is classically internal, hence, it is not targeted by performing a deep sclerectomy, however, there is a significant amount of evidence supporting its success in PCG.71, 72
The use of anti-metabolites, such as mitomycin C (MMC), in PCG patients has been reported in some studies to improve success rates of surgery, quoted as 52–82%.73, 74 The use of MMC is preferred in refractory cases, aphakic eyes, and older children.75, 76, 79 Although some are doubtful about the application of MMC in children, evidence suggests that its use is safe and postoperative complications encountered are similar to those seen in adults.76, 77 These complications include prolonged hypotony, scleral and conjunctival fragility and endophthalmitis.74, 75, 76, 77
Glaucoma drainage devices (GDD), cyclocryotherapy or cyclodestruction may be used to control IOP when initial surgical procedures have failed. The data of using GDD have revealed a 28% to 49% reduction in the mean IOP and a success rate ranging from 63% to 97% after one year from surgery.80, 82, 83, 84 However, tube-migration with its related complications can be encountered and these were reported at a higher frequency among Hispanic ethnicity and female patients.81
The traditional cyclocryotherapy was associated with a very low success rate of around 30%, postoperative inflammation and phthisical changes.82 The use of cyclophotocoagulation was also found to be associated with severe complications, including hypotony, progression of vision loss, retinal detachment, and vitreous hemorrhage.83, 84 Therefore, this modality is usually reserved for cases with poor potential after failure of multiple surgeries. Finally, micropulse trans-scleral diode laser was recently introduced as a viable alternative cyclodestructive procedure that has a higher safety profile with satisfactory efficacy.85, 86, 87 However, its efficacy is often transient and several sessions may be necessary.85, 86, 87
Conclusion
Early and accurate diagnosis of PCG is vital, so that appropriate management can be initiated before irreversible damage to the optic nerve takes place. The hereditary and genetic characteristics of the disease allow the conduction of genetic counseling based on the risk of recurrence and, in targeted situations, a molecular diagnosis, possibly antenatal. Due to the low incidence of PCG, studies in the literature are usually retrospective, nonrandomized, and have limited sample sizes. Hence, ophthalmologists should be aware of the limitations of these types of studies and interpret the results with caution. An optimal management plan should be individualized in each patient based on his clinical presentation. Finally, there is a need to conduct well-designed large multi-center randomized clinical trials to fill the gaps in the literature regarding the efficacy, success rates, and postoperative complications of different surgical procedures of PCG.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Allingham R.R., Damji K., Freeman S., Moroi S., Shafranov G. Congenital glaucomas and developmental glaucomas with associated anomalies. In: Allingham R.R., Damji K.F., Freedman S., Moroi A.E., Rhee D.J., editors. Shields Textbook of Glaucoma. 5 ed. Lippincott Williams & Wilkins; Philadelphia, PA: 2005. pp. 235–271. [Google Scholar]
- 2.Francois J. Congenital glaucoma and its inheritance. Ophthalmologica. 1972;181:61–73. doi: 10.1159/000309028. [DOI] [PubMed] [Google Scholar]
- 3.Stricker S. The New Sydenham Society; London: 1873. Manual, of human and comparative histology; p. 562. [Google Scholar]
- 4.Pagenstecher HE, Genth C. Atlas der pathologischen Anatomie des Augenapfels: Atlas of the pathological anatomy of the eyeball. Wiesburg 58 bl.,38 pl. (kobberstikk) ill; 1875.
- 5.Anderson J.R. Cambridge University Press; London: 1939. Hydrophthalmia or congenital glaucoma. [Google Scholar]
- 6.Barkan O. Technique of goniotomy. Arch Ophthalmol. 1938;19:217–221. [Google Scholar]
- 7.Weinreb R.G., Papadopoulos M., Grigg J. Kugler Publications; Amsterdam, The Netherlands: 2013. Childhood glaucoma. World glaucoma association consensus series 9. [Google Scholar]
- 8.Hoskins H.D., Jr., Shaffer R.N., Hetherington J. Anatomical classification of the developmental glaucomas. Arch Ophthalmol. 1984;102:1331–1336. doi: 10.1001/archopht.1984.01040031081030. [DOI] [PubMed] [Google Scholar]
- 9.Waring G.O., 3rd, Rodrigues M.M., Laibson P.R. Anterior chamber cleavage syndrome. A stepladder classification. Surv Ophthalmol. 1975;20:3–27. doi: 10.1016/0039-6257(75)90034-x. [DOI] [PubMed] [Google Scholar]
- 10.Gencik A. Epidemiology and genetics of primary congenital glaucoma in Slovakia: description of a form of primary congenital glaucoma in gypsies with autosomal recessive inheritance and complete penetrance. Dev Ophthalmol. 1989;16:76–115. [PubMed] [Google Scholar]
- 11.Gencik A., Gencikova A., Ferák V. Population genetical aspects of primary congenital glaucoma: I. Incidence, prevalence, gene frequency, and age of onset. Hum Genet. 1982;61:193–197. doi: 10.1007/BF00296440. [DOI] [PubMed] [Google Scholar]
- 12.MacKinnon J.R., Giubilato A., Elder J.E., Craig J.E., Mackey D.A. Primary infantile glaucoma in an Australian population. Clin Experiment Ophthalmol. 2004;32(1):14–18. doi: 10.1046/j.1442-9071.2004.00750.x. [DOI] [PubMed] [Google Scholar]
- 13.Sarfarazi M., Stoilov I., Schenkman J.B. Genetics and biochemistry of primary congenital glaucoma. Ophthalmol Clin North Am. 2003;16(4):543–554. doi: 10.1016/s0896-1549(03)00062-2. [DOI] [PubMed] [Google Scholar]
- 14.Tamcelik N., Atalay E., Bolukbasi S., Capar O., Ozkok A. Demographic features of subjects with congenital glaucoma. Indian J Ophthalmol. 2014;62(5):565–569. doi: 10.4103/0301-4738.126988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jaffar M.S. Raven Press; New York, NY: 1988. Care of the Infantile Glaucoma Patient. Ophthalmology Annual; pp. 15–37. [Google Scholar]
- 16.Al-Rajhi A., Awad A., Badeeb O. Causes of blindness in students attending school for the blind in Saudi Arabia. Saudi J Ophthalmol. 2003;17:276–280. [Google Scholar]
- 17.Alanazi F.F., Song J.C., Mousa A. Primary and secondary congenital glaucoma: baseline features from a registry at King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia. Am J Ophthalmol. 2013;155:882–889. doi: 10.1016/j.ajo.2012.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Liu B., Huang W., He M., Zheng Y. An investigation on the causes of blindness and low vision of students in blind school in Guangzhou. Yan Ke Xue Bao. 2007;23(2):117–120. [PubMed] [Google Scholar]
- 19.Fung D.S., Roensch M.A., Kooner K.S., Cavanagh H.D., Whitson J.T. Epidemiology and characteristics of childhood glaucoma: results from the Dallas Glaucoma Registry. Clin Ophthalmol. 2013;7:1739–1746. doi: 10.2147/OPTH.S45480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fan B.J., Wiggs J.L. Glaucoma: genes, phenotypes, and new directions for therapy. J Clin Invest. 2010;120:3064–3072. doi: 10.1172/JCI43085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sarfarazi M., Akarsu A.N., Hossain A. Assignment of a locus (GLC3A) for primary congenital glaucoma (buphthalmos) to 2p21 and evidence for genetic heterogeneity. Genomics. 1995;30:171–177. doi: 10.1006/geno.1995.9888. [DOI] [PubMed] [Google Scholar]
- 22.Akarsu A.N., Turacli M.E., Aktan S.G. A second locus (GLC3B) for primary congenital glaucoma (buphthalmos) maps to the 1p36 region. Hum Mol Genet. 1996;5:1199–1203. doi: 10.1093/hmg/5.8.1199. [DOI] [PubMed] [Google Scholar]
- 23.Sarfarazi M., Stoilov I. Molecular genetics of primary congenital glaucoma. Eye. 2000;14:422–428. doi: 10.1038/eye.2000.126. [DOI] [PubMed] [Google Scholar]
- 24.Vincent A.L., Billingsley G., Buys Y. Digenic inheritance of early-onset glaucoma: CYP1B1, a potential modifier gene. Am J Hum Genet. 2002;70:448–460. doi: 10.1086/338709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Libby R.T., Smith R.S., Savinova O.V. Modification of ocular defects in mouse developmental glaucoma models by tyrosinase. Science. 2003;299:578–581. doi: 10.1126/science.1080095. [DOI] [PubMed] [Google Scholar]
- 26.Bejjani B.A., Stockton D.W., Lewis R.A. Multiple CYP1B1 mutations and incomplete penetrance in an inbred population segregating primary congenital glaucoma suggest frequent de novo events and a dominant modifier locus. Hum Mol Genet. 2000;9:367–374. doi: 10.1093/hmg/9.3.367. [DOI] [PubMed] [Google Scholar]
- 27.Abu-Amero K.K., Osman E.A., Mousa A. Screening of CYP1B1 and LTBP2 genes in Saudi families with primary congenital glaucoma: genotype-phenotype correlation. Mol Vis. 2011;17:2911–2919. [PMC free article] [PubMed] [Google Scholar]
- 28.Badeeb O.M., Micheal Sh., Koenekoop R.K., den Hollander A.I., Hedrawi M.T. CYP1B1 mutations in patients with primary congenital glaucoma from Saudi Arabia. BMC Medical Genet. 2014;15:109. doi: 10.1186/s12881-014-0109-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dietlein T.S., Jacobi P.C., Krieglstein G.K. Assessment of diagnostic criteria in management of infantile glaucoma. An analysis of tonometry, optic disc cup, corneal diameter and axial length. Int Ophthalmol. 1996;20:21–27. doi: 10.1007/BF00212940. [DOI] [PubMed] [Google Scholar]
- 30.Beck A.D. Diagnosis and management of pediatric glaucoma. Ophthalmol Clin North Am. 2001;14:501–512. doi: 10.1016/s0896-1549(05)70248-0. [DOI] [PubMed] [Google Scholar]
- 31.Wenzel M., Krippendorff U., Hunold W., Reim M. Corneal endothelial damage in congenital and juvenile glaucoma. Klin Monatsbl Augenheilkd. 1989;195:344–348. doi: 10.1055/s-2008-1050052. [DOI] [PubMed] [Google Scholar]
- 32.Erkkila H., Laatikainen L. Characteristics of optic disc in healthy school children. Acta Ophthalmol (Copenh) 1979;57:914–921. doi: 10.1111/j.1755-3768.1979.tb01858.x. [DOI] [PubMed] [Google Scholar]
- 33.Kessing S.V., Gregersen E. The distended disc in early stages of congenital glaucoma. Acta Ophthalmol (Copenh) 1977;55:431–435. doi: 10.1111/j.1755-3768.1977.tb06119.x. [DOI] [PubMed] [Google Scholar]
- 34.Quigley H.A. The pathogenesis of reversible cupping in congenital glaucoma. Am J Ophthalmol. 1977;84:358–370. doi: 10.1016/0002-9394(77)90680-8. [DOI] [PubMed] [Google Scholar]
- 35.Levy J., Lifshitz T., Rosen S., Tessler Z., Biedner B.Z. Is the tono-pen accurate for measuring intraocular pressure in young children with congenital glaucoma? J AAPOS. 2005 Aug;9(4):321–325. doi: 10.1016/j.jaapos.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 36.Sihota R., Tuli D., Dada T., Gupta V., Sachdeva M.M. Distribution and determinants of intraocular pressure in a normal pediatric population. J Pediatr Ophthalmol Strabismus. 2006;43:14–18. doi: 10.3928/01913913-20060101-01. [DOI] [PubMed] [Google Scholar]
- 37.Pensiero S., Da Pozzo S., Perissutti P., Cavallini G.M., Guerra R. Normal intraocular pressure in children. J Pediatr Ophthalmol Strabismus. 1992;29:79–84. doi: 10.3928/0191-3913-19920301-05. [DOI] [PubMed] [Google Scholar]
- 38.Ausinsch B., Munson E.S., Levy N.S. Intraocular pressures in children with glaucoma during halothane anesthesia. Ann Ophthalmol. 1977;9:1391–1394. [PubMed] [Google Scholar]
- 39.Morin J.D., Bryars J.H. Causes of loss of vision in congenital glaucoma. Arch Ophthalmol. 1980;98:1575–1576. doi: 10.1001/archopht.1980.01020040427005. [DOI] [PubMed] [Google Scholar]
- 40.Kaur K., Mandal A.K., Chakrabari S. Primary congenital glaucoma and the involvement of CYP1B1. MEAJO. 2011;18:7–16. doi: 10.4103/0974-9233.75878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panicker S.G., Mandal A.K., Reddy A.B., Gothwal V.K., Hasnain S.E. Correlations of genotype with phenotype in Indian patients with primary congenital glaucoma. Invest Ophthalmol Vis Sci. 2004;45:1149–1156. doi: 10.1167/iovs.03-0404. [DOI] [PubMed] [Google Scholar]
- 42.Faiq M., Sharma R., Dada R., Mohanty K., Saluja D., Dada T. Genetic, Biochemical and clinical insights into primary congenital glaucoma. J Current Glau Prac. 2013;7(2):66–84. doi: 10.5005/jp-journals-10008-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gedde S.J., Schiffman J.C., Feuer W.J. Treatment outcomes in the tube versus trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153 doi: 10.1016/j.ajo.2011.10.026. 789e2-803e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lewis R.A. Bimatoprost sustained-release implants for glaucoma therapy: 6-month results from a phase I/II clinical trial. Am J Ophthalmol. 2017 Mar;175:137–147. doi: 10.1016/j.ajo.2016.11.020. [DOI] [PubMed] [Google Scholar]
- 45.Barkan O. Surgery of congenital glaucoma. Review of 196 eyes operated by goniotomy. Am J Ophthalmol. 1953;36:1523–1534. doi: 10.1016/0002-9394(53)91780-2. [DOI] [PubMed] [Google Scholar]
- 46.deLuise V.P., Anderson D.R. Primary infantile glaucoma (congenital glaucoma) Surv Ophthalmol. 1983;28:1–19. doi: 10.1016/0039-6257(83)90174-1. [DOI] [PubMed] [Google Scholar]
- 47.Ho C.L., Walton D.S. Primary congenital glaucoma: 2004 update. J Pediatr Ophthalmol Strabismus. 2004;41:271–288. doi: 10.3928/01913913-20040901-11. [DOI] [PubMed] [Google Scholar]
- 48.Bowman R.J., Dickerson M., Mwende J., Khaw P.T. Outcomes of goniotomy for primary congenital glaucoma in East Africa. Ophthalmology. 2011;118:236–240. doi: 10.1016/j.ophtha.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 49.Sharaawy T., Bhartiya S. Surgical management of glaucoma: evolving paradigms. Indian J Ophthalmol. 2011;59:123–130. doi: 10.4103/0301-4738.73692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Broughton W.L., Parks M.M. An analysis of treatment of congenital glaucoma by goniotomy. Am J Ophthalmol. 1981;91:566–572. doi: 10.1016/0002-9394(81)90054-4. [DOI] [PubMed] [Google Scholar]
- 51.Shaffer R.N. Prognosis of goniotomy in primary infantile glaucoma (trabeculodysgenesis) Trans Am Ophthalmol Soc. 1982;80:321–325. [PMC free article] [PubMed] [Google Scholar]
- 52.Gramer E.M., Tausch Kraemer C. Time of diagnosis, reoperations and long-term results of goniotomy in the treatment of primary congenital glaucoma: a clinical study. Int Ophthalmol. 1996–1997;;20(1–3):117–123. doi: 10.1007/BF00212957. [DOI] [PubMed] [Google Scholar]
- 53.Al-Hazmi A., Awad A., Zwaan J., Al-Mesfer S.A., Al-Jadaan I., Al-Mohammed A. Correlation between surgical success rate and severity of congenital glaucoma. Br J Ophthalmol. 2005;89(4):449–453. doi: 10.1136/bjo.2004.047761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Medow N.B., Sauer H.L. Endoscopic goniotomy for congenital glaucoma. J Pediatr Ophthalmol Strabismus. 1997;34(4):258–259. doi: 10.3928/0191-3913-19970701-18. [DOI] [PubMed] [Google Scholar]
- 55.Bayraktar S., Koseoglu T. Endoscopic goniotomy with anterior chamber maintainer: surgical technique and 1-year results. Ophthalmic Surg Lasers. 2001;32(6):496–502. [PubMed] [Google Scholar]
- 56.Kulkarni S.V., Damji K.F., Fournier A.V., Pan I., Hodge W.G. Endoscopic goniotomy: early clinical experience in congenital glaucoma. J Glaucoma. 2010;19(4):264–269. doi: 10.1097/IJG.0b013e3181b21ede. [DOI] [PubMed] [Google Scholar]
- 57.Smith R. A new technique for opening the canal of Schelmm. Preliminary report. Br J Ophthalmol. 1960;44:370–373. doi: 10.1136/bjo.44.6.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X., Du S., Fan Q., Peng S., Yu M., Ge J. Long-term surgical outcomes of primary congenital glaucoma in China. Clinics (Sao Paulo) 2009;64(6):543–551. doi: 10.1590/S1807-59322009000600009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Anderson D.R. Trabeculotomy compared to goniotomy for glaucoma in children. Ophthalmol. 1983;90(7):805–806. doi: 10.1016/s0161-6420(83)34484-5. [DOI] [PubMed] [Google Scholar]
- 60.McPherson S.D., Jr, Berry D.P. Goniotomy vs external trabeculotomy for developmental glaucoma. Am J Ophthalmol. 1983;95(4):427–431. doi: 10.1016/0002-9394(83)90260-x. [DOI] [PubMed] [Google Scholar]
- 61.Meyer G., Schwenn O., Pfeiffer N., Grehn F. Trabeculotomy in congenital glaucoma. Graefes Arch Clin Exp Ophthalmol. 2000;238:207–213. doi: 10.1007/s004170050345. [DOI] [PubMed] [Google Scholar]
- 62.Mendicino M.E., Lynch M.G., Drack A. Long-term surgical and visual outcomes in primary congenital glaucoma: 360 degrees trabeculotomy versus goniotomy. JAAPOS. 2000;4(4):205–210. doi: 10.1067/mpa.2000.106201. [DOI] [PubMed] [Google Scholar]
- 63.Filous A., Brunova B. Results of the modified trabeculotomy in the treatment of primary congenital glaucoma. JAAPOS. 2002;6(3):182–186. doi: 10.1067/mpa.2002.123431. [DOI] [PubMed] [Google Scholar]
- 64.Trabeculectomy Cairns JE. Preliminary report of a new method. Am J Ophthalmol. 1968;66:673–679. [PubMed] [Google Scholar]
- 65.Fulcher T., Chan J., Lanigan B., Bowell R., O’Keefe M. Long-term follow-up of primary trabeculectomy for infantile glaucoma. Br J Ophthalmol. 1996;80(6):499–502. doi: 10.1136/bjo.80.6.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawrence S.D., Netland P.A. Trabeculectomy versus combined trabeculotomy-trabeculectomy in pediatric glaucoma. J Pediatr Ophthalmol Strabismus. 2012;49(6):359–365. doi: 10.3928/01913913-20120710-06. [DOI] [PubMed] [Google Scholar]
- 67.Dureau P., Dollfus H., Cassegrain C., Dufier J.L. Long-term results of trabeculectomy for congenital glaucoma. J Pediatr Ophthalmol Strabismus. 1998;35:198–202. doi: 10.3928/0191-3913-19980701-05. [DOI] [PubMed] [Google Scholar]
- 68.Demailly P., Lavat P., Kretz G., Jeanteur-Lunel M.N. Non-penetrating deep sclerectomy (NPDS) with or without collagen device (CD) in primary open-angle glaucoma: middle-term retrospective study. Int Ophthalmol. 1996;20:131–140. doi: 10.1007/BF00212959. [DOI] [PubMed] [Google Scholar]
- 69.Zimmerman T.J., Kooner K.S., Ford V.J., Olander K.W., Mandlekorn R.M., Rawlings F.E. Effectiveness of nonpenetrating trabeculectomy in aphakic patients with glaucoma. Ophthalmic Surg. 1984;15:44–50. [PubMed] [Google Scholar]
- 70.Al-Obeidan S.A., Eel-D Osman, Dewedar A.S., Kestelyn P., Mousa A. Efficacy and safety of deep sclerectomy in childhood glaucoma in Saudi Arabia. Acta Ophthalmol. 2014;92(1):65–70. doi: 10.1111/j.1755-3768.2012.02558.x. [DOI] [PubMed] [Google Scholar]
- 71.Feusier M., Roy S., Mermoud A. Deep sclerectomy combined with trabeculectomy in pediatric glaucoma. Ophthalmology. 2009 Jan;116(1):30–38. doi: 10.1016/j.ophtha.2008.08.039. [DOI] [PubMed] [Google Scholar]
- 72.Luke C., Dietlein T.S., Jacobi P.C., Konen W., Krieglstein G.K. Risk profile of deep sclerectomy for treatment of refractory congenital glaucomas. Ophthalmology. 2002;109:1066–1071. doi: 10.1016/s0161-6420(02)01077-1. [DOI] [PubMed] [Google Scholar]
- 73.Freedman S.F., McCormick K., Cox T.A. Mitomycin C-augumented trabeculectomy with postoperative wound modulation in pediatricglaucoma. JAAPOS. 1999;3(2):117–124. doi: 10.1016/s1091-8531(99)70082-0. [DOI] [PubMed] [Google Scholar]
- 74.Sidoti P.A., Belmonte S.J., Liebmann J.M., Ritch R. Trabeculectomy with mitomycin-C in the treatment of pediatric glaucomas. Ophthalmol. 2000;107(3):422–429. doi: 10.1016/s0161-6420(99)00130-x. [DOI] [PubMed] [Google Scholar]
- 75.Jayaram H., Scawn R., Pooley F. Long-term outcome of trabulectomy augmented with mitomycin C undertaken within the first two years of life. Ophthalmology. 2015;122(11):2216–2222. doi: 10.1016/j.ophtha.2015.07.028. [DOI] [PubMed] [Google Scholar]
- 76.al-Hazmi A., Zwaan J., Awad A., al-Mesfer S., Mullaney P.B., Wheeler D.T. Effectiveness and complications of mitomycin C use during pediatric glaucoma surgery. Ophthalmology. 1998;105(10):1915–1920. doi: 10.1016/S0161-6420(98)91041-7. [DOI] [PubMed] [Google Scholar]
- 77.Scuderi G., Iacovello D., Pranno F., Plateroti P., Scuderi L. Pediatric Glaucoma: a literature’s review and analysis of surgical Results. Biomed Res Int. 2015;2015 doi: 10.1155/2015/393670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ozkiris A., Tamcelik N. Long-term results of trabeculectomy with different concentrations of mitomycin C in refractory developmental glaucoma. J Pediatr Ophthalmol Strabismus. 2005;42(2):97–102. doi: 10.3928/01913913-20050301-04. [DOI] [PubMed] [Google Scholar]
- 79.Dave P., Senthil S., Choudhari N., Sekhar G.C. Outcomes of Ahmed valve implant following a failed initial trabeculotomy and trabeculectomy in refractory primary congenital glaucoma. Middle East Afr J Ophthalmol. 2015;22(1):64–68. doi: 10.4103/0974-9233.148351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Razeghinejad M.R., Kaffashan S., Nowroozzadeh M.H. Results of Ahmed glaucoma valve implantation in primary congenital glaucoma. J AAPOS. 2014;18(6):590–595. doi: 10.1016/j.jaapos.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Panarelli J.F., Banitt M.R., Gedde S.J., Shi W., Schiffman J.C., Feuer W.J. A retrospective comparison of primary baerveldt implantation versus trabeculectomy with mitomycin C. Ophthalmology. 2016;123(4):789–795. doi: 10.1016/j.ophtha.2015.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Al Faran M.F. Tomey KF, al Mutlaq FA. Cyclocryotherapy in selected cases of congenital glaucoma. Ophthalmic Surg. 1990;21(11):794–798. [PubMed] [Google Scholar]
- 83.Wagle N.S., Freedman S.F., Buckley E.G., Davis J.S., Biglan A.W. Long-term outcome of cyclocryotherapy for refractory pediatric glaucoma. Ophthalmol. 1998;105(10):1921–1926. doi: 10.1016/S0161-6420(98)91042-9. [DOI] [PubMed] [Google Scholar]
- 84.Al-Haddad C.E., Freedman S.F. Endoscopic laser cyclophoto-coagulation in pediatric glaucoma with corneal opacities. JAAPOS. 2007;11(1):23–28. doi: 10.1016/j.jaapos.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 85.Aquino M.C., Barton K., Tan A.M. Micropulse versus continuous wave transscleral diode cyclophotocoagulation in refractory glaucoma: a randomized exploratory study. Clin Experiment Ophthalmol. 2015;43(1):40–46. doi: 10.1111/ceo.12360. [DOI] [PubMed] [Google Scholar]
- 86.Elhefney E.M., Mokbel T.H., Hagras S.M., AlNagdy A.A., Ellayeh A.A., Mohsen T.A. Micropulsed diode laser cyclophotocoagulation in recurrent pediatric glaucoma. Eur J Ophthalmol. 2019;Jul 1 doi: 10.1177/1120672119858226. 1120672119858226. [DOI] [PubMed] [Google Scholar]
- 87.Tan A.M., Chockalingam M., Aquino M.C., Lim Z.I., See J.L., Chew P.T. Micropulse transscleral diode laser cyclophoto-coagulation in the treatment of refractory glaucoma. Clin Experiment Ophthalmol. 2010;38(3):266–272. doi: 10.1111/j.1442-9071.2010.02238.x. [DOI] [PubMed] [Google Scholar]