Abstract
Chitosan derivatives are reported as anticoagulants in the literature. This work was undertaken to develop novel chitosan derivatives as anticoagulants. The sulfonated derivatives of chitosan were formed by the reaction of chitosan derivatives with chlorosulfonic acid in N,N-dimethylformamide. The structures of these derivatives were established by FTIR and 1H NMR spectra. The prepared derivatives were evaluated for their in vivo anticoagulant effects by the tail bleeding method in Wistar rats utilizing nicoumalone as a standard drug. The results revealed that the sulfonation of the chitosan increases its anticoagulant activity. The developed compounds exhibited faster onset of action and potency than nicoumalone after one hour of the drug administration. The sulphated N-alkyl derivatives of chitosan were more potent anticoagulants than sulfated quaternary derivatives/sulfated chitosan. It is also suggested to develop analogs of Ethyl chitosan sulfate (4b) and Benzyl chitosan sulfate (4c), which may provide some more fruitful anticoagulants having faster onset of action as well as longer duration of action and possessing a balanced hydrophilic/lipophilic character.
Keywords: Synthesis, Chitosan derivatives, Sulfonated chitosan, Anticoagulant activity
1. Introduction
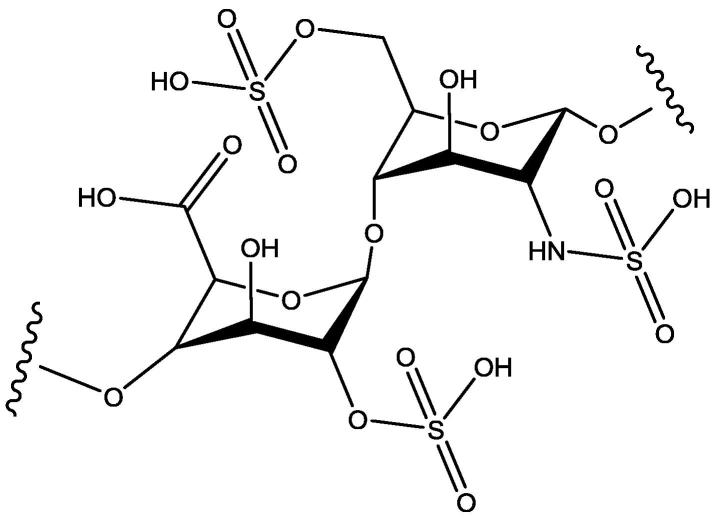
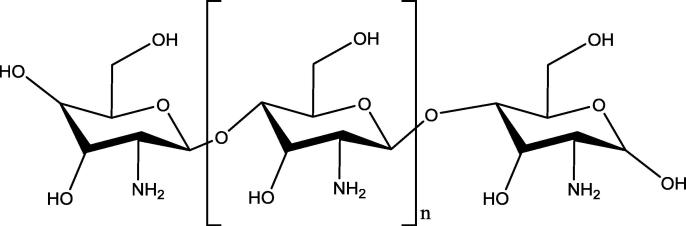
Many drugs have been developed for the treatment of medical conditions related to blood coagulation process, including saccharides like heparin (Fig. 1) and fondaparinux. However, anticoagulation therapies with these drugs need continuous monitoring to avoid bleeding side effects and the allergic adverse events (Franchini et al., 2016). Anticoagulants are clinically used in different medical conditions like thrombosis and have the maximum annual growth rate among the top ten treatment area. This is because of the increased elderly population and the increased risks of cardiovascular events (Fan et al., 2018). This prompts the researchers to strive to develop new and better anticoagulants. Chitosan (Fig. 2) is a natural polyaminosaccharide with a non-toxic, non-allergenic, biocompatible, and biodegradable characteristic. These properties of chitosan make it a good pharmaceutical excipient and a carrier for the preparation of prodrugs, as an accelerator of wound healing, and as a physiological substance due to its anticancer, antimicrobial, immune-modulator, anti-cholesterolemic, and antioxidant properties (Mustafa et al., 2015, Szymańska and Winnicka, 2015, Yadu et al., 2017, Cheung et al., 2015, Ngo and Kim, 2014, Szymańska and Winnicka, 2012, Vladimir et al., 2006, Avadi et al., 2004). Also, chitosan is a polymer having a primary amino group, which is a possible site for substitution in the chitosan and N-alkyl chitosan had previously been reported to have antibacterial activity (Avadi et al., 2004). The chitosan has a similar close structure like heparin (Heise et al., 2018). Based on this feature, many chitosan derivatives as anticoagulants have been synthesized (Vongchan et al., 2002, Vonghan et al., 2003, Vikhoreva et al., 2005, Huang et al., 2003, Xiong et al., 2011, Baumann and Faust, 2001, Shagdarova et al., 2016, Subhapradha et al., 2013, Yang et al., 2013, Drozd et al., 2019, Song et al., 2018, Ouerghemmi et al., 2018, Drozd et al., 2017, Skorik et al., 2017, Park et al., 2004, Ragab et al., 2018). Recently (Heise et al., 2018), it has been stated that incorporation of the sulfate groups in the structure of chitosan improve its compatibility with blood and increase its anticoagulation effects by its complexing ability with the blood. This makes the sulfonated chitosan derivatives a good template to develop newer anticoagulants. Encouraged by the anticoagulant potential of the sulfonated chitosan derivatives, the authors aimed to synthesize sulfonated chitosan derivatives (Fig. 3) having expected anticoagulant activity (see Table 1).
Fig. 1.
Heparin.
Fig. 2.
Chitosan.
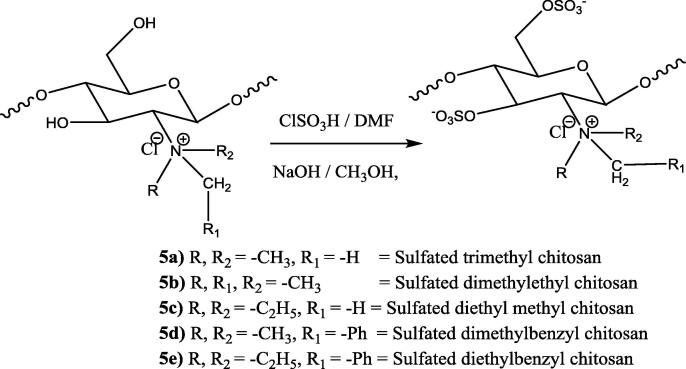
Fig. 3.
General structure of the synthesized sulfonated chitosan derivatives.
Table 1.
The general structure of N-alkyl/aryl chitosan derivatives.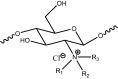
| Compounds | Code | R1 | R2 | R3 | %Yield |
|---|---|---|---|---|---|
| Chitosan | – | H | H | H | – |
| Methyl Chitosan | 1a | CH3 | H | H | 88% |
| Ethyl Chitosan | 1b | C2H5 | H | H | 80% |
| Benzyl Chitosan | 1c | CH2Ph | H | H | 85% |
| Trimethyl Chitosan | 2a | CH3 | CH3 | CH3 | 90% |
| Diethyl Methyl Chitosan | 2b | CH3 | C2H5 | C2H5 | 92% |
| Dimethyl Ethyl Chitosan | 2c | C2H5 | CH3 | CH3 | 86% |
| Dimethyl Benzyl Chitosan | 2d | CH2Ph | CH3 | CH3 | 83% |
| Diethyl Benzyl Chitosan | 2e | CH2Ph | C2H5 | C2H5 | 91% |
2. Experimental
2.1. Materials and methods
Chitosan was a gift from the Central Marine Fisheries Research Institute. All the chemicals were obtained from Central Drug House (CDH), SD Fine and Rankem, India. Synthetic work for the derivatization of the chitosan was carried in the following steps:
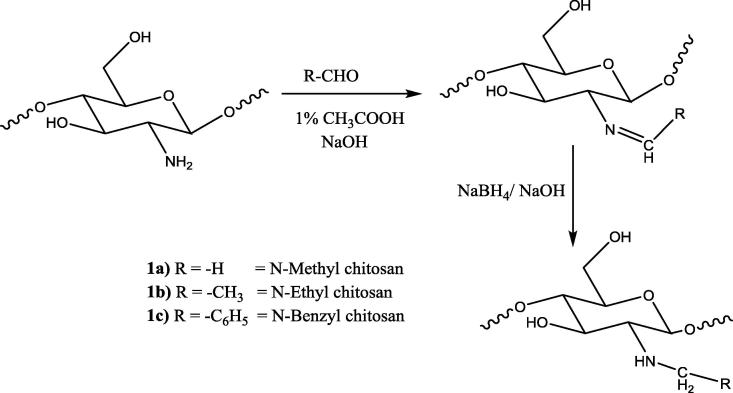
2.2. Synthesis of N-alkyl/aryl chitosan (1a-c)
Synthesis of N-alkyl/aryl chitosan was two-step reaction procedure. The first step of N-alkyl/aryl chitosan synthesis involved Schiff base formation as an intermediate. In the second step, the reduction of the Schiff base (R—CH N—) provided N-alkyl/aryl chitosan. The N-methyl chitosan (1a) was prepared by the following procedure. Chitosan (500 mg) was dissolved in 20 ml of 1% acetic acid solution (pH = 3.8–3.9), and 5 ml of formaldehyde (37%) was added to the solution. The pH of the solution was adjusted to 4.5 with 5% sodium hydroxide solution after 1 h stirring. Sodium borohydride solution (10%, 2 ml) was added for the reduction of the Schiff base, and the mixture was stirred for 1.5 h. A precipitate of N-methyl chitosan was obtained after adding 1 M sodium hydroxide solution by adjusting the pH to 10. The precipitate was washed with distilled water and dried in an oven at 60 °C. The N-ethylchitosan (1b), and N-benzylchitosan (1c) were prepared with similar reaction conditions using acetaldehyde and benzaldehyde, respectively (Avadi et al., 2004).
2.3. Quaternization of N-alkyl/aryl chitosan (2a-e)
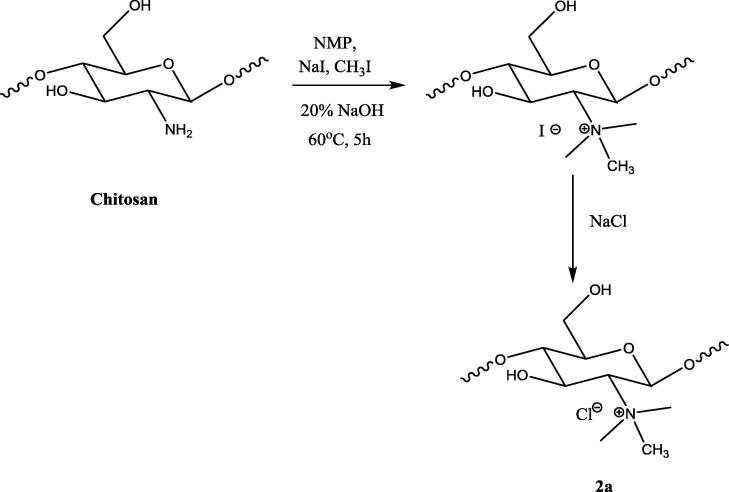
2.3.1. Synthesis of N-trimethyl chitosan (2a)
Synthesis of trimethyl chitosan was one step reaction procedure. The chitosan was alkylated with methyl iodide to obtain N-trimethyl chitosan. This reaction was based on the principle of nucleophilic substitution reaction, wherein the amine protons were substituted with the methyl group of the methyl iodide in the presence of sodium iodide in basic water/NMP medium. Chitosan (750 mg) was dispersed in 8 ml of N-Methyl-2-pyrrolidone (NMP) and stirred for 4 h. Sodium hydroxide (20% w/v, 1 ml), 3 ml of methyl iodide and 400 mg of sodium iodide were added to the dispersion. The reaction mixture was stirred for 5 h at 60 °C. The precipitate of N-trimethyl chitosan iodide was obtained by the addition of acetone. The I− salt was converted to the Cl- salt by adding 10% NaCl. The Cl- salt was isolated and dried at 60 °C.
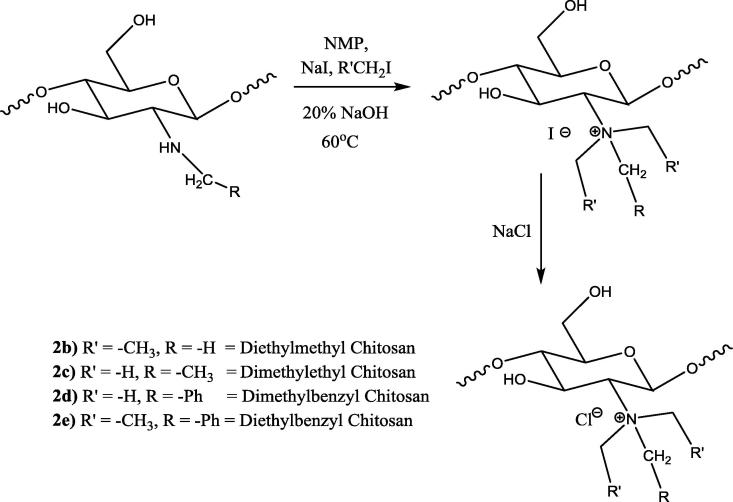
2.3.2. Synthesis of dimethyl ethyl chitosan (2b)
N-ethyl chitosan (300 mg) was dispersed in a water/NMP solvent system (20 ml). Methyl iodide (3 ml) and sodium iodide (0.5 g) were added, and the mixture was stirred for four hours at 60 °C. A precipitate of dimethyl ethyl chitosan iodide was obtained by adding acetone, which was converted to the Cl- salt by adding 10% NaCl. The Cl- salt was isolated and dried at 60 °C.
2.3.3. Synthesis of diethyl methyl chitosan (2c)
N-methyl chitosan (200 mg) was dispersed in NMP (20 ml) and stirred for 4 h. Sodium hydroxide (20% w/v, 1 ml), ethyl iodide (3 ml) and sodium iodide (400 mg) were added to the dispersion. The mixture was stirred for 5 h at 60 °C. A precipitate of diethyl methyl chitosan iodide was obtained by adding acetone, which was converted to Cl- salt using 10% NaCl. The Cl- salt was isolated and dried at 60 °C.
2.3.4. Synthesis of DialkylBenzyl chitosan (2d & 2e)
The dimethylbenzyl chitosan (2d) and diethyl benzyl chitosan (2e) were prepared by reacting N-benzylchitosan with methyl iodide and ethyl iodide, respectively, by following the same procedure of dimethyl ethyl chitosan (2b) and diethyl methyl chitosan (2c).
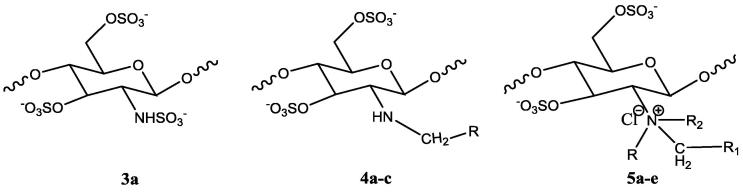
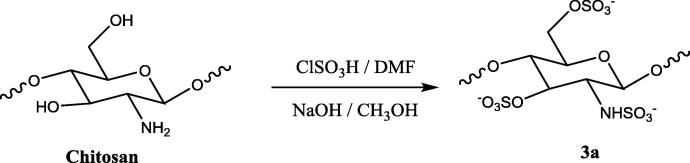
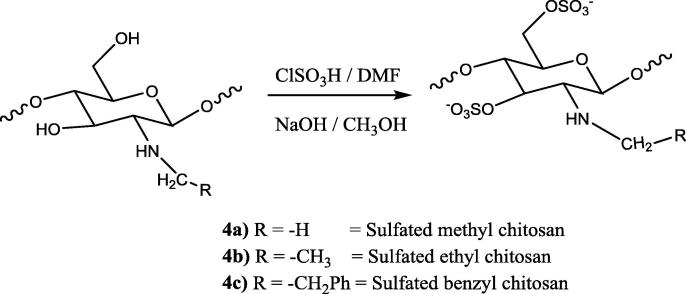
2.4. Sulfonation of chitosan and chitosan derivatives (3a, 4a-c & 5a-e)
Synthesis of sulfated chitosan and its derivatives was one step reaction procedure. The mechanism of the reaction was the electrophilic substitution reaction in which proton was substituted by —SO3H group (Morrison and Boyd. 1997). All the quaternary derivatives of chitosan and the chitosan were sulfonated with chlorosulfonic acid. Chitosan or chitosan derivatives (1 g) were dispersed in dimethylformamide (DMF). The sulfonating complex (4.5 ml chlorosulphonic acid in 30 ml of DMF) was added fractionally, and the mixture was stirred at room temperature for 5 h. The reaction mixture was neutralized with 20% NaOH solution, wherein a precipitate of sulfated chitosan was obtained. Sulfated chitosan and sulphated chitosan derivatives were dissolved in water and reprecipitated with methanol twice (Cristiane, 2003).
3. Spectroscopic evaluation
3.1. Chitosan sulfate (3a)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (2° amine); 1H NMR (500 MHz, DMSO‑d6/CDCl3) δ in ppm: 2.1 (—NH, singlet), 2.6 (CH at 1 position, 3-CH at C-1 & C-3 position), 5.4–5.5 (—CH at C-2 & C-4).
3.2. Methyl chitosan sulfate (4a)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (2° amine), 2960 (—CH3 group); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.1 (—NH attached at C-3 position), 2.5 (—CH3 at —NH group), 3.0 (CH at C-1 and C-3 position), 3.5 (—CH2 at C-6 position side chain), 4.2 (—CH at C-1).
3.3. Ethyl chitosan sulfate (4b)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (2° amine), 2960 (—CH3 group), 2862 (—CH2 group); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.1 (—NH attached at C-3 position), 3.0 (CH at C-1 and C-3 position), 3.5 (—CH2 at C-6 position side chain), 4.2 (—CH at C-1).
3.4. Benzyl chitosan sulfate (4c)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (2° amine), 2862 (—CH2 group), 3100 (Aromatic ring); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.0 (—NH at C-3 position), 3.0 (—CH at C-1 and C-3 position), 3.9 (—CH2 at NHCH2Ph & C-6 CH2), 5.5 & 5.6 (—CH at C-2 & C-4 position), 7.1 (—CH of benzene).
3.5. Trimethyl chitosan sulfate (5a)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (Disappear due to quternatisation), 2960 (—CH3 group), 3100 (aromatic CH str), 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.0 (Due to un-quaternized amino group), 2.9 (—CH at C-1 position), 3.1 (—CH3 of quaternary amino group), 3.6 (—CH2 at C-6 side chain), 4.1 (—CH at C-6 and C-3), 5.4–5.5 (—CH at C-2 and C-4).
3.6. Dimethyl ethyl chitosan sulfate (5b)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (Disappear due to quternatisation), 2960 (—CH3 group); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 1.0 (—CH2CH3 from quaternary amino group), 2.9 (due to un-quaternized amino group), 3.0 (—CH2CH3 and CH3 at C6 side chain), 3.6 (—CH2 at C-6 side chain), 4.0 (CH at C-6 and C-3), 5.5–5.6 (—CH at C-2 & C-4).
3.7. Diethyl methyl chitosan sulfate (5c)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (Disappear due to quternatisation), 2960 (—CH3 group), 2862 (—CH2 group); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 1.0 (CH2CH3 from quaternary amino group), 2.0 (due to un-quaternized amino group), 3.1 (CH2 at C-6 side chain), 3.5 (—CH2 at C-6 side chain), 4.0 (C-6 and C-3), 5.5–5.6 (—CH at C-2 & C-4 position).
3.8. Dimethyl benzyl chitosan sulfate (5d)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (Disappear due to quternatisation), 2960 (—CH3 group), 3100 (aromatic str); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.0 (Due to un-quaternized amino group), 3.0 (—CH3 at a quaternary amino group), 3.3 (—CH2 at the C-6 side chain), 4.5 (-CH2Ph), 5.4–5.5 (—CH at C-2 & C-4), 7.1 (—CH from the benzene ring).
3.9. Diethyl benzyl chitosan sulfate (5e)
IR (KBr) ν max/cm−1: 872 & 1140 (Saccharide structure), 1245 (—N—C str), 810 (—C—O—S str), 1240 (—S O), 3350 (Disappear due to quternatisation), 2960(—CH3 group), 2862 (—CH2 group); 1H NMR (500 MHz, DMSO‑d6/CDCl3): 2.0 (due to unquaternized amino group), 3.0 (—CH2 at C-6 side chain), 5.4–5.5 (—CH at C-2 and C-4), 7.1 (—CH from benzene ring).
4. Animals and experimental procedure
Mature Wistar rats weighing 150–250 g were procured from Laboratory Animal Resource, Division of Animal Genetics, IVRI, Izatnagar, (Reg. No. CPC–196) and acclimatized to laboratory condition at Animal House of IFTM, Moradabad (India) at room temperature (24 ± 2 °C) with a 12 h/12 h/light/dark cycle and 70% relative humidity). All rats were treated following the guideline for the Care and Use of Laboratory Animals (NIH Publication No.86-23, revised 1985) with the permission of the institutional ethical committee (Proposal No.11). The animals were kept in polypropylene cages and maintained on a balanced ration provided by the Feed Technology Unit, Division of Animal Nutrition, IVRI, Izzatnagar, India.
4.1. Tail bleeding method
For the pharmacological study, 55 animals were divided into 11 groups and each group comprised of five rats. Following treatment was given to each group of animals.
Group I: Distilled water (Normal control) was given.
Group II: Standard drug (Nicoumalone) was given at 10 mg/ml.
Group III: Sulfated chitosan (3a) was given at 10 mg/ml.
Group IV: Sulfated methyl chitosan (4a) was given at 10 mg/ml.
Group V: Sulfated ethyl chitosan (4b) was given at 10 mg/ml.
Group VI: Sulfated benzylchitosan (4c) was given at 10 mg/ml.
Group VII: Sulfated trimethyl chitosan (5a) was given at 10 mg/ml.
Group VIII: Sulfated dimethyl ethyl chitosan (5b) was given at 10 mg/ml.
Group IX: Sulfated diethyl methyl chitosan (5c) was given at 10 mg/ml.
Group X: Sulfated dimethyl benzyl chitosan (5d) was given at 10 mg/ml.
Group XI: Sulfated diethyl benzyl chitosan (5e) was given at 10 mg/ml.
The hemorrhagic activity was measured by the tail bleeding model (Saito et al., 2016). The total bleeding time is defined as the sum of the duration of all the bleeding episodes from the injection of the drug until termination of the study. Blood loss was determined by the measurement of the accumulated amount of haemoglobin in the saline from the time of injection of the drug. The haemoglobin measurement was performed by the addition of haemoglobin reagent, and the resulting cyanomethhemoglobin was measured spectrophotometrically at 540 nm. Haemoglobin content, clotting time and the total bleeding time were measured after 1 h and 3 h of the treatment. Data were analyzed by comparing with the control and standard drug (Nicoumalone). Clotting time was measured in open capillary (Hoover-Plow et al., 2006). The SPSS software was used to determine statistically significant values (p-value < 0.05).
5. Results
The structures of the synthesized chitosan derivatives (Scheme 1, Scheme 2a, Scheme 2b, Scheme 3a, Scheme 3b, Scheme 3c, Scheme 1, Scheme 2a, Scheme 2b, Scheme 3a, Scheme 3b, Scheme 3c) were established based on their FTIR and 1H NMR analysis, which is provided in the experimental section of these compounds. Table 2 provides the clotting time, bleeding time, and the Hb content analysis of the sulphated chitosan derivatives (3a, 4a-c, 5a-e), and the standard drug (nicoumalone).
Scheme 1.
Synthesis of N-alkyl/aryl chitosan derivatives (1a-c).
Scheme 2a.
Synthesis of N-trimethyl chitosan (2a).
Scheme 2b.
Synthesis of quaternized N-alkyl/aryl chitosan derivatives (2b-e).
Scheme 3a.
Synthesis of sulfated chitosan (3a).
Scheme 3b.
Synthesis of N-alkyl/aryl sulphated chitosan derivatives (4a-c).
Scheme 3c.
Synthesis of quaternized N-alkyl/aryl sulphated chitosan derivatives (5a-e).
Table 2.
Clotting time, bleeding time, and the Hb content of the sulphated chitosan derivatives (3a, 4a-c, 5a-e).
| Treatment | Clotting time in sec |
Bleeding time in sec |
Absorbance |
|||
|---|---|---|---|---|---|---|
| After 1 h (% Activity#) |
After 3 h (% Activity#) |
After 1 h (% Activity#) |
After 3 h (% Activity#) |
After 1 h (% Absorbance) |
After 3 h (% Absorbance) |
|
| Control | 69.33 ± 5.31* (100%) |
67.33 ± 7.02* (100%) |
190.50 ± 6.70* (100%) |
188.66 ± 8.71* (100%) |
1.17 ± 0.067* (100%) | 1.18 ± 0.058* (100%) |
| Nicoumalone | 98.66 ± 6.31* (142.30%) |
133.16 ± 5.67* (197.77%) |
225.67 ± 5.83* (118.46%) |
283.50 ± 7.42* (150.27%) |
1.24 ± 0.052* (105.9%) |
1.79 ± 0.075* (151.69%) |
| 3a | 102.30 ± 6.31* (147.55%) |
85.66 ± 5.23* (127.22%) |
236.66 ± 5.68* (124.23%) |
232.66 ± 6.50* (123.32%) |
1.34 ± 0.063* (114.52%) |
1.23 ± 0.073* (104.23%) |
| 4a | 116.66 ± 5.04* (168.26%) |
102.6 ± 6.12* (152.38%) |
261.50 ± 6.33* (137.27%) |
250.83 ± 7.35* (132.95%) |
1.59 ± 0.067* (135.89%) |
1.51 ± 0.055* (127.96%) |
| 4b | 117.50 ± 5.68* (169.47%) |
105.0 ± 7.23* (155.94%) |
275.50 ± 4.98* (144.61%) |
262.66 ± 6.77* (139.22%) |
1.75 ± 0.070* (149.57%) |
1.56 ± 0.066* (132.20%) |
| 4c | 123.66 ± 3.77* (178.36%) |
110.83 ± 6.5* (164.60%) |
277.50 ± 5.23* (145.66%) |
265.33 ± 4.63* (140.63%) |
1.82 ± 0.056* (155.55%) |
1.72 ± 0.048* (145.76%) |
| 5a | 104.33 ± 5.03* (150.48%) |
94.33 ± 5.34* (140.10%) |
240.67 ± 6.12* (126.33%) |
210.66 ± 7.12* (111.66%) |
1.28 ± 0.040* (109.40%) |
1.22 ± 0.06* (103.38%) |
| 5b | 105.30 ± 5.24* (151.88%) |
95.83 ± 6.23* (142.32%) |
246.50 ± 5.35* (129.39%) |
218.66 ± 5.70* (115.90%) |
1.38 ± 0.046* (117.94%) |
1.30 ± 0.055* (110.16%) |
| 5c | 110.30 ± 4.85* (159.09%) |
98.16 ± 6.51* (145.78%) |
248.33 ± 5.68* (130.35%) |
224.66 ± 5.71* (119.08%) |
1.42 ± 0.053* (121.36%) |
1.39 ± 0.054* (117.79%) |
| 5d | 112.0 ± 4.24* (161.54%) |
100.83 ± 7.11* (149.75%) |
255.67 ± 5.90* (134.20%) |
240.33 ± 5.64* (127.38%) |
1.44 ± 0.046* (123.07%) |
1.41 ± 0.067* (119.49%) |
| 5e | 116.30 ± 5.46* (167.74%) |
103.50 ± 5.98* (153.72%) |
258.30 ± 6.50* (135.59%) |
249.30 ± 5.60* (132.14%) |
1.45 ± 0.052* (123.93%) |
1.46 ± 0.054* (123.72%) |
p < 0.0.
% Activity with respect to control.
6. Discussion
The sulphated polysaccharides possess varied physiological effects. Heparin is one of the best examples due to its medical application as an anticoagulant. Heparin exerts its anticoagulant effect by its sulphated pentasaccharide moiety, which is supposed to inhibit factor Xa (Rabenstein, 2002, Weitz and Weitz, 2010). Chitosan has structural similarity with heparin and fondaparinux. The incorporation of the sulfate groups in the structure of chitosan improves its compatibility with blood by its complexing ability with the blood components (Heise et al., 2018). The anticoagulant activity of the chitosan and its derivatives is also greatly influenced by many factors such as degree of sulfonation (Xiong et al., 2011, Shagdarova et al., 2016, Subhapradha et al., 2013, Yang et al., 2013, Drozd et al., 2019, Song et al., 2018, Ouerghemmi et al., 2018, Drozd et al., 2017, Skorik et al., 2017, Ragab et al., 2018). Further, the N-alkylation of chitosan to primary or secondary chitosan derivatives and finally to quaternary chitosan derivatives increase the water solubility of the chitosan derivatives (Mustafa et al., 2015, Szymańska and Winnicka, 2015, Yadu et al., 2017, Cheung et al., 2015, Avadi et al., 2004). Based on the literature, the authors developed newer chitosan derivatives as anticoagulants. The titled chitosan derivatives (3a, 4a-c, and 5a-e) were prepared by the processes described in Scheme 1, Scheme 2a, Scheme 2b, Scheme 3a, Scheme 3b, Scheme 3c, Scheme 1, Scheme 2a, Scheme 2b, Scheme 3a, Scheme 3b, Scheme 3c. These compounds were characterized based on their FTIR and 1H NMR analysis. Results from the tail bleeding method of the sulphated chitosan derivatives showed that the sulphates of the chitosan have anticoagulant activity. The synthesized chitosan derivatives are supposed to bear similar anticoagulant mechanism of action like heparin and fondaparinux due to similarity in their structures (Rabenstein, 2002, Weitz and Weitz, 2010). Table 2 mentions the clotting time, bleeding time, and the Hb content of standard (nicoumalone) and the synthesized chitosan derivatives. The clotting time, bleeding time, and the Hb content of nicoumalone taken in the experiment increased at 3 h at its peak. This indicates the slow onset of action and longer duration of action of nicoumalone in comparison to the synthesized compounds. From the results of the clotting time, bleeding time and the Hb content analysis, it is evident that the chitosan sulfate (3a), secondary amino chitosan sulphates (4a-c), and the quaternary chitosan sulfates (5a-e) caused a remarkable and better increment of the clotting time, bleeding time, and the Hb content than nicoumalone after one hour of the drug administration. The order of potency of the compounds followed the pattern as 4c > 4b > 4a > 5e > 5d > 5c > 5b > 5a > 3a > nicoumalone after one hour of the drug administration. This indicated that all the synthesized derivatives had a faster onset of action and potency than nicoumalone. This may be because of the expected higher water solubility of the synthesized derivative because of the presence of sulphate group and the quaternary ammonium group (Heise et al., 2018, Shagdarova et al., 2016, Drozd et al., 2019, Song et al., 2018). However, after 3 h of drug administration the potency of compounds was less than nicoumalone. This indicated that the duration of action of the synthesized compounds was less than nicoumalone. This may be because the synthesized compounds have more prevalent hydrophilic groups and less prevalent hydrophobic groups. The prevalence of the hydrophilic groups might be responsible for the increased rate of elimination of these compounds. Among the quaternary chitosan derivatives (5a-e), 5e was the most potent, and among the secondary amine chitosan derivatives (4a-c), 4c was most potent. This may be because of the presence of lipophilic phenyl moiety in their structure plus hydrophilic quaternary ammonium/sulfate groups, which might be providing a balance in the hydrophilic and the lipophilic character of 4c and 5e. The dimethylbenzyl chitosan sulphate (5d), and diethyl benzyl chitosan sulfate (5e) were more potent in comparison to other quaternary chitosan derivatives but were not more potent as secondary chitosan derivatives (4a-c). This may be because of the better complex-forming ability of the secondary chitosan derivatives (4a-c) with the blood components, for example, factor Xa (Heise et al., 2018). It is expected that the development of the similar compounds like 4b and 4c may provide some more fruitful anticoagulants having faster onset of action, potency, and longer duration of action by their complex-forming ability with the blood components, for example, factor Xa, and having a balanced hydrophilic/lipophilic character. Accordingly, further the work is in progress in our laboratories.
7. Conclusion
Sulfonation of the chitosan increases its anticoagulant activity. The N-alkyl derivatives of chitosan were more potent than its sulfated quaternary derivatives and the sulfated chitosan. Quaternization of the chitosan increase its water solubility but also decreases its anticoagulant effect. The better anticoagulation effects of the secondary sulfated chitosan derivatives (4a-c) might be attributed to their better complex-forming ability with the blood components, for example, factor Xa. Because of the biocompatible and non-allergenic nature of chitosan, it may provide an interesting template to provide heparin and fondaparinux like anticoagulants with better potency and lesser side effects. Special emphasis should be given to developing secondary sulfated chitosan derivatives rather than their sulfated quaternary derivatives.
Declaration of Competing Interest
The authors declared that there is no conflict of interest.
Acknowledgements
The authors are thankful to the Department of Pharmaceutical Chemistry, IFTM, University, Moradabad, Uttar Pradesh, India, Faculty of Pharmacy, Northern Border University, Rafha, Saudi Arabia, Department of Pharmacy, GRD (PG) IMT, Dehradun, India, Department of Pharmaceutical chemistry, Himalayan Institute of Pharmacy and Research, Dehradun, India for providing technical support and facilities to carry out this work.
Footnotes
Peer review under responsibility of King Saud University.
References
- Avadi M.R., Sadeghi A.M.M., Tahzibi A., Bayati K., Pouladzadeh M., Zohuriaan-Mehr M.J., Rafiee-Tehrani M. Diethylmethyl chitosan as an antimicrobial agent: Synthesis, characterization and antibacterial effects. Eur. Polym. J. 2004;40:1355–1361. doi: 10.1016/j.eurpolymj.2004.02.015. [DOI] [Google Scholar]
- Baumann H., Faust V. Concepts for improved regioselective placement of O-sulfo, N-sulfo, N-acetyl, and N-carboxymethyl groups in chitosan derivatives. Carbohydr. Res. 2001;331:43–57. doi: 10.1016/S0008-6215(01)00009-X. [DOI] [PubMed] [Google Scholar]
- Cheung R.C., Ng T.B., Wong J.H., Chan W.Y. Chitosan: an update on potential biomedical and pharmaceutical applications. Mar. Drugs. 2015;13:5156–5186. doi: 10.3390/md13085156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristiane R.M., Giacommo R., Marco A.D.P. Synthesis in pilot plant scale and physical properties of sulfonate polystyrene. J. Braz. Chem. Soc. 2003;14:797–802. doi: 10.1590/S0103-50532003000500015. [DOI] [Google Scholar]
- Drozd N.N., Logvinova Y.S., Shagdarova B.T., Il'ina A.V., Varlamov V.P. Analysis of the action of quaternized chitosans with different molecular weight on anticoagulant activity of heparins in vitro. Bull. Exp. Biol. Med. 2019;167:279–283. doi: 10.1007/s10517-019-04509-w. [DOI] [PubMed] [Google Scholar]
- Drozd N.N., Shagdarova B.T., Il'ina A.V., Varlamov V.P. Effects of Chitosan Derivative N-[(2-Hydroxy-3-Trimethylammonium)Propyl]Chloride on Anticoagulant Activity of Guinea Pig Plasma. Bull. Exp. Biol. Med. 2017;163:340–343. doi: 10.1007/s10517-017-3799-6. [DOI] [PubMed] [Google Scholar]
- Fan P., Gao Y., Zheng M., Xu T., Schoenhagen P., Jin Z. Recent progress and market analysis of anticoagulant drugs. J. Thorac. Dis. 2018;10:2011–2025. doi: 10.21037/jtd.2018.03.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franchini M., Liumbruno G.M., Bonfanti C., Lippi G. The evolution of anticoagulant therapy. Blood Transfus. 2016;14:175–184. doi: 10.2450/2015.0096-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heise K., Hobisch M., Sacarescu L., Maver U., Hobisch J., Reichelt T., Sega M., Fischer S., Spirk S. Low-molecular-weight sulfonated chitosan as template for anticoagulant nanoparticles. Int. J. Nanomed. 2018;13:4881–4894. doi: 10.2147/IJN.S172230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoover-Plow J., Shchurin A., Hart E., Sha J., Hill A.E., Singer J.B., Nadeau J.H. Genetic background determines responses to hemostats and thrombosis. BMC Blood Disord. 2006;6:1–6. doi: 10.1186/1471-2326-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang R., Du Y., Yang J., Hong L. Influence of functional groups on the in vitro anticoagulant activity of chitosan sulfate. Carbohydr. Res. 2003;338:483–489. doi: 10.1016/S0008-6215(02)00505-0. [DOI] [PubMed] [Google Scholar]
- Morrison, R.T., Boyd, R.N., 1997. Organic chemistry, Sixth ed. Prentice-Hall, pp. 517–527.
- Mustafa A., Cadar E., Sîrbu R. Pharmaceutical Uses of Chitosan in the Medical Field. Eur. J. Interdisciplin. Studies. 2015;1:35–40. doi: 10.26417/ejis.v3i1.p35-40. [DOI] [Google Scholar]
- Ngo D.H., Kim S.K. In: Kim S.K., editor. Vol. 73. Research. Academic Press; Waltham, MA, USA: 2014. Antioxidant effects of chitin, chitosan, and their derivatives; pp. 15–31. (Advances in Food and Nutrition). [DOI] [PubMed] [Google Scholar]
- Ouerghemmi S., Dimassi S., Tabary N., Leclercq L., Degoutin S., Chai F., Pierlot C., Cazaux F., Ung A., Staelens J.N., Blanchemain N., Martel B. Synthesis and characterization of polyampholytic aryl-sulfonated chitosans and their in vitro anticoagulant activity. Carbohydr. Polym. 2018;196:8–17. doi: 10.1016/j.carbpol.2018.05.025. [DOI] [PubMed] [Google Scholar]
- Park P.J., Je J.Y., Jung W.K., Ahn C.B., Kim S.K. Anticoagulant activity of heterochitosan and their oligosaccharides sulfates. Eur. Food Res. & Technol. 2004;219:529–533. doi: 10.1007/s00217-004-0977-3. [DOI] [Google Scholar]
- Rabenstein D.L. Heparin and heparan sulfate: structure and function. Nat. Prod. Rep. 2002;19:312–331. doi: 10.1039/B100916H. [DOI] [PubMed] [Google Scholar]
- Ragab T., El-Bassyouni G.T., Helmy W., Taie H., Refaat A., Ibrahim M.A., Abd El-Hmeed E., Esawy M.A. Evaluation of multifunction bioactivities of extracted chitosan and their UV/ozone derivatives. J. App. Pharm. Sci. 2018;8:053–062. doi: 10.7324/JAPS.2018.81008. [DOI] [Google Scholar]
- Saito M.S., Lourenco A.L., Kang H.C., Rodrigues C.R., Cabral L.M., Castro H.C., Satlher P.C. New approaches in tail-bleeding assay in mice: improving an important method for designing new anti-thrombotic agents. Inter. J. Exp. Pathol. 2016;97:285–292. doi: 10.1111/iep.12182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shagdarova B.T., Drozd N.N., Il’ina A.V., Logvinova Y.S., Varlamov V.P. Neutralization of anticoagulant activity of heparin by N-[(2-hydroxy-3-trimethylammonium) propyl] chloride derivatives of chitosan. Appl. Biochem. Microbiol. 2016;52:445–451. doi: 10.1134/S0003683816040141. [DOI] [PubMed] [Google Scholar]
- Skorik Y.A., Kritchenkov A.S., Moskalenko Y.E., Golyshev A.A., Raik S.V., Whaley A.K., Vasina L.V., Sonin D.L. Synthesis of N-succinyl- and N-glutaryl-chitosan derivatives and their antioxidant, antiplatelet, and anticoagulant activity. Carbohydr. Polym. 2017;166:166–172. doi: 10.1016/j.carbpol.2017.02.097. [DOI] [PubMed] [Google Scholar]
- Song W., Zeng Q., Yin X., Zhu L., Gong T., Pan C. Preparation and anticoagulant properties of heparin-like electrospun membranes from carboxymethyl chitosan and bacterial cellulose sulfate. Int. J. Biol. Macromol. 2018;120:1396–1405. doi: 10.1016/j.ijbiomac.2018.09.133. [DOI] [PubMed] [Google Scholar]
- Subhapradha N., Suman S., Ramasamy P., Saravanan R., Shanmugam V., Srinivasan A., Shanmugam A. Anticoagulant and antioxidant activity of sulfated chitosan from the shell of donacid clam Donax scortum (Linnaeus, 1758) Int. J. Nutr. Pharmacol. Neurol. Dis. 2013;3:39–45. http://www.ijnpnd.com/text.asp?2013/3/1/39/106990 [Google Scholar]
- Szymańska E., Winnicka K. Preparation and in vitro evaluation of chitosan microgranules with clotrimazole. Acta Pol. Pharm. Drug Res. 2012;69:509–513. https://www.ptfarm.pl/pub/File/Acta_Poloniae/2012/3/509.pdf [PubMed] [Google Scholar]
- Szymańska E., Winnicka K. Stability of chitosan-a challenge for pharmaceutical and biomedical applications. Mar. Drugs. 2015;13:1819–1846. doi: 10.3390/md13041819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vikhoreva G., Bannikova G., Stolbushkina P., Panov A., Drozd N., Makarov V., Varlamov V., Galbraikh L. Preparation and anticoagulant activity of low molecular weight sulfated chitosan. Carbohydr, Poly. 2005;62:327–332. doi: 10.1016/j.carbpol.2005.05.022. [DOI] [Google Scholar]
- Vladimir E.T., Evgeniya A.S., Valery G.B., Igor A.Y., Javier P.G., Hans-Börje J., Luis V.L.L., Jesus S., Denis V.G., Inna D.A., Valery P.V. Bactericidal and antifungal activities of a low molecular weight chitosan and its N-/2(3)-(dodec-2-enyl)succinoyl/-derivatives. Carbohydr. Poly. 2006;64:66–72. doi: 10.1016/j.carbpol.2005.10.021. [DOI] [Google Scholar]
- Vongchan P., Sajomsang W., Subyen D., Kongtawelart P. Anticoagulant activity of sulfated chitosan. Carbohydr. Res. 2002;337:1239–1242. doi: 10.1016/S0008-6215(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Vonghan P., Sajomsang W., Kasinrerk W., Subyen D., Kongtawelart P., Charoensatapron Anticoagulant activities of the chitosan poly sulfate synthesized from marine crab shell by semi heterogenous condition. Sci. Asia. 2003;29:115–120. doi: 10.2306/scienceasia1513-1874.2003.29.115. [DOI] [Google Scholar]
- Weitz D.S., Weitz J.I. Update on heparin: what do we need to know? J. Thromb. Thrombolysis. 2010;29:199–207. doi: 10.1007/s11239-009-0411-6. [DOI] [PubMed] [Google Scholar]
- Xiong W.Y., Yi Y., Liu H.Z., Wang H., Liu J.H., Ying G.Q. Selective carboxypropionylation of chitosan: synthesis, characterization, blood compatibility, and degradation. Carbohydr. Res. 2011;346:1217–1223. doi: 10.1016/j.carres.2011.03.037. [DOI] [PubMed] [Google Scholar]
- Yadu, N.V.K., Raghvendrakumar, M., Aswathy, V., Parvathy, P., Sunija, S., Neelakandan, M.S., Nitheesha, S., Vishnu, K.A., 2017. Chitosan as promising materials for biomedical application. Review. Res. Dev. Mat. Sci. 2, RDMS.000543. doi: 10.31031/RDMS.2017.02.000543.
- Yang J., Luo K., Li D., Yu S., Cai J., Chen L., Du Y. Preparation and characterization and in vitro anticoagulant activity of highly sulfated chitosan. Inter. J. Biol. Macromol. 2013;52:25–31. doi: 10.1016/j.ijbiomac.2012.09.027. [DOI] [PubMed] [Google Scholar]