Abstract
Plantago major L. which is a medicinal plant with important biological activities, commonly used as traditional medicine. Potential inhibitory activities of the aqueous extract and three isolated constituents calceorioside B (1), homoplantaginin (hispidulin-7-O-glucoside) (2) and plantamajoside (3) from the aerial parts of Plantago major subsp. major L. (Plantaginaceae) have been tested against hyaluronidase, collagenase, and elastase, which play critical roles in wound pathogenesis. Even though, the extract (27.04%), and among the isolated compounds, calceorioside B (41.16%) exerted significant inhibition against hyaluronidase enzyme, homoplantaginin and plantamajoside were found to be inactive. Similar results were obtained from collagenase enzyme inhibition test. The extract (21.92%) and calceorioside B (28.34%) also caused notable inhibition in this test. However, no remarkable inhibition was observed in the presence of elastase enzyme. The experimental data revealed that P. major subsp. major displayed remarkable inhibitory activity against hyaluronidase and collagenase enzymes. In vitro enzyme activity of P. major subsp. major is reported for the first time in the current study.
Keywords: Collagenase, Elastase, Enzyme inhibition, Hyaluronidase, In vitro, Plantago major, Plantaginaceae
1. Introduction
Plantago major L. (Plantaginaceae) belongs to the genus Plantago, which is represented by 21 species in Turkey and 2 of them are endemic (Davis, 1982). Many species of Plantago genus have been documented as medicinal plants in numerous countries including Turkey for centuries (Baytop, 1999, Jankovic et al., 2012, Goncalves and Romano, 2016). P. major (common plantain) is the most known and widely used species in traditional medicine for treatment of wound, abscess, acnes, diabetes, and cancer (Yesilada et al., 1995, Sezik et al., 1997, Sezik et al., 2001, Goncalves and Romano, 2016, Kuranel et al., 2016). Due to conspicuous veins on the leaves, P. major is named as “sinirli ot” in Turkey. There are three subspecies of P. major; P. major subsp. major, P. major subsp. intermedia and P. major subsp. winteri (Adom et al., 2017). P. major subsp. major and P. major subsp. intermedia have been commonly used as a traditional medicine in Anatolia (Baytop, 1999). The presence of iridoid glucosides, phenylethanoid glycosides, flavonoids, terpenoids, phenolic acids and polysaccharides in Plantago species has been reported up to date (Jankovic et al., 2012, Harput et al., 2012, Grubesic et al., 2013, Goncalves and Romano, 2016, Adom et al., 2017).
Though there has been an extensive investigation going on discovery of new collagenase, elastase and hyaluronidase enzyme inhibitory compounds of both synthetic and natural origins, a great essential still remains for new inhibitors of these enzymes owing to either side effects or low efficacy of present inhibitors. Further, the number of the current these enzyme inhibitors is quite limited, and new inhibitors are in demand mainly for cosmetics industry and wound healer. To date, we have investigated a large number of medicinal plants as well as natural compounds using several in vivo and in vitro experiments and as a result of these efforts we have find different collagenase, elastase, hyaluronidase enzyme inhibitors such as Eucalyptus globulus Labill., Marsdenia erecta R. Br., Podospermum canum C.A. Mey. etc. (Tumen et al., 2017, Acıkara et al., 2019). As part of our ongoing efforts on this road, in the current study we have aimed to investigate potential enzyme inhibitory activity of the aqueous extract and the isolated constituents (1–3) from the aerial parts of Plantago major subsp. major L.
2. Materials and methods
2.1. Chemicals
Column chromatography was accomplished using polyamide (polyamide 6, 50–160 µm, Sigma-Aldrich, St. Louis, MO, USA), silica gel (Kieselgel 60, 70–230 mesh, Merck, Darmstadt, Germany), Sephadex LH-20 (GE Healthcare, Chicago, IL, USA) and LiChroprep C18 (40–63 µm, Merck). Thin layer chromatography (TLC) was carried out on pre-coated Kieselgel 60 F254, 0.2 mm aluminum plates (Merck). Chloroform (CHCl3), methanol (MeOH) and ethyl acetate (EtOAc) were obtained from Merck. Medium pressure liquid chromatography (MPLC) was performed on Buchi (3.5 × 45 cm) glass columns filled with LiChroprep C18 using Buchi Pump Module C-605 peristaltic pumps and Buchi Fraction Collector C-660 (Buchi AG, Flawil, Switzerland). NMR spectra were recorded for 13C NMR and 1H NMR by a Bruker AVANCE600 spectrometer (Billerrica, MA, USA) at 150 MHz and 600 MHz, respectively.
2.2. Plant material
P. major subsp. major L. was collected from Maçka, Trabzon, Turkey, in June 2009. The voucher specimen, identified by Serdar Aslan (Department of Biology, Faculty of Sciences, Gazi University, Ankara, Turkey), has been deposited at the Herbarium of the Faculty of the Pharmacy, Hacettepe University, Ankara, Turkey [HUEF 09009].
2.3. Extraction, fractionation and purification procedure
The air-dried and powdered aerial parts of the plant (65 g) were extracted with MeOH (3 × 500 mL) at 40 °C for 4 h. The combined extracts were concentrated under vacuum at 40 °C to obtain 15.4 g of crude MeOH extract. Crude extract was dissolved in distilled water and partitioned with petroleum ether to remove non-polar compounds. After removal of the petroleum ether phase, aqueous phase was evaporated and lyophilized to give 13.1 g of the aqueous extract.
11.0 g of the aqueous extract of aerial parts was chromatographed over a polyamide column to get five fractions (Fr. A: 0% MeOH; Fr. B: 25% MeOH; Fr. C: 50% MeOH; Fr. D: 75% MeOH; Fr. E: 100% MeOH) using increasing concentrations of methanol in H2O (0, 25, 50, 75 and 100%).
Fr. B (1 g) was subjected to MPLC using 0–100% MeOH as a solvent system to obtain compound 3, plantamajoside (400 mg) with 35% MeOH.
Fr. C (164 mg), was applied to C-18 silica gel vacuum liquid chromatography (VLC) eluted with different concentrations of MeOH in H2O (0–100% MeOH) to get compound 2, homoplantaginin (43.2 mg) with 40–45% MeOH.
Fr. D (250 mg), was also applied to C-18 silica gel vacuum liquid chromatography with increasing concentrations of MeOH in H2O (0–100% MeOH) and compound 1, calceorioside B (34 mg) was yielded with 40% MeOH.
Structure elucidation of the isolated compounds was carried out by 1H-, 13C NMR, DEPT and 2D NMR (COSY, HSQC, HMBC) spectroscopy techniques.
2.4. Enzyme inhibitory activity studies
2.4.1. Hyaluronidase inhibitory activity assessment
The inhibition of hyaluronidase enzyme activity was evaluated by measuring the amount of N-acetylglucosamine released from sodium hyaluronate. 50 µL bovine hyaluronidase (7900 units/mL) dissolved in 0.1 M acetate buffer (pH 3.6) was mixed with 50 µL of different concentrations of the extracts dissolved in 5% DMSO. 50 µL of 5% DMSO was used instead of the extracts for the control group. After 20 min of incubation at 37 °C, 50 µL of calcium chloride (12.5 mM) was added to the mixture and again incubated for another 20 min in same conditions. 250 µL sodium hyaluronate (1.2 mg/mL) was added and incubated for 40 min in same way. After incubation, the mixture was treated with 50 µL of 0.4 M NaOH and 100 µL of 0.2 M sodium borate and then incubated for 3 min inside the boiling water bath. 1.5 mL of p-dimethylaminobenzaldehyde solution was added to the reaction mixture after cooling to room temperature and was further incubated at 37 °C for 20 min for color formation. The absorbance of this colored solution was measured at 585 nm (Beckmann Dual Spectrometer; Beckman, Fullerton, CA, USA) (Lee et al., 2008, Sahasrabudhe and Deodhar, 2010, Tumen et al., 2017).
2.4.2. Collagenase inhibitory activity assessment
The test samples were dissolved in DMSO. Clostridium histolyticum (ChC) was dissolved in 50 mM Tricine buffer (with 0.4 M NaCl and 0.01 M CaCl2, pH 7.5). 2 mM N-[3-(2-Furyl)acryloyl]-Leu-Gly-Pro-Ala (FALGPA) solution was prepared in the same buffer. 25 µL buffer, 25 µL test sample and 25 µL enzyme were added to each well and then incubated for 15 min. 50 µL substrate was added to the mixture to immediately measure the decrease of the optical density (OD) at 340 nm using a spectrometer.
The ChC inhibitory activity of each sample was calculated according to the following formula:
where ODcontrol and ODsample represent the optical densities in the absence and presence of sample, respectively (Barrantes and Guinea, 2003, Tumen et al., 2018).
2.4.3. Elastase inhibitory activity assessment
Test sample solutions and human neutrophil elastase enzyme (HNE) (17 mU/mL) were mixed in 0.1 M Tris-HCl buffer (pH 7.5), then incubated at 25 °C for 5 min. N-Methoxysuccinyl-Ala-Ala-Pro-Val p-nitroanilide (MAAPVN) was added to the mixture and incubated at 37 °C for 1 h. Soybean trypsin inhibitor (1 mg/mL) was added to stop the reaction and the optical density due to the formation of p-nitroaniline was immediately measured at 405 nm. The HNE inhibitory activities were calculated as described in the ChC inhibitory activity (Melzig et al., 2001, Tumen et al., 2017).
2.5. Statistical analysis of the data
The data obtained from in vitro enzyme inhibition assays were expressed as the standard deviation (S.D). One-way analysis of variance (ANOVA) was used to evaluate statistical differences between the reference and the test material groups. Dunnett’s multiple comparison tests were used as post hoc tests. The values of p < 0.05 was considered to be statistically significant [*p < 0.05; **p < 0.01; ***p < 0.001, ****p < 0.0001].
3. Results
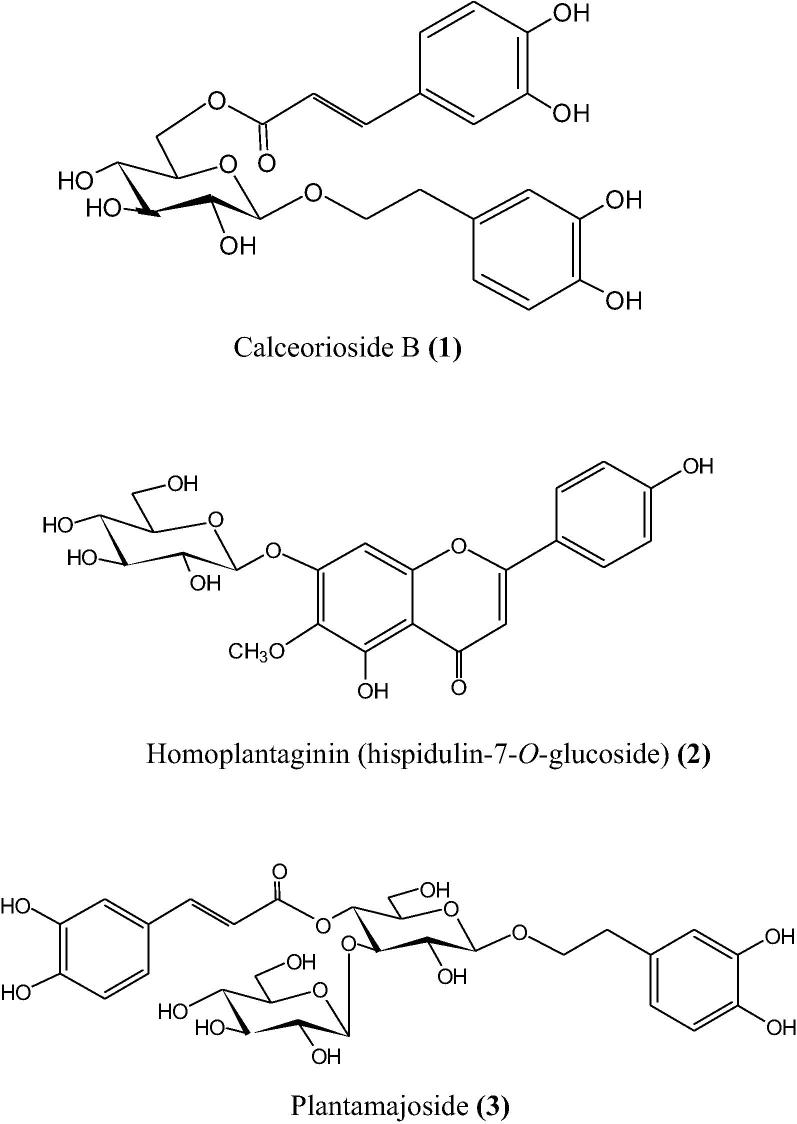
Our phytochemical studies on the aqueous extract prepared from the aerial parts of P. major subsp. major resulted in the isolation of three previously known compounds (Fig. 1). The chemical structures of these compounds were identified as calceorioside B (1), homoplantaginin (2) and plantamajoside (3) by comparing their spectroscopic data with the relevant literatures previously published (Zheng et al., 2002, Dawa et al., 2009, Maggi et al., 2009, Jensen et al., 2011).
Fig. 1.
Chemical structures of the isolated compounds 1–3.
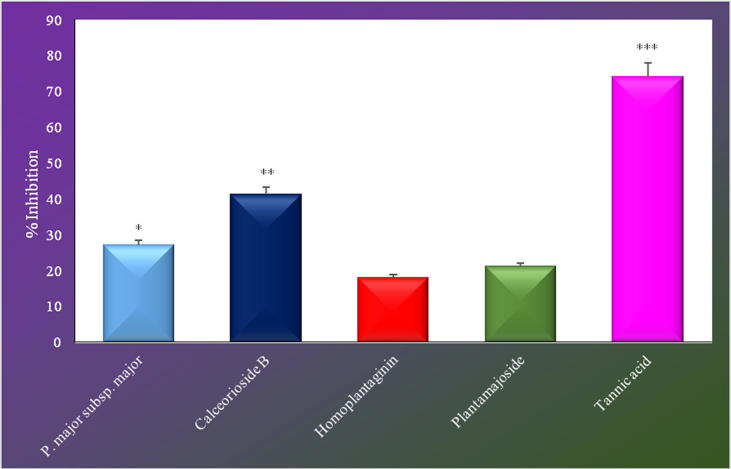
Despite the extract (27.04%), and among the isolated compounds, calceorioside B (41.16%) exerted significant inhibition at 100 μg/mL when compared to reference compound, homoplantaginin and plantamajoside were found to be inactive in the hyaluronidase enzyme inhibition test at the same concentration (Fig. 2).
Fig. 2.
Hyaluronidase inhibitory activity of the extracts and isolated compounds from Plantago major subsp. major.
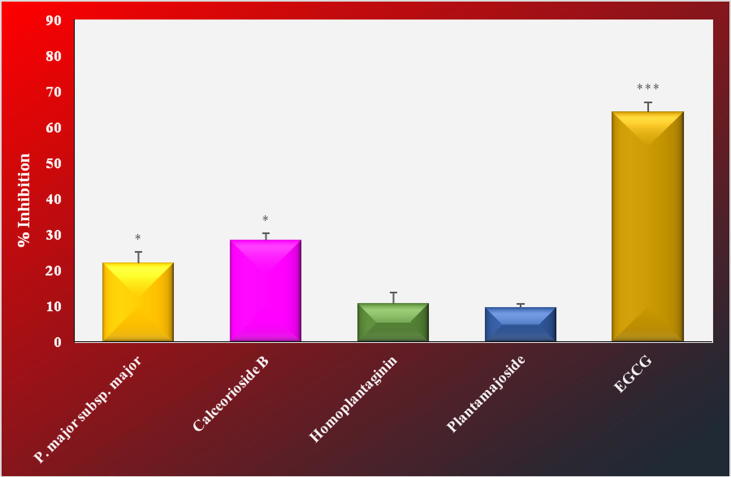
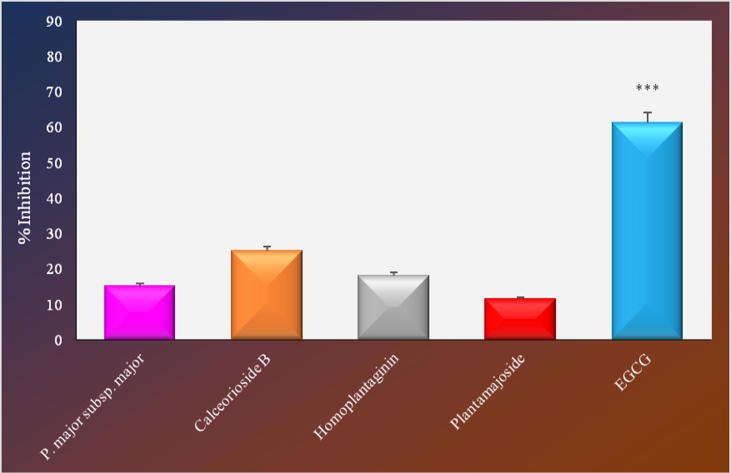
Similar results were obtained from collagenase enzyme inhibition test. The extract (21.92%) and calceorioside B (28.34%) also caused notable inhibition in this test (Fig. 3). However, no remarkable inhibitory activity was observed for both the extract and the isolated compounds in the presence of the enzyme elastase (Fig. 4).
Fig. 3.
Collagenase inhibitory activity of the extracts and isolated compounds from Plantago major subsp. major.
Fig. 4.
Elastase inhibitory activity of the extracts and isolated compounds from Plantago major subsp. major.
4. Discussion
The wound healing process comprises physiological events such as coagulation, formation of granulation tissue, reepithelization and remodeling of the extracellular matrix (ECM) (Xue and Jackson, 2015). Recent research has shown that the only function of the ECM is not to provide passive physical support for cells and that the ECM is also involved in tissue repair. Undistorted ECM molecules have been found to play an active role in the wound healing process with the ability to transform signals important for cellular processes in connection with growth factor activation (Agren and Werthén, 2007). The ECM molecules in the dermis consist of collagen, elastin, proteoglycans and glycosaminoglycans. Glycosaminoglycans interact with proteins and hyaluronic acid is the predominant glycosaminoglycan in the skin. Collagen, which is the most abundant protein in the ECM, plays a significant role in all phases of wound healing process. Elastin is another protein found in the ECM and gives elasticity to skin and other tissue (Haraway, 2006).
Nevertheless, inflammatory response accelerates the synthesis of dermal enzymes leading to degradation of ECM. Hyaluronic acid is depolymerized by hyaluronidase, elastase hydrolyzes fibrin and elastin fibers, and matrix metalloproteinases-1 particularly breaks the type I collagen. It has been suggested that the expression of dermal enzymes and the down-regulation of fiber synthesis play a major role in the process of skin wound (Mukherjee et al., 2011).
The injured tissues increase the formation of reactive oxygen products and reduce various enzymatic and non-enzymatic free radical scavengers and the presence of reactive oxygen radicals adversely affects the wound healing process (Ben Djemaa et al., 2016). In addition, excess of reactive oxygen species (ROS) causes inflammation, death of cells, tissue damage and reduction of the healing process, so antioxidant substances reduce the likelihood of these adverse events. Therefore, they seem important for the successful management of wounds (Ktari et al., 2017).
Calceorioside B (1), is a phenylethanoid glycoside isolated from several medicinal plants which possess anticancer, antioxidant and neuroprotective potentials (Ali et al., 2012). Harput et al. reported calceorioside A showed strong radical scavenging effects against DPPH, nitric oxide (NO) and superoxide (SO) anion radicals comparable to known antioxidants (Harput et al., 2012). The matrix metalloproteinase enzymes have degenerative effects on the structural proteins but metalloproteinase inhibitors may reduce the severity of the injury and contribute the healing process. Thus, inhibition of hyaluronidase, collagenase and elastase enzymes, which break down these components, could be useful for the wound healing process. Indeed, it was previously reported that it is vital to minimize the level of these breakdown enzymes (Edwards et al., 2004). In the present study, calceorioside B was found to have both hyaluronidase and collagenase inhibitory activities which could clearly explain its wound healer potential.
Homoplantaginin (hispidulin-7-O-glucoside) (2) a flavonoid glucoside, is another phytochemical constituent markedly decreased the levels of tumor necrosis factor-α (TNF-α) and interleukin-1 (IL-1) in Bacillus Calmette–Guérin/lipopolysaccharide-induced hepatic injury model of mice (Qu et al., 2009). Wu et al. reported that homoplantaginin ameliorates endothelial insulin resistance by inhibiting inflammation and modulating cell signalling via the IKKβ/IRS-1/pAkt/peNOS pathway (Wu et al., 2012). Akram et al. demonstrated that homoplantaginin inhibited NO and PGE2 production, and iNOS and COX-2 protein expression through heme oxygenase-1 (HO-1) induction via activation of nuclear factor erythroid 2–related factor2 (Nrf2) (Akram et al., 2015). In another study, palmitic acid-induced inflammation was inhibited by homoplantaginin through interacting with reactive oxygen species (ROS) sensitive thioredoxin protein. Protein and mRNA levels of inflammatory mediators (interleukin-1 beta, intercellular cell adhesion molecule-1, and monocyte chemotactic protein-1) were decreased by homoplantaginin. It has been indicated that homoplantaginin could protect endothelial cells from palmitic acid insult by restoring impaired NO generation (He et al., 2016). Weng and Wang reported that homoplantaginin isolated from Salvia plebeia had also low antioxidant activity (Weng and Wang, 2000). However, in the current study, homoplantaginin did not show inhibitory activity on all tested enzymes.
Previous bioactivity experiments carried out on plantamajoside (3), another isolated phenylethanoid glycoside, revealed that this phytochemical has antioxidant (Choi et al., 2008), antibiotic (Shoyama et al., 1986, Ravn and Brimer, 1988;; Ravn et al., 1989, Jimenez and Riguera, 1994, Deyama et al., 2006) and anti-inflammatory activities (Murai et al., 1995) and has effect on nitrite formation, and iNOS expression induced by LPS (Oh et al., 2005). Additionally, there are many studies showing that plantamajoside has inhibitory activity on cAMP phosphodiesterase (Ravn et al., 1990, Jimenez and Riguera, 1994, Skari et al., 1998, Nishibe, 2002, Deyama et al., 2006) and 5-lipoxygenase enzymes (Ravn et al., 1990) and also enzymatic lipid peroxidation (Skari et al., 1999). However, in our study, no effect on hyaluronidase, collagenase or elastase enzyme was determined.
Of all the compounds tested, calceorioside B (1) has been considered the active compound of P. major subsp. major due to its inhibitory effect on collagenase and hyaluronidase in wound healing. Since excess of oxidative stress causes wound occurring, and wound healing process consists of different stages of inflammation; compounds with antioxidant and anti-inflammatory effects are also important for the treatment of wounds (Schreml et al., 2011, Balekar et al., 2012). However, results obtained in this study are compatible with previous investigations on antioxidant and anti-inflammatory effects of some phenylethanoid glycosides (Lee et al., 2008, Zhao et al., 2015, Genc et al., 2019). In addition, the current study supports the wound healing usage of Plantago genus (Zubair et al., 2012, Kovac et al., 2015, Zubair et al., 2016, Goncalves and Romano, 2016). On the other hand, in vitro wound healing related enzyme activity of P. major subsp. major and isolated three compounds are reported for the first time in this study.
5. Conclusion
Consequently, aqueous extract of aerial parts of P. major subsp. major and only calceorioside B among three compounds isolated from the plant have been revealed to exert a prominent collagenase and hyaluronidase enzyme inhibitory activity. The highest inhibitory effect of calceorioside B compared to the extract showed the lack of synergistic effect between the extract and tested compounds. In this study, we disclose the first in vitro study on hyaluronidase and collagenase inhibitory effects of calceorioside B, which might be a promising precursor model for enzyme-inhibiting molecules with wound healer potential.
Declaration of Competing Interest
The authors declare that there are no conflicts of interest.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Yasin Genc, Email: yasin.genc@hacettepe.edu.tr.
Fatma Tugce Guragac Dereli, Email: tugceguragac@gazi.edu.tr.
Iclal Saracoglu, Email: isaracog@hacettepe.edu.tr.
Esra Kupeli Akkol, Email: esrak@gazi.edu.tr.
References
- Acıkara O.B., Ilhan M., Kurtul E., Šmejkal K., Kupeli Akkol E. Inhibitory activity of Podospermum canum and its active components on collagenase, elastase and hyaluronidase enzymes. Bioorg. Chem. 2019 doi: 10.1016/j.bioorg.2019.103330. [DOI] [PubMed] [Google Scholar]
- Adom M.B., Taher M., Mutalabisin M.F., Amri M.S., Kudos M.B.A., Sulaiman M.W.A.W., Sengupta P., Susanti D. Chemical constituents and medical benefits of Plantago major. Biomed. Pharmacother. 2017;96:348–360. doi: 10.1016/j.biopha.2017.09.152. [DOI] [PubMed] [Google Scholar]
- Agren M.S., Werthén M. The extracellular matrix in wound healing: a closer look at therapeutics for chronic wounds. Int. J. Low. Extrem. Wounds. 2007;6(2):82–97. doi: 10.1177/1534734607301394. [DOI] [PubMed] [Google Scholar]
- Akram M., Syed A.S., Kim K.A., Lee J.S., Chang S.Y., Kim C.Y., Bae O.N. Heme oxygenase 1-mediated novel antiinflammatory activities of Salvia plebeia and its active components. J. Ethnopharmacol. 2015;174:322–330. doi: 10.1016/j.jep.2015.08.028. [DOI] [PubMed] [Google Scholar]
- Ali R., Mirza Z., Ashraf G.M.D., Kamal M.A., Ansari S.A., Damanhouri G.A., Abuzenadah A.M., Chaudhary A.G., Sheikh I.A. New anticancer agents: recent developments in tumor therapy. Anticancer Res. 2012;32(7):2999–3005. [PubMed] [Google Scholar]
- Balekar N., Katkam N.G., Nakpheng T., Jehtae K., Srichana T. Evaluation of the wound healing potential of Wedelia trilobata (L.) leaves. J. Ethnopharmacol. 2012;141(3):817–824. doi: 10.1016/j.jep.2012.03.019. [DOI] [PubMed] [Google Scholar]
- Barrantes E., Guinea M. Inhibition of collagenase and metalloproteinases by aloins and aloe gel. Life Sci. 2003;72(7):843–850. doi: 10.1016/s0024-3205(02)02308-1. [DOI] [PubMed] [Google Scholar]
- Baytop T. Nobel Press; İstanbul: 1999. Therapy with Medicinal Plants in Turkey (Past and Present) [Google Scholar]
- Ben Djemaa F.G., Bellassoued K., Zouari S., El Feki A., Ammar E. Antioxidant and wound healing activity of Lavandula aspic L. ointment. J. Tissue Viability. 2016;25(4):193–200. doi: 10.1016/j.jtv.2016.10.002. [DOI] [PubMed] [Google Scholar]
- Choi S.U., Jung S.H., Lee H.S., Park K., Yun W., Lee B.S.K.W. Glycation inhibitory activity and the identification of an active compound in Plantago asiatica extract. Phytother. Res. 2008;22:323–329. doi: 10.1002/ptr.2316. [DOI] [PubMed] [Google Scholar]
- Davis P.H. Edinburgh University Press; Edinburgh: 1982. Flora of Turkey and the East Aegean Islands. [Google Scholar]
- Dawa Z.M., Bai Y., Zhou Y., Gesang S.L., Ping A., Ding L.S. Chemical constituents of the whole plants of Saussurea medusa. J. Nat. Med. 2009;63(3):327–330. doi: 10.1007/s11418-009-0320-1. [DOI] [PubMed] [Google Scholar]
- Deyama T., Kobayashi H., Nishibe S., Tu P. Isolation, structure elucidation and bioactivities of phenylethanoid glycosides from Cistanche, Forsythia and Plantago plants. Bioactive Nat. Prod. 2006;33:645–674. [Google Scholar]
- Edwards J.V., Howley P., Cohen I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004;284(1–2):1–12. doi: 10.1016/j.ijpharm.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Genc Y., Harput U.S., Saracoglu I. Active compounds isolated from Plantago subulata L. via wound healing and anti-inflammatory activity guided studies. J. Ethnopharmacol. 2019;241 doi: 10.1016/j.jep.2019.112030. [DOI] [PubMed] [Google Scholar]
- Goncalves S., Romano A. The medicinal potential of plants from the genus Plantago (Plantaginaceae) Ind. Crop Prod. 2016;83:213–226. [Google Scholar]
- Grubesic R.J., Srecnik G., Kremer D., Rodriguez J.V., Nikolic T., Vladimir-Knezevic S. Simultaneous RP-HPLC-DAD separation, and determination of flavonoids and phenolic acids in Plantago L. species. Chem. Biodivers. 2013;10(7):1305–1316. doi: 10.1002/cbdv.201200210. [DOI] [PubMed] [Google Scholar]
- Haraway, G.D., 2006. The extracellular matrix in wound healing (accessed 14 May 2019).
- Harput U.S., Genc Y., Saracoglu I. Cytotoxic and antioxidative activities of Plantago lagopus L. and characterization of its bioactive compounds. Food Chem. Toxicol. 2012;50(5):1554–1559. doi: 10.1016/j.fct.2012.01.019. [DOI] [PubMed] [Google Scholar]
- He B.Q., Zhang B.B., Wu F.H., Wang L.Y., Shi X.J., Qin W.W., Lin Y.N., Ma S.P., Liang J.Y. Homoplantaginin inhibits palmitic acid-induced endothelial cells inflammation by suppressing TLR4 and NLRP3 inflammasome. J. Cardiovasc. Pharmacol. 2016;67(1):93–101. doi: 10.1097/FJC.0000000000000318. [DOI] [PubMed] [Google Scholar]
- Jankovic T., Zdunic G., Beara I., Balog K., Pljevljakusic D., Stesevic D., Savikin K. Comparative study of some polyphenols in Plantago species. Biochem. Syst. Ecol. 2012;42:69–74. [Google Scholar]
- Jensen S.R., Opitz S.E.W., Gotfredsen C.H. A new phenylethanoid triglycoside in Veronica beccabunga L. Biochem. Syst. Ecol. 2011;39(3):193–197. [Google Scholar]
- Jimenez C., Riguera R. Phenylethanoid glycosides in plants: structure and biological activity. Nat. Prod. Rep. 1994;11(6):591–606. doi: 10.1039/np9941100591. [DOI] [PubMed] [Google Scholar]
- Kovac I., Durkac J., Holly M., Jakubcova K., Perzelova V., Mucaji P. Plantago lanceolata L. water extract induces transition of fibroblasts into myofibroblasts and increases tensile strength of healing skin wounds. J. Pharm. Pharmacol. 2015;67(1):117–125. doi: 10.1111/jphp.12316. [DOI] [PubMed] [Google Scholar]
- Ktari, N., Trabelsi, I., Bardaa, S., Triki, M., Bkhairia, I., Ben Slama-Ben Salem, R., Nasri, M., Ben Salah, R., 2017. Antioxidant and hemolytic activities, and effects in rat cutaneous wound healing of a novel polysaccharide from fenugreek (Trigonella foenum-graecum) seeds, Int. J. Biol. Macromol. 95, 625-634 [DOI] [PubMed]
- Kuranel E., Akkol E.K., Suntar I., Gursoy S., Keles H., Aktay G. Investigating biological activity potential of Plantago lanceolata L. in healing of skin wounds by a preclinical research, Turk. J. Pharm. Sci. 2016;13(2):135–144. [Google Scholar]
- Lee T.H., Huang N.K., Lai T.C., Yang A.T.Y., Wang G.J. Anemonin, from Clematis crassifolia, potent and selective inducible nitric oxide synthase inhibitor. J. Ethnopharmacol. 2008;116(3):518–527. doi: 10.1016/j.jep.2007.12.019. [DOI] [PubMed] [Google Scholar]
- Maggi A., Taskova R., Gotfredsen C.H., Bianco A., Jensen S.R. Chemical markers in Veronica sect. Hebe. III. Biochem. Syst. Ecol. 2009;37(6):731–736. [Google Scholar]
- Melzig M.F., Loser B., Ciesielski S. Inhibition of neutrophil elastase activity by phenolic compounds from plants. Pharmazie. 2001;56(12):967–970. [PubMed] [Google Scholar]
- Mukherjee P.K., Maity N., Nema N.K., Sarkar B.K. Bioactive compounds from natural resources against skin aging. Phytomedicine. 2011;19(1):64–73. doi: 10.1016/j.phymed.2011.10.003. [DOI] [PubMed] [Google Scholar]
- Murai M., Tamayama Y., Nishibe S. Phenylethanoids in the herb of Plantago lanceolata and inhibitory effect on arachidonic acid-induced mouse ear edema. Planta Med. 1995;61(5):479–480. doi: 10.1055/s-2006-958143. [DOI] [PubMed] [Google Scholar]
- Nishibe S. The plant origins of herbal medicines and their quality evaluation. Yakugaku Zasshi-J. Pharm. Soc. Jpn. 2002;122(6):363–379. doi: 10.1248/yakushi.122.363. [DOI] [PubMed] [Google Scholar]
- Oh J.W., Lee J.Y., Han S.H., Moon Y.H., Kim Y.G., Woo E.R., Kang K.W. Effects of phenylethanoid glucosides from Digitalis purpurea L. on the expression of inducible nitric oxide synthase. J. Pharm. Pharmacol. 2005;57:903–910. doi: 10.1211/0022357056451. [DOI] [PubMed] [Google Scholar]
- Qu X.J., Xia X., Wang Y.S., Song M.J., Liu L.L., Xie Y.Y., Cheng Y.N., Liu X.J., Qiu L.L., Xiang L., Gao J.J., Zhang X.F., Cui S.X. Protective effects of Salvia plebeia compound homoplantaginin on hepatocyte injury. Food Chem. Toxicol. 2009;47(7):1710–1715. doi: 10.1016/j.fct.2009.04.032. [DOI] [PubMed] [Google Scholar]
- Ravn H., Brimer L. Structure and antibacterial activity of plantamajoside, a caffeic acid sugar ester from Plantago major subsp. major. Phytochemistry. 1988;27(11):3433–3437. [Google Scholar]
- Ravn, H.W., Andary, C., Kova ´cs, G., Mølgaard, P., 1989. Caffeic acid esters as in vitro inhibitors of plant pathogenic bacteria and fungi, Biochem. Syst. Ecol. 17, 175–184.
- Ravn H., Nishibe S., Sasahara M., Li X.B. Phenolic compounds from Plantago asiatica. Phytochemistry. 1990;29(11):3627–3631. [Google Scholar]
- Sahasrabudhe A., Deodhar M. Anti-hyaluronidase, anti-elastase activity of Garcinia indica. Int. J. Bot. 2010;6:299–303. [Google Scholar]
- Schreml S., Landthaler M., Schaeferling M., Babilas P. A new star on the H2O2rizon of wound healing? Exp. Dermatol. 2011;20(3):229–231. doi: 10.1111/j.1600-0625.2010.01195.x. [DOI] [PubMed] [Google Scholar]
- Sezik, E. Yesilada, E., Tabata, M., Honda, G., Takaishi, Y., Fujita, T., Tanaka, T., Takeda, Y., 1997. Traditional medicine in Turkey. VIII. Folk medicine in East Anatolia; Erzurum, Erzincan, Agri, Kars, Igdir provinces, Econ. Bot. 51(3), 195–211.
- Sezik E., Yesilada E., Honda G., Takaishi Y., Takeda Y., Tanaka T. Traditional medicine in Turkey X. Folk medicine in Central Anatolia. J. Ethnopharmacol. 2001;75(2–3):95–115. doi: 10.1016/s0378-8741(00)00399-8. [DOI] [PubMed] [Google Scholar]
- Shoyama Y., Matsumoto M., Nishioka I. Four caffeoyl glycosides from callus tissue of Rehmannia glutinosa. Phytochemistry. 1986;25(7):1633–1636. [Google Scholar]
- Skari, K.P., Malterud, K.E., Haugli, T., 1998. Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major, a medicinal plant. In: Kum-pulainen, J.T., Salonene, J.T. (Eds.) Proceedings of the Second International Conference on Natural Antioxidants and Anticarcinogens in Nutrition, Health and Disease, Royal Society of Chemistry, Helsinki, Cambridge, pp. 200–202.
- Skari K.P., Malterud K.E., Haugli T. Radical scavengers and inhibitors of enzymatic lipid peroxidation from Plantago major, a medicinal plant. Roy. Soc. Ch. 1999;240:200–202. [Google Scholar]
- Tumen I., Guragac F.T., Keles H., Reunanen M., Kupeli-Akkol E. Characterization and wound repair potential of essential oil Eucalyptus globulus Labill. Fresenius Environ. Bull. 2017;26(11):6390–6399. [Google Scholar]
- Tumen I., Kupeli-Akkol E., Pranovich A., Reunanen M., Yaman B. Chemical composition and wound healing activity of inflorescences, leaves, wood, and bark of Marsdenia erecta R. Br. (Apocynaceae) Fresenius Environ. Bull. 2018;27(8):5590–5598. [Google Scholar]
- Weng X.C., Wang W. Antioxidant activity of compounds isolated from Salvia plebeia. Food Chem. 2000;71(4):489–493. [Google Scholar]
- Wu F.H., Wang H., Li J., Liang J.Y., Ma S.P. Homoplantaginin modulates insulin sensitivity in endothelial cells by inhibiting inflammation. Biol. Pharm. Bull. 2012;35(7):1171–1177. doi: 10.1248/bpb.b110586. [DOI] [PubMed] [Google Scholar]
- Xue M.L., Jackson C.J. Extracellular matrix reorganization during wound healing and its impact on abnormal scarring. Adv. Wound Care. 2015;4(3):119–136. doi: 10.1089/wound.2013.0485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yesilada E., Honda G., Sezik E., Tabata M., Fujita T., Tanaka T., Takeda Y., Takaishi Y. Traditional medicine in Turkey. V. Folk medicine in the inner Taurus Mountains. J. Ethnopharmacol. 1995;46(3):133–152. doi: 10.1016/0378-8741(95)01241-5. [DOI] [PubMed] [Google Scholar]
- Zhao J., Guo J., Zhang Y., Meng D.L., Sha Z. Chemical constituents from the roots and stems of Stauntonia brachyanthera Hand-Mazz and their bioactivities. J. Funct. Foods. 2015;14:374–383. [Google Scholar]
- Zheng X.K., Li J., Feng W.S. Studies on phenylethanoid glycosides from Corallodiscus flabellate. Chinese Traditional Herbal Drugs. 2002;33(10):881–883. [Google Scholar]
- Zubair M., Ekholm A., Nybom H., Renvert S., Widen C., Rumpunen K. Effects of Plantago major L. leaf extracts on oral epithelial cells in a scratch assay. J. Ethnopharmacol. 2012;141(3):825–830. doi: 10.1016/j.jep.2012.03.016. [DOI] [PubMed] [Google Scholar]
- Zubair M., Nybom H., Lindholm C., Brandner J.M., Rumpunen K. Promotion of wound healing by Plantago major L. leaf extracts–ex-vivo experiments confirm experiences from traditional medicine. Nat. Prod. Res. 2016;30(5):622–624. doi: 10.1080/14786419.2015.1034714. [DOI] [PubMed] [Google Scholar]