Abstract
Purpose
To evaluate the prevalence of glaucoma and its determinants among adult Saudi Residents aged 40 years and older in the Riyadh Governorate (except the Capital).
Methods
A cluster-based sample of randomly selected citizens from six primary health center catchment areas were examined between 2014 and 2015. Data were collected on their glaucoma management. Assessment included measurement of intraocular pressure, optic nerve head evaluation and gonioscopy. Glaucoma suspects were referred for visual field testing.
Result
A total of 940 citizens were examined and 124 had glaucoma. The prevalence of glaucoma was 5.6% [95% Confidence interval (CI): 5.43–5.75] with an estimated 3758 cases of glaucoma in study area. Males had a significantly higher prevalence (7.62%) than females (3.48%). Glaucoma was not significantly associated to diabetes [Odds ratio (OR) = 1.1; (95% CI: 0.8–1.7); P = 0.5]. The variation in the prevalence of glaucoma by age group was not significant (P = 0.2). Open angle of anterior chamber was in 78% of glaucoma cases. The coverage of glaucoma management was 27.8%. Among known glaucoma patients were 69% were treatment-complaint. Of 124 glaucoma patients, 29 (23.5%) were aware of their diagnosis. Mild and moderate visual impairment was in 67% and 8 (6.5%) glaucoma patients while one (0.8%) patient was bilateral blind.
Conclusion
The prevalence of glaucoma was high. Identified determinants should be noted and accordingly a public health approach for early detection and adequate management is recommended.
Keywords: Glaucoma, Glaucoma suspect, Prevalence, Survey, Visual impairment
Introduction
Glaucoma is one of the leading causes of irreversible visual disability in the adult population worldwide.1 The global prevalence of glaucoma in 40 years and plus population is 3.57% [95% confidence interval (CI): 2.09–5.82]. Individuals with glaucoma in this age-group are likely to increase from 64.3 million in 2013 to 76 million in 2020.2 Therefore, the World Health Organization (WHO) included glaucoma as the priority blinding eye disease. The WHO has recommended data collection on visual impairment (VI) and the epidemiological trends on eye diseases including glaucoma.3 Hence, evidence-based policy making at the national and subnational levels is essential to combat this public health issue.
Saudi Arabia has a population of 31.5 M (21.1 M Saudi Nationals) and the Gross Domestic Product (GDP) of 20,723 US $.4 The prevalence of blindness among the population aged 50 years and older in Saudi Arabia in 2010 was projected to be 3.3%.5 Eid et al reported 17.7% glaucoma rate in a hospital based study of western region of Saudi Arabia.6 Al-Shaaln et al. in a primary health center (PHC) based study reported found 5.8% prevalence of glaucoma in northern Saudi Arabia.7 Although the hospital based magnitude and determinants of glaucoma are known in some parts of Saudi Arabia, community based prevalence of glaucoma remains unknown.
We present the prevalence and determinants of glaucoma and the coverage of glaucoma services among 40 years and older Saudi population of Riyadh Governorate (except the Capital region). This study was a part of a community based prevalence survey conducted for the blindness and blinding eye diseases in the study area.
Materials and methods
The Institutional Research Board (IRB) at our center approved this survey (P-1309). The Ministry of Health, Riyadh governorate, also approved and supported this survey. This study adhered to the guidelines of the Declaration of Helsinki. The survey was conducted between 2013 and 2015.
Saudi residents aged 40 years and older in the study area comprised the study population. Randomly selected families from the PHC registry were invited to participate in the study.
To represent around 80,000 individual of the population aged 40 years and older, in the study area, we assumed the prevalence of glaucoma was 4.75%.8 To achieve 95% confidence interval (CI), 2% acceptable error margin and a design effect of 2, we need to recruit 865 individuals. This was a cluster-based survey. Of the 291 PHCs in the study area, we randomly selected 7 clusters. We aimed to assess approximately 125 participants from each cluster.
Four ophthalmologists, three clinical coordinators and one epidemiologist were involved in the survey. Data were collected on demographics including, age, gender and residence. The participants were asked about diagnoses of glaucoma, diabetes, use of glaucoma medication, surgery for glaucoma and use of glaucoma medication in last three days. A family history of glaucoma was also recorded.
The PHC had provided two rooms, one for vision assessment and other for eye examination by ophthalmologist. The visual status was noted using a Lea Symbol chart placed in light box and held 3-meter away from the participant. Presenting uncorrected visual acuity (UCVA) and the best-corrected visual acuity (BCVA) was recorded in LogMAR notation for each eye. The anterior segment of the eye was assessed with the slit lamp bio-microscope (Topcon Corp., Tokyo, Japan). Applanation tonometer was used to measure intraocular pressure (IOP). The anterior chamber angle was evaluated using a four-mirror gonio-lens (Volk Optical, Inc., Mentor, OH, USA). The central retina and other components of the posterior segment of eye were evaluated using +90 D lens (Volk Optical, Inc., Mentor, OH, USA). Non-mydriatic digital fundus camera (Topcon Corp., Tokyo, Japan) was used to obtain digital images of macula and optic nerve head (ONH). The vertical and horizontal cup-to-disc (CD) ratio was calculated by evaluating the ONH and its image. In this study, a patient was labeled as glaucoma if he/she had a CD ratio greater than 0.7 along with other evidences suggestive of glaucomatous cupping,9 notching of vessels emerging from the cup, splinter hemorrhage, thinning of the neuroretinal rim, nerve fiber layer defect, presence of overpass phenomenon in eyes with CD ratio greater than 0.5 and IOP greater than 21 mmHg.10 Glaucoma suspects were defined as eyes with IOP greater than 22 mmHg or CD ratio between 0.5 and 0.7, without other signs of ONH changes. Glaucoma suspects were referred for further assessment including visual field testing.
Based on the BCVA in the better eye, visual impairment (VI) status was graded as, bilateral blind (Vision > 1.3 LogMAR), severe VI (vision > 1 to 1.29), moderate VI (>0.5 to < 1.0) and mild VI (<0 to < 0.49).11
A number of measures were undertaken to ensure high standard of the survey. They included: training of field staff, standard operating procedure survey manual, auditing the data at the end of each cluster visit, supervision of the field activities and periodic calibration of the equipment.
The data was analysed using Statistical Package for Social Sciences (SPSS 23) (IBM, NY, USA). For qualitative variables, frequencies and the percentage proportions were calculated. For quantitative variables, histogram was plotted. If the curves were normal, the mean and standard deviations were calculated. If the data were not normally distributed, the median and 25% quartiles were calculated. We estimated the crude rate and then projected the number of individuals with glaucoma in the population in the smallest unit, age group and gender. Based on these numbers and using population above 40 years as a denominator, the adjusted prevalence rates for the study area were calculated by gender, age groups and clusters. The 95% CI of the prevalence rate was also calculated. To associate the glaucoma rates to other known risk factors, open epi software was used to find the Odds ratio (OR), the 95% CI and the two- sided ‘P’ value. For more than two independent variables in the subgroups, chi-square values, degree of freedom and the two-sided P value were calculated. P values less than 0.05 were considered statistically significant.
Results
Nine hundred and fifty participants were enrolled of whom, 890 were examined, with a response rate of 93.7%. A comparison of study population and examined population is presented in Table 1. Younger age group males were underrepresented. Females ‘less than 50 years of age’ and ‘more than 74 years’ were underrepresented in this survey. Therefore, age-sex standardized prevalence rates were calculated to represent the glaucoma magnitude in the study population. One hundred and twenty (13.5%) participants had undergone surgery in one eye and 116 (13%) were using spectacles suggesting that they had visited an optometrist or ophthalmologist for impaired vision.
Table 1.
Proportion of study population and examined sample by gender and age group.
| Male |
Age group (years) | Female |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Population |
Examined |
A-B/A | Population |
Examined |
A-B/A | |||||
| Number | % (A) | Number | % (B) | Number | % | Number | % | |||
| 10,967 | 27.1 | 70 | 14.2 | 0.5 | 40–44 | 10,716 | 27.7 | 94 | 23.7 | 0.14 |
| 8903 | 22.0 | 76 | 15.4 | 0.3 | 45–49 | 8326 | 21.6 | 62 | 15.7 | 0.27 |
| 6596 | 16.3 | 82 | 16.6 | −0.02 | 50–54 | 6090 | 15.8 | 90 | 22.7 | −0.44 |
| 4492 | 11.1 | 73 | 14.8 | −0.3 | 55–59 | 4356 | 11.3 | 44 | 11.1 | 0.01 |
| 3480 | 8.6 | 80 | 16.2 | −0.9 | 60–64 | 3315 | 8.6 | 47 | 11.9 | −0.38 |
| 1862 | 4.6 | 26 | 5.3 | −0.1 | 65–69 | 1850 | 4.8 | 20 | 5.1 | −0.05 |
| 2064 | 5.1 | 38 | 7.7 | −0.5 | 70–74 | 1773 | 4.6 | 23 | 5.8 | −0.27 |
| 2064 | 5.1 | 49 | 9.9 | −0.9 | 75+ | 2197 | 5.7 | 16 | 4.0 | 0.29 |
| 40,428 | 100.0 | 494 | 100.0 | – | Total | 38,622 | 100.0 | 396 | 100.0 | - |
There were 124 participants with glaucoma in at least one eye. The age-sex adjusted prevalence of glaucoma was 5.6% (95% CI: 5.43–5.75). Hence, there could be 3758 individuals with glaucoma in this age group in the study area.
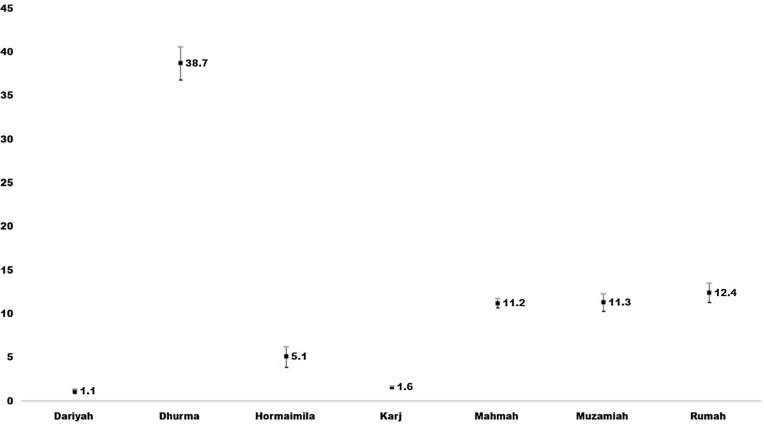
There were 22 (1.3%) individuals classified as glaucoma suspects who could not be confirmed at the PHCs with visual field testing. If we assume that all 22 glaucoma suspects had true glaucoma, the prevalence rate could increase from 5.59% to 6.58%. The prevalence of glaucoma in the subgroups is presented in Table 2. Males and individuals aged 60 years and more had comparatively higher rates of glaucoma (Table 2). Durmah, Rumah, Muzamiah and Mahmah clusters had higher rates of glaucoma (Fig. 1). However in view of large population, Karj and Mahmah are projected to have nearly three-fourths of glaucoma cases. The prevalence of glaucoma among diabetics was 58/388 [14.9% (95% CI: 11.4–18.5)]. There were 65 (13.5%) glaucoma cases among 483 nondiabetics. Glaucoma and diabetes were not statistically significantly associated. [OR = 1.1; (95% CI: 0.8–1.7) P = 0.5].
Table 2.
Prevalence of Glaucoma in the Saudi population of Riyadh Governorate (except the capital) aged 40 years and older (Riyadh eye survey 2015).
| Examined | Number of glaucoma cases | Adjusted prevalence | 95% confidence interval | Projected number in population | ||
|---|---|---|---|---|---|---|
| Total | 890 | 124 | 5.59 | 5.43–5.75 | 3758 | |
| Gender | Male | 494 | 78 | 7.62 | 7.36–7.88 | 2416 |
| Female | 396 | 46 | 3.48 | 3.30–3.66 | 1342 | |
| Age group | 40–49 | 302 | 33 | 2.9 | 2.8–3.1 | 1144 |
| 50–59 | 289 | 37 | 2.4 | 2.2–2.6 | 510 | |
| 60–69 | 173 | 29 | 10.7 | 10.1–11.3 | 1120 | |
| 70 and plus | 126 | 25 | 12.2 | 11.4–12.9 | 984 | |
Fig. 1.
Prevalence of Glaucoma in 7 clusters of Riyadh Governorate (except the capital) aged 40 years and older.
Of the 124 individuals diagnosed with glaucoma, 29 (23.4%) knew that they had glaucoma in one of their eyes. In the remaining 95 glaucoma cases, glaucoma was detected for the first time during the survey.
Of the known 124 glaucoma cases, 25 (86.2%) patients were using topical glaucoma medication. Sixteen (55%) patients had undergone surgery or laser treatment in the past. On the day of survey, 20 (69%) had used glaucoma medication. In 15 (51%) patients, glaucoma medications were used after previous glaucoma surgery.
Among 124 known glaucoma patients, 18 (14.5%) had a family history of glaucoma. One of 22 glaucoma suspects, had a positive family history of glaucoma. In 733 individuals without glaucoma, 89 (12.1%) had a positive family history of glaucoma. Data on family history of glaucoma was unknown for 33 participants. The family history of glaucoma was not significantly associated to glaucoma [OR = 1.42 (95% CI: 0.8–2.5), P = 0.2].
The mean central corneal thickness (CCT) among glaucoma cases was 472 ± 139 μm. CCT in non-glaucomatous eyes was 537.4 ± 88.3 μm. The mean difference in CCT between glaucomatous and non-glaucomatous eyes was 65.4 μm (range, 11.7–119 μm), which was statistically significant (P = 0.01).
The anterior chamber angle was reviewed by gonioscopy in only 23 glaucoma cases. Eighteen (78%) cases had a grade 3 or 4 angle suggestive of open angle glaucoma.
The BCVA based grading of VI status suggested that two (1.6%) individuals had vision impairment of category 3, 4 and 5. Category 1 VI was noted in 15 (12.1%) glaucoma cases and 107 (86.3%) glaucoma cases were noted as category 0.
Table 3 presents the community-based prevalence of glaucoma in some earlier published data.
Table 3.
Magnitude of glaucoma based on community surveys in different countries.
| Year | Authors | Sample size | Prevalence rate + 95% CI | Remarks | Reference |
|---|---|---|---|---|---|
| 2005–06 | Khandekar et al. | 3324 | 4.75 (4–5.5) | POAG 41% Age 30+ |
8 |
| 2009 | Al Mansouri et al. | 3149 | 1.73 (1.69–1.77) | POAG – 67% Age: 40+ |
12 |
| 2010–11 | Pakrawan et al. | 1990 | 4.4 (3.3–5.4) | POAG 68% Age: 40–80 |
13 |
| 2008 | Palimkar et al. | 7438 | 3.68 (3.3–4.1) | POAG: 13% Age 35+ |
14 |
| 2004 | Rahman et al. | 2347 | 2.1 (1.5–2.9) | POAG: 75% Age 40+ |
15 |
| 2007 | Casson et al. | 1997 | 4.9 (4.1–5.7) | POAG: 38% Age: 40+ |
16 |
| 2000 | Buhrmann et al. | 3268 | 4.16 (3.5–4.9) | POAG 74.5% Age: 40+ |
17 |
| 2006–08 | Bundenz et al. | 5603 | 6.5 (5.8–7.1) | POAG 94.5% Age: 40+ |
19 |
POAG = primary open angle.
Discussion
This was the first community-based glaucoma survey in Saudi Arabia. Glaucoma was prevalent in more than 1 in 20 Saudis aged 40 years and older resident of Riyadh governorate (except the capital). Males had a higher risk of glaucoma compared to females. In the current study, less than one-fourth of the individuals with glaucoma knew about their disease. More than two-third had good compliance for using glaucoma medications. The existing eye services could identify and manage less than one third of glaucoma cases. VI grade and family history of glaucoma were not associated to glaucoma in our study population. The eye service providers in the area need to address the burden of 3758 glaucoma cases in the study population.
The prevalence of glaucoma in the current study area is similar to that reported in community-based studies for similar age groups and from different countries.12, 13, 14, 15, 16, 17
The distribution of glaucoma by subtype in the current study indicated that the majority of cases have primary open angle glaucoma (POAG). In study of Singaporean Chinese, POAG comprised 47% of glaucoma cases.18 Globally, POAG is projected to comprise three-fourths of all glaucoma cases by 2020.1 A study of West African adults reported that POAG comprised of 95% of all glaucoma cases.19 The proportion of POAG found in the current study is lower than West African adults but much higher than the Far East Asian population. Noncompliance to the medical treatment among Saudi glaucoma patients was 31%. This was lower than that noted in Oman; a neighboring gulf country, but was within range of 5–80% as noted in a review by Olthoff et al.20, 21 In addition to identifying cases of glaucoma in the community, the national task force for the Prevention of Blindness in Saudi Arabia will have to focus on strategies to improve the compliance to glaucoma treatment.
In our survey, the ratio of medical versus surgical management of glaucoma was 1:0.6 among known glaucoma patients. Whether medical or surgical management of adult POAG is the better strategy remains debatable.22 Conventionally, surgery is advised if medications fail to control IOP and glaucomatous damage is progressing.23 In the current study, half of cases that underwent surgery remained on glaucoma medications. This high rate of surgical failure is a matter of concern. The underlying causes of surgical failure warrants further investigation to improve the expectations of surgical treatment among glaucoma patients.
In the current study, there was no association of glaucoma to diabetes in the adult Saudi population. With the rising prevalence of glaucoma in the UK, use of telemedicine for glaucoma and diabetic retinopathy screening is a strategy to detect cases more efficiently.24 With the advent of smartphone based apps to screen for diabetic retinopathy and glaucoma (including visual field testing), a combined screening is a possibility.25
In the present survey, three-fourth of cases of glaucoma were not aware of their disease and were not treated for glaucoma by ophthalmologists. Unfortunately many of these first time detected glaucoma cases in the survey had visited ophthalmologists and optometrist for other eye ailments. Perhaps implementation of standard operating procedures for comprehensive eye assessment would have resulted in early detection of these glaucoma cases. A number of tools to detect and manage glaucoma are available in eye care services in the Ministry of Health, Saudi Arabia.26 They should be optimally used to improve the Optic Nerve Head evaluation and field of vision testing to confirm the diagnosis of glaucoma.27
We noted a significant positive association of thinner corneal thickness to glaucoma. This observation concurs with Francis et al.28 The subsequent risk of developing glaucoma in eyes with thinner CCT is higher in glaucoma suspects with normal IOP.29 Thus, assessment of CCT in comprehensive glaucoma assessment is encouraged.
In the current study, there was no significant association of glaucoma with positive family history of glaucoma. In another study, POAG diagnosed on visual field changes were associated to a positive family history of glaucoma.30 Macmonnes31 proposed that negative family histories can often be unreliable due to large numbers of undiagnosed glaucoma cases. This observation may explain our non-significant association of family history with glaucoma.
Only one individual with glaucoma was bilaterally blind in our study. The rest of the glaucoma cases had either mild or moderate VI in the better eye. In 2010, a study from other regions in Saudi Arabia reported that 8% of global blindness and 2% of global VI were due to glaucoma.32 This implies that glaucoma cases especially newly detected either had minimum functional changes of the central retina or did not have other macular comorbidities. From Saudi patient’s perspective, VI is the key to seek an ophthalmic consultation. This behavior practice could explain why three-fourth of glaucoma cases with limited VI were identified for the first time during this survey. It also suggests that any screening project of the elderly population with the foundation of vision examination is less likely to identify glaucoma cases and even the identified cases of glaucoma with less vision problem may not seek expert opinion unless they are properly counseled.
This study, provided estimates of glaucoma as blinding disease in addition to a VI survey and focused on the population aged 40 years and older that comprises nearly 15% of the Saudi population. This is in contrast to the Rapid Assessment of Avoidable Blindness (RAAB) surveys conducted in four regions of Saudi Arabia targeting individuals aged 50 years and older, which constitutes less than 5% of the Saudi population. Future approaches should thus focus on the “at-risk” population. We believe the at-risk population for glaucoma, and diabetic retinopathy is 40 years and older. We also recommend similar surveys in the other four zones of Saudi Arabia to confirm the evidence generated in Riyadh (central zone of the Kingdom of Saudi Arabia) and prepare a national Action Plan for glaucoma, which is an important eye component of Sustainable Developmental Goals.33
There were some limitations of this study. The type of glaucoma in all the cases could not be determined as gonioscopy was not possible in all cases.
Additionally, the goniolens was damaged during the survey and two clusters could not undergo assessment of the anterior chamber angle. In Karj and Dariyah clusters, we could not enrolled required sample size despite prolonging the cluster coverage. This could have resulted in higher projections of glaucoma in these clusters. Additionally, the labelling of cases of glaucoma although suitable for community based survey was not based on international definitions that are used in clinical practices.9 The absence of visual field defects from the criteria indicates a number of cases without increased IOP and ONH changes not suggestive of glaucoma might have been underrepresented. Boland et al have highlighted the limitations of the existing tools for visual field testing.34 However, the projection of the prevalence of glaucoma from this survey are adequate to plan public health initiatives to combat visual disabilities due to glaucoma in the study area.
Glaucoma is prevalent in one out of 20 Saudis aged 40 years or older in the central province. Three out of four cases were detected for the first time during this survey. A public health approach for early detection and adequate management is required.
Financial support
None.
Conflict of interest
The authors declared that there is no conflict of interest
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Quigley H.A., Broman A.T. The number of people with glaucoma worldwide in 2010 and 2020. Br J Ophthalmol. 2006;90(3):262–267. doi: 10.1136/bjo.2005.081224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tham Y.C., Li X., Wong T.Y., Quigley H.A., Aung T., Cheng C.Y. Global prevalence of glaucoma and projections of glaucoma burden through 2040: a systematic review and meta-analysis. Ophthalmology. 2014;121(11):2081–2090. doi: 10.1016/j.ophtha.2014.05.013. [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Glaucoma: Disease control and prevention of visual impairment in Global initiative for the elimination of avoidable blindness. Action Plan 2006–2011. Geneva, Switzerland. World Health Organization; 2007. p. 37–9. [Last accessed on 15 January 2018] <http://www.iapb.org/sites/iapb.org/files/VISION2020ActionPlan2006-2011.pdf>.
- 4.Ministry of Health, Saudi Arabia. Statistical book 2015. Demographic and finance indicators. Published in Riyadh, KSA; 2016. [Accessed on 15 January 2018] <https://www.moh.gov.sa/en/Ministry/Statistics/book/Documents/StatisticalBook-1436.pdf>.
- 5.International Agency for the Prevention of Blindness. The GVD Map (Adult >50) in Vision Atlas. [Last accessed on 15 January 2018]. <http://atlas.iapb.org/>.
- 6.Eid T.M., el-Hawary I., el-Menawy W. Prevalence of glaucoma types and legal blindness from glaucoma in the western region of Saudi Arabia: a hospital- based study. Int Ophthalmol. 2009;29(6):477–483. doi: 10.1007/s10792-008-9269-4. [DOI] [PubMed] [Google Scholar]
- 7.Al-Shaaln F.F., Bakrman M.A., Ibrahim A.M., Aljoudi A.S. Prevalence and causes of visual impairment among Saudi adults attending primary health care centers in northern Saudi Arabia. Ann Saudi Med. 2011;31(5):473–480. doi: 10.4103/0256-4947.84624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khandekar R., Jaffer M.A., Al Raisi A. Oman Eye Study 2005: prevalence and determinants of glaucoma. East Mediterr Health J. 2008;14(6):1349–1359. [PubMed] [Google Scholar]
- 9.American Academy of Ophthalmology. Primary Open Angle Glaucoma. PPP - 2015. by Prum BE, Rosenberg LF, Gedde SA, et al. [accessed on 7/2/2017] <https://pdfs.semanticscholar.org/0c9d/fcbd1f2ccad3a77c74b818da397767fb7cb4.pdf>.
- 10.Smith S.D., Singh K., Lin S.C. Evaluation of the anterior chamber angle in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2013;120(10):1985–1997. doi: 10.1016/j.ophtha.2013.05.034. [DOI] [PubMed] [Google Scholar]
- 11.World Health Organization. International Statistical Classification of Diseases and Related Health Problems – 11th Revision. WHO, Geneva, Switzerland 2016. Diseases of eye and adnexa. [Accessed on 7 January 2018] <https://icd.who.int/browse10/2016/en#/H53-H54> [chapter VII].
- 12.Al-Mansouri F.A., Kanaan A., Gamra H. Prevalence and determinants of glaucoma in citizens of Qatar aged 40 years or older: a community-based survey. Middle East Afr J Ophthalmol. 2011;18(2):141–149. doi: 10.4103/0974-9233.80703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pakravan M., Yazdani S., Javadi M.A. A population-based survey of the prevalence and types of glaucoma in central Iran: the Yazd eye study. Ophthalmology. 2013;120(10):1977–1984. doi: 10.1016/j.ophtha.2013.02.029. [DOI] [PubMed] [Google Scholar]
- 14.Palimkar A., Khandekar R., Venkataraman V. Prevalence and distribution of glaucoma in central India (Glaucoma Survey 2001) Indian J Ophthalmol. 2008;56(1):57–62. doi: 10.4103/0301-4738.37597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rahman M.M., Rahman N., Foster P.J. The prevalence of glaucoma in Bangladesh: a population based survey in Dhaka division. Br J Ophthalmol. 2004;88(12):1493–1497. doi: 10.1136/bjo.2004.043612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casson R.J., Newland H.S., Muecke J. Prevalence of glaucoma in rural Myanmar: the Meiktila Eye Study. Br J Ophthalmol. 2007;91(6):710–714. doi: 10.1136/bjo.2006.107573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buhrmann R.R., Quigley H.A., Barron Y., West S.K., Oliva M.S., Mmbaga B.B.O. Prevalence of Glaucoma in a rural east African population. Invest Ophthalmol Vis Sci. 2000;41(1):40–48. [PubMed] [Google Scholar]
- 18.Baskaran M., Foo R.C., Cheng C.Y. The prevalence and types of glaucoma in an Urban Chinese Population: The Singapore Chinese Eye Study. JAMA Ophthalmol. 2015;133(8):874–880. doi: 10.1001/jamaophthalmol.2015.1110. [DOI] [PubMed] [Google Scholar]
- 19.Budenz D.L., Barton K., Whiteside-de Vos J. Survey Study Group. Prevalence of glaucoma in an urban West African population: the Tema Eye Survey. JAMA Ophthalmol. 2013;131(5):651–658. doi: 10.1001/jamaophthalmol.2013.1686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khandekar R., Shama Mel-S., Mohammed A.J. Noncompliance with medical treatment among glaucoma patients in Oman–a cross-sectional descriptive study. Ophthal Epidemiol. 2005;12(5):303–309. doi: 10.1080/09286580500224602. [DOI] [PubMed] [Google Scholar]
- 21.Olthoff C.M., Schouten J.S., van de Borne B.W., Webers C.A. Noncompliance with ocular hypotensive treatment in patients with glaucoma or ocular hypertension an evidence-based review. Ophthalmology. 2005;112:953–961. doi: 10.1016/j.ophtha.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 22.Burr J., Azuara-Blanco A., Avenell A., Tuulonen A. Medical versus surgical interventions for open angle glaucoma. Cochrane Database Syst Rev. 2012;9:CD004399. doi: 10.1002/14651858.CD004399.pub3. [DOI] [PubMed] [Google Scholar]
- 23.SooHoo J.R., Seibold L.K., Radcliffe N.M., Kahook M.Y. Minimally invasive glaucoma surgery: current implants and future innovations. Can J Ophthalmol. 2014;49(6):528–533. doi: 10.1016/j.jcjo.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 24.Sim D.A., Mitry D., Alexander P. The evolution of teleophthalmology programs in the United Kingdom: beyond Diabetic Retinopathy Screening. J Diab Sci Technol. 2016;10(2):308–317. doi: 10.1177/1932296816629983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rajalakshmi R., Arulmalar S., Usha M. Validation of smartphone based retinal photography for diabetic retinopathy screening. PloS One. 2015;10(9) doi: 10.1371/journal.pone.0138285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Al Motowa S., Khandekar R., Al-Towerki A. Resources for eye care at secondary and tertiary level government institutions in Saudi Arabia. Middle East Afr J Ophthalmol. 2014;21(2):142–146. doi: 10.4103/0974-9233.129761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sharma P., Sample P.A., Zangwill L.M., Schuman J.S. Diagnostic tools for glaucoma detection and management. Surv Ophthalmol. 2008;53(6):S17–S32. doi: 10.1016/j.survophthal.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Francis B.A., Varma R., Chopra V., Lai M.Y., Shtir C., Azen S.P. Los Angeles Latino Eye Study Group. Intraocular pressure, central corneal thickness, and prevalence of open-angle glaucoma: the Los Angeles Latino Eye Study. Am J Ophthalmol. 2008;146(5):741–746. doi: 10.1016/j.ajo.2008.05.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rogers K. Britannica Educational Publishing; New York, NY: 2011. The eye, the physiology of human perception. [Google Scholar]
- 30.Kang J.H., Loomis S.J., Rosner B.A., Wiggs J.L., Pasquale L.R. Comparison of risk factor profiles for primary open-angle glaucoma subtypes defined by pattern of visual field loss: a prospective study. Invest Ophthalmol Vis Sci. 2015;56(4):2439–2448. doi: 10.1167/iovs.14-16088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McMonnies C.W. Glaucoma history and risk factors. J Optom. 2017;10(2):71–78. doi: 10.1016/j.optom.2016.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pascolini D., Mariotti S.P. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96(5):614–618. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 33.Eye Health, Vision care and Sustainable Developmental Goals; Vision 2020 Australia a reference document; 2015. [Accessed on 26 Dec 2017] <http://www.vision2020australia.org.au/uploads/resource/175/Eye-health-%20vision-care-and-the-Sustainable-Development-Goals.pdf>.
- 34.Boland M.V., Gupta P., Ko F., Zhao D., Guallar E., Friedman D.S. Evaluation of frequency-doubling technology perimetry as a means of screening for glaucoma and other eye diseases using the National Health and Nutrition Examination Survey. JAMA Ophthalmol. 2016;134(1):57–62. doi: 10.1001/jamaophthalmol.2015.4459. [DOI] [PubMed] [Google Scholar]