Abstract
Acute or chronic wounds are one of the most common health problems worldwide and medicinal drugs or traditional remedies are often used in wound healing. Further studies regarding wound treatment are rapidly continuing. Vitexin is a phenolic compound, which is found in many medicinal plants, has different pharmacological effects such as anti-inflammatory, analgesic and antioxidant. In the present study, it is aimed to investigate the wound healing effect of formulation prepared as chitosan-based gel with vitexin in vivo and in vitro. Cytotoxicity and wound healing assays were used for in vitro and excisional wound model is used for in vivo studies. Extracted tissues from wound area were histologically examined. Wound healing process was monitored on 7, 14 and 21st days. When wound construction was evaluated, chitosan-based gel formulation containing vitexin demonstrated significant effect compared to control group. Histological examinations demonstrated that skin regeneration was promoted by vitexin formulation. Significant cell proliferation was observed with vitexin/chitosan dispersion in the wound healing assay performed with NIH 3T3 and HaCaT cells. In conclusion, our test substance chitosan-based gel formulation containing vitexin significantly accelerated wound healing both in vivo and in vitro.
Keywords: Vitexin, Chitosan, Wound healing assay, Excisional wound model
1. Introduction
Skin is a protective barrier for the human body against the environmental attacks such as infections, injuries and incisions. A wound can be described as the deterioration of anatomical and functional integrity of the epithelial tissue of the skin. Acute, chronic, dry or infected wound types may develop due to various physical and mechanical factors, or different reasons such as animal bites, sharp object injuries and trauma (Mekonnen et al., 2013, Dreifke et al., 2015). Wound healing has various processes such as coagulation, inflammation, collagen production, and epithelial formation. After the platelets contact with collagen in wound area, platelets release clotting factor and growth factors. After that, neutrophils migrate to the wound site and remove the microbes from damaged tissue. The macrophages are also contributing to this phase. Microorganisms can cause the release of pro-inflammatory cytokines such as interleukin-1 and TNF-alfa in inflammatory phase. If this situation persists for a long time, the wound may enter a chronic state and fail to heal angiogenesis, granulation tissue production, collagen deposition, and epithelialization occur in the proliferative phase. The final stage of wound healing is maturation. In this phase, collagen cross-linking, remodeling, and wound contraction occur (Lodhi and Singhai, 2013, Lodhi et al., 2016, Gonzalez et al., 2016).
It is known that some plants or ingredients which affect one or more of these stages, especially topically, are used for wound care in folk medicine (Demirezer, 2007, Działo et al., 2016). Medical plants contain different biologically active compounds most of which are known as flavonoids. Flavonoids are a large group of phenolic compounds which are widely distributed in the plant kingdom. Vitexin (8-β-D-glucopyranosyl-apigenin), a C-glycosylated flavone, present in various medicinal and other plants such as Crataegus and Vitex spp., Passiflora spp., Pennisetum glaucum R. Br., Phyllostachys nigra, Munro (bamboo leaves), has showed different pharmacological activities including antinociceptive (Demir Özkay and Can, 2013, Zhu et al., 2016), anti-oxidant (Kim et al., 2005), antispasmodic (Gilani et al., 2006), anti-inflammatory (Borghi et al., 2013), antiviral, antibacterial (Aslam et al., 2015) and cardioprotective (Che et al., 2016) effects. It has been reported that vitexin could be inhibited productions of NO, TNF-alfa, and myeloperoxidase releasing from activated human peripheral blood neutrophils (Nikfarjam et al., 2017). And also, Borghi et al. (2013) have revealed that vitexin has free-radical scavenger ability and inhibited the production of hyperalgesic cytokines such as TNF-α, IL-1β, IL-6. These molecules cause the release of adhesion molecules, such as selectins and integrins, and thus affect many ways of tissue repair, such as angiogenesis and re-epithelization (Gonzalez, et al., 2016).
Chitosan is a biocompatible, biodegradable, non-irritant and non-toxic amino polysaccharide obtained by deacetylation of chitin. It is convenient for pharmaceutical and biomedical applications. It is informed that chitosan has antibacterial, anti-inflammatory and antioxidant effects besides tissue-adhesive property due to its polycationic nature and makes a good wound healing material (Hoemann et al., 2005, Aoyagi et al., 2007, Periayah et al., 2016). It was reported that simulation of the migration of polymorphonuclear as well as mononuclear cells and acceleration of the re-epithelization and normal skin regeneration is the mechanism of action of chitosan in tissue engineering (Fouda et al. 2009). Based on this information, it is thought that the combination of vitexin and chitosan, which have similar properties, in tissue repair will accelerate recovery. In the present study, it is aimed to investigate the wound healing effect of formulation prepared as chitosan-based gel with vitexin in vivo and in vitro.
2. Material and methods
2.1. Materials
Vitexin, chitosan (highly viscous;2-amino-2-deoxy-(14)-β-D-glucopyranan, high molecular weight with 800–2000 cPs viscosity values with> 75% deacetylated), acetic acid, Dulbecco's Modified Eagle's Medium (DMEM) Fetal Bovine Serum (FBS), penicillin– streptomycin (10,000 units penicillin and 10 mg streptomycin/mL), 0.25% (w/v) Trypsin-EDTA solution, Phosphate Buffered Saline (PBS), Thiazolyl Blue Tetrazolium Bromide (MTT) and Spectrophotometry-grade Dimethyl Sulfoxide (DMSO) were purchased from Sigma-Aldrich (Germany). CytoSelect™ 24-Well Wound Healing Assay was purchased from Cell Bioblas INC, USA.
2.2. Formulations
2.2.1. Preparation of the chitosan gel formulations
During the pre-formulation studies, blank formulations and drug loaded formulations were prepared. Gel formulations were prepared with three different chitosan concentrations (1.0% [R1]; 1.5% [R2]; 2% [R3] w/v). Briefly, chitosan was dissolved in acetic acid solution (0.5%, v/v) under a magnetic stirrer at 250 rpm for 4 h. For the drug loaded formulations, vitexin was briefly dissolved in 50 µL DMSO and then added up to the final concentration of 10 mg mL−1 of the transparent chitosan solution. Formulations were kept at room temperature overnight in well closed vials for the removal of air bubbles.
2.2.2. pH analyses
pH values of the formulations prepared were recorded by a WTW Profi Lab (pH 597, Weilheim, Germany) at 25 ± 1 °C. The pH values of all formulations were analyzed at the 0th time. Depending on the result of 4.1 ± 0.1 pH value, R1 formulation (chitosan-based gel formulation containing vitexin) was selected as the suitable formulation for in vivo studies. All analyses were repeated in triplicate. R1 and F1 (blank formulation-vitexin-free chitosan) formulations were kept at three different conditions (25 ± 1 ◦C, 4 ± 1 ◦C, and 40 ± 1 ◦C + 60% Relative Humidity (RH)) for better evaluation of the stability of the chitosan gel during the storage period of one-month (Değim et al., 2011, Yenilmez et al., 2015).
2.2.3. Rheological analyses
For better evaluation of the stability and spreadability capacity of the gel formulations prepared rheological behaviors of the formulations were determined by a cone plate rheometer (Brookfield RVDV-III + CP, Middleboro, USA). Briefly, about 0.5 g of the tested formula was applied to the plate and the measurements were carried out at 250 rpm at 25 ± 1 ◦C. Results were recorded only when the torque was within the acceptable range (10–100%) (Ruel-Gariepy et al., 2002, Yenilmez et al., 2015).
2.3. In vitro experiments
2.3.1. Cytotoxicity assay
The cytotoxicity assay was performed on NIH-3T3 mouse embryonic fibroblast cells by the MTT (3-(4, 5-dimethyl thiazol-2yl)-2, 5-diphenyl tetrazolium bromide) assay. Briefly, the cells in DMEM medium containing 10% FBS and 1% penicillin–streptomycin were seeded into 96 well plates and incubated at 37 °C for 24–48 h in a humidified atmosphere containing 5% CO2. When the desired cell confluence was reached, fresh medium which contained different concentrations of vitexin, chitosan and vitexin containing chitosan dispersion (R1) was added to the wells. At the end of the incubation time, the MTT dye was added and after re-incubation, formazan crystals were dissolved in DMSO. Absorbance of the wells was measured at 570 nm using a spectrometric microplate reader (BioTek Cytation 5, England). Results were expressed as the percentage of inhibition compared with control cells in which cell survival was presumed 100%. All cell viability assays were performed in triplicate (Gencer et al, 2010).
2.3.2. Wound healing assay
The CytoSelect ™ 24-Well Wound Healing Assay (Cat no: CBA 120) was used to test wound healing activity of vitexin in vitro. The study was performed according to the manufacturers' optimized protocols (http-1, 2019). Briefly, using sterile forceps, the desired number of inserts were placed in the plate wells with their “wound field” aligned in the same direction. The cell suspension containing 0.5–1.0 × 106 cells/ml in media containing 10% FBS was added to each well by carefully inserting the pipet tip through the open end at the top of the insert. Cells were cultured until they form a monolayer around the insert. The insert was removed, leaving about 0.9 mm open “wound field” between the cells. The study was performed in two plate for 0 and 72 h incubation. 0th time point indicated that the insert should be removed after 24 h of incubation. At this time vitexin, chitosan and vitexin/chitosan dispersion (R1) were added to second plate wells and incubated for 72 h. At the end of the time point, the wells were stained with cell stain solution. And then the wound closure was monitored with a light microscope (4x magnification) and imaged under brightfield microscope.
2.4. In vivo experiments
2.4.1. Animals
Male Sprague–Dawley rats (160–180 g) were purchased from Osmangazi University (Eskisehir, Turkey) were used in the experiments. All animals were housed in a well-ventilated room with 12-h light/dark cycles at 22 ± 1 °C and allowed free access to food and water ad libitum. The animal study was approved by the Local Ethics Committee of Osmangazi University (Approval Number: 588–2017). Animal care and research protocols were based on the principles and guidelines adopted by the Guide for the Care and Use of Laboratory Animals (NIH publication No: 85–23, revised in 1985).
2.4.2. Excisional wound model
The animals were anesthetized with xylazine (2% Alfazines) and 0.08 cm3 ketamine (10% Ketasols). The dorsal hairs of the rats were removed by shaving. The circular wound (10–12 mm) was created on the dorsal interscapular region of each animal by excising the skin with scissors. After the wound was created, animals were placed in separate cages to prevent any damages by interactions in between. Animal treatment groups are formed as: control (no treatment), positive control (Madecassol® treatment), vehicle-treated group (chitosan treatment), formulation treated group (chitosan-based gel formulation containing vitexin treatment). Progressive changes in wound areas at the 0th, 7th, 14th and 21st days of treatment were photographed with camera and measured with caliper. All test materials were administered every morning and evening (Akkol et al., 2011, Süntar et al., 2013).
2.4.3. Histopathological study
The animals were anesthetized, and the wound area was removed on the 7th, 14th and 21st days of the study from rats for histological analysis. Extracted tissues were stained with hematoxylin and eosin stain (H & E) and Masson Trichrome. Histopathologic changes in dermis and epidermis were detected under microscope. A systematic examination of 20 random fields under 40X magnification was performed in order to semi-quantitatively score the following histological variables (0:none; 1:mild; 2:moderate; 3:severe). Reepithelization, collagen tissue disorganization, neutrophil infiltration, hemorrhage, granulation tissue formation and angiogenesis were taken into consideration while evaluating the histopathological changes in microscopic tissue examination. Scoring was performed by considering these criteria (İşeri et al., 2010, Abbas et al., 2018).
2.5. Statistical analyses
Statistical analyses were performed by using SPSS 18.0 and GraphPad Prism version 5.0 software. Measurement of wound contraction assessment, the differences between groups were analyzed by two-way analysis of variance (ANOVA) and the results were expressed as means ± SEM. Histological analysis were evaluated by Kruskal-Wallis one-way ANOVA on Ranks Teat and results were expressed as means ± SD. Cytotoxicity studies were evaluated with two-way ANOVA and the results were expressed as means ± SEM. Differences were considered significant when P ≤ 0.05.
3. Results
3.1. Formulation analysis
The results of pH measurements and rheological analyses of formulations were shown in Table 1, Table 2.
Table 1.
Changes in pH values of the gel formulations prepared during the storage period of 1 months (mean ± SE; RH = relative humidity).
| Storage Condition | Storage Day | R1 (Chitosan-based gel formulation containing vitexin | F1 (Blank Gel Formulation) |
|---|---|---|---|
| 25 ± 1 ◦C | 0 | 4.1 ± 0.1 | 3.6 ± 0.05 |
| 30 | 4.1 ± 0.1 | 3.6 ± 0.1 | |
| 4 ± 1 ◦C | 0 | 4.1 ± 0.1 | 3.6 ± 0.05 |
| 30 | 4.1 ± 0.1 | 3.7 ± 0.05 | |
| 40 ± 1 ◦C 60% | 0 | 4.1 ± 0.1 | 3.6 ± 0.05 |
| 30 | 4.2 ± 0.05 | 3.8 ± 0.1 | |
Table 2.
Rheological analyses of the R1 and F1 formulations during the storage period of 1 months (RH = relative humidity).
| Storage Condition | Storage Day | R1 (Chitosan-based gel formulation containing vitexin | F1 (Blank Gel Formulation) |
|---|---|---|---|
| mPa | mPa | ||
| 25 ± 1 ◦C | 0 | 9645 | 9021 |
| 30 | 9785 | 9112 | |
| 4 ± 1 ◦C | 0 | 9645 | 9021 |
| 30 | 9756 | 9345 | |
| 40 ± 1 ◦C 60% | 0 | 9645 | 9021 |
| 30 | 9753 | 9456 | |
3.2. Experiments
3.2.1. Cytotoxicity assay
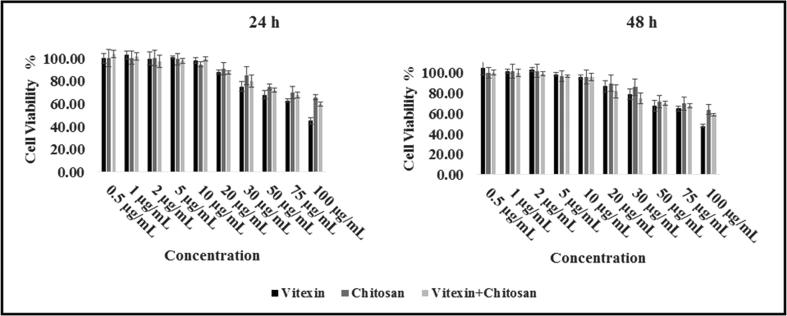
The dispersions were applied to the cells for 24 and 48 h, with a concentration of 0.5, 1, 2, 5, 10, 20, 30, 50, 75 and 100 μg/mL. As a result, it was observed that the cell viability did not drop below 45% even at the highest dose for 48 h. The results after 24 and 48 h of the cytotoxicity test are shown in Fig. 1. In the study, the IC50 value for vitexin was 90 μg/mL. In addition, vitexin was dissolved in DMSO. DMSO in the used concentration was seen to have no significant effect in cytotoxicity assay (Hebling et al., 2015).
Fig. 1.
NIH-3T3 cell viability results from cells exposed to different concentrations of vitexin, chitosan and vitexin/chitosan dispersions (R1) after 24 h and 48 h incubation. *(Ι) on figure indicated standard deviation, SD.
3.2.2. Wound healing assay
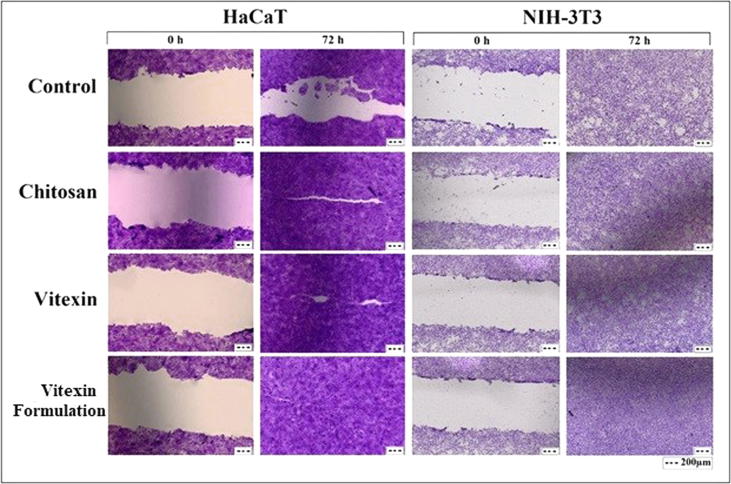
In this study, NIH-3T3 (mouse embryo fibroblast cell line) and HaCaT (human epidermal keratinocyte cell line) cells were selected. The vitexin, chitosan and vitexin/chitosan dispersions were used as 1 μg of vitexin in each well and incubated for 0–72 h in the growth medium. Vitexin and the dispersions were applied after the insert was removed. The wells containing only DMEM medium were used as controls. According to results there were no visually obvious differences between vitexin and chitosan dispersions containing vitexin, but after 72 h, a significant cell migration was observed especially in HaCaT cells and the space was tightly closed. In the NIH-3T3 cells, there was still some space in the control, and a significant cell proliferation was observed in vitexin, chitosan, vitexin/chitosan dispersion (Fig. 2).
Fig. 2.
Results of wound healing assay performed with NIH-3T3 (mouse embryo fibroblast cell line) and HaCaT (human epidermal keratinocyte cell line) cells.
3.2.3. Wound healing construction
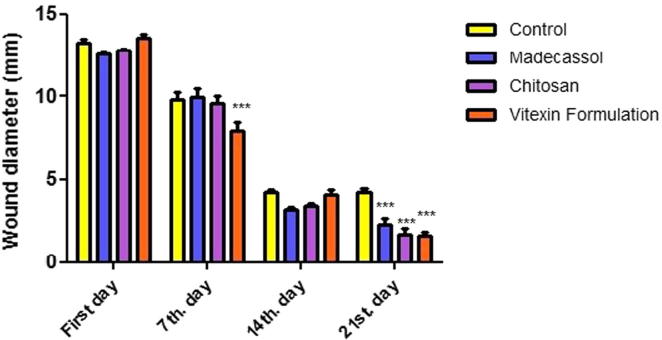
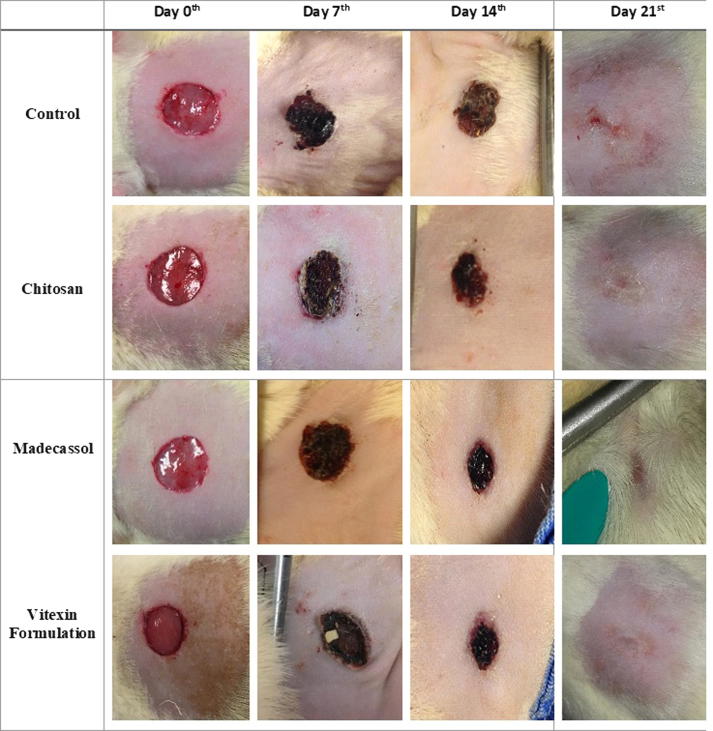
The changes in the size of wounds were shown in Fig. 3. Differentiation of wound area photographed on the 7th, 14th, 21st days and were shown in Fig. 4. When compared to the control group, progressive healing was observed on wounds in rats treated with vitexin formulation. According to our macroscopic results, it is determined that vitexin formulation was almost as potent as positive control Madecassol®.
Fig. 3.
Wound construction of the macroscopic measurements. Data are means ± SEM. ***P < 0.001 compared to the control group (n = 6–7).
Fig. 4.
Digital imaging of circular excision wound areas at 0, 7, 14, 21st days.
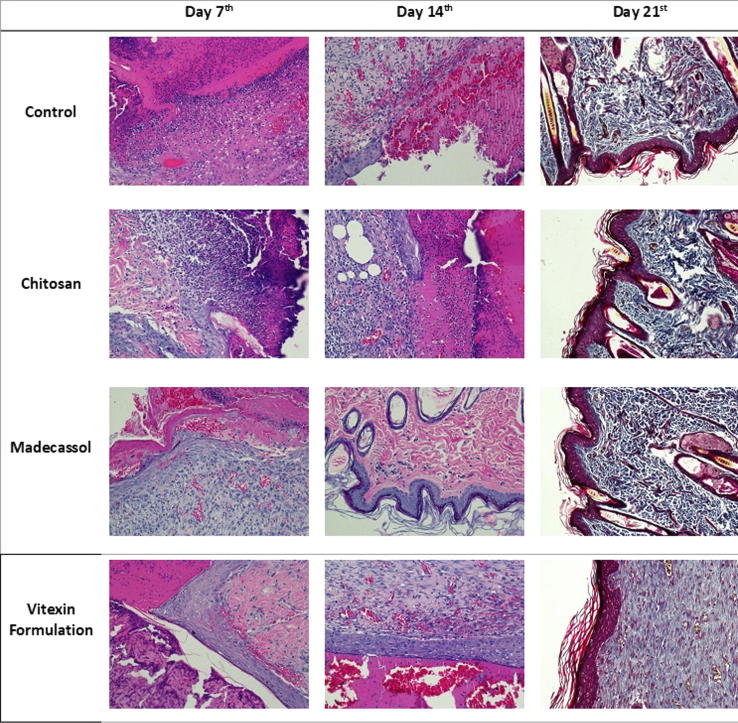
3.2.4. Histopathological results
Histological examinations demonstrated that epidermal and dermal regeneration was promoted by vitexin formulation. When 7-day groups were evaluated, reepithelization was observed in rats in the formulation-treated group. In statistical evaluation of the results of scoring, results from formulation-treated groups were significantly better than the control and chitosan-treated groups. When reepithelization and wound healing were compared in 14-day groups, statistically better results were obtained from the Madecassol® and formulation groups than the control group. Significantly better results were observed in the vitexin formulation group in comparison with the chitosan-treated group. There were no statistically significant changes in the evaluation of 21-day groups. Wound healing was detected in all groups. It was found that vitexin formulation provide reepithelization and wound healing in shorter time (Table 3, Fig. 5).
Table 3.
Scoring of wound healing in histological examination. Mean of epidermal-dermal regeneration, granulation and angiogenesis. Data are means ± SD. aaaP < 0.001 compared to the control group. bbbP < 0.001 compare to the chitosan group.
| Mean ± SD | Median (25–75) | P | Multiple comparison | ||
|---|---|---|---|---|---|
| 7thDay | Control | 14,50 ± 0,84 | 15,00 (13,75–15,00) | 0,001 | 1–3, 2–3 |
| Chitosan (F1) | 13,83 ± 0,98 | 14,00 (13,50–14,25) | |||
| Vitexin Formulation (R1) | 8,33 ± 0,82aaa, bbb | 8,50 (7,75–9,00) | |||
| Madecassol | 10,83 ± 1,47 | 10,50 (9,75–12,25) | |||
| 14thDay | Control | 9,83 ± 1,17 | 10,00 (8,75–11,00) | 0,001 | 1–3, 1–4, 2–3 |
| Chitosan (F1) | 9,33 ± 0,52 | 9,00 (9,00–10,00) | |||
| Vitexin Formulation (R1) | 4,00 ± 1,10aaa, bbb | 4,00 (3,50–5,00) | |||
| Madecassol | 4,83 ± 1,33aaa | 5,00 (3,75–5,50) | |||
| 21stDay | Control | 3,83 ± 0,75 | 4,00 (3,00–4,25) | 0,214 | Ns. |
| Chitosan (F1) | 3,67 ± 1,03 | 4,00 (2,75–4,25) | |||
| Vitexin Formulation (R1) | 2,67 ± 1,21 | 2,00 (2,00–3,50) | |||
| Madecassol | 3,17 ± 1,60 | 2,50 (200–4,50) | |||
Fig. 5.
Histological analyses of wound area samples at days 7, 14 and 21.
4. Discussion
Wound healing is known to be a complex and dynamic process that usually involves distinct phases marking the healing stages and requires multiple cell types to complete a variety of cellular activities (Lopez-Jornet et al., 2014). The first phase in wound healing is hemostasis, followed by inflammation phase, proliferative phase and maturation phase. Herbal drugs, along with other drugs are used in wound healing for a long period of time (Maver et al., 2015). Plants affect some of these phases in wound healing by accelerating or easing. In this context, vitexin is one of the well-known flavonoids found in medicinal plants such as Passiflora spp and Crataegus spp. Vitexin has been shown to have many different pharmacological activities such as antioxidant, anti-inflammatory, and analgesic effects (Kim et al., 2005, Borghi et al., 2013, Özkay and Can, 2013). In present study it is aimed to investigate the in vivo and in vitro effects of chitosan-based gel formulation containing vitexin in wound healing. Inflammation phase that starts following the hemostasis phase, is an important and necessary phase for wound healing. In this phase, inflammatory cells (neutrophiles, macrophages etc.) immigrate to the wound area and they ensure the removal of microorganisms and tissue residues in the environment. Inflammatory cells are also responsible for the stimulation of fibroblasts and epithelial cells (Nguyen et al., 2017). Inflammation may be acute or chronic. Acute inflammation contributes to wound healing, while chronic inflammation delays wound healing and prolongs this process (Hadagali and Chua, 2014). Vitexin flavonoid used in this study has been shown to have antimicrobial and anti-inflammatory properties in various studies (Gökbulut et al., 2010, Chen et al., 2019). Likewise, chitosan in the formulation, is also known to have antioxidant and anti-inflammatory activities (Qiao et al., 2011). When evaluating the results of this study, it is observed that chitosan-based gel formulation containing vitexin was more effective compared to control and madecassol-treated groups, especially in 7 and 14th days (Fig. 3, Fig. 4, Fig. 5 and Table 3). The tissue renewal in epithelial and dermal tissues were more significant compared to control and chitosan groups, especially in histopathological changes. As seen in histological examinations, it has been seen that chitosan-based gel formulation containing vitexin increases reepithelization and vascularization in damaged tissue (Table 3, Fig. 5) and this has also been accelerating the wound healing. Similarly, when wound area diameter shrinkage was examined, recovery was faster (Fig. 3).
The authors believe that their formulation with vitexin which is known to have both antioxidant and anti-inflammatory effects and chitosan accelerate wound healing by exhibiting synergistic activity. Antioxidant substances accelerate wound healing by removing the reactive oxygen species and inflammation products formed during wound healing. In addition, they are thought to contribute to wound healing by preventing the prolongation of the inflammation phase with their anti-inflammatory effects. In a study performed by Kant et al., it was observed that curcumin accelerated wound contraction, decreased the expressions of inflammatory mediators (tumor necros factor-α, interleukin-1β, etc.) and also increased the inflammatory cytokine levels (Kant et al., 2014). It is demonstrated that the anti-inflammatory effect of vitexin is exhibited via the inhibition of cytokines, nitric oxide and prostaglandin E2 release (Rosa et al., 2016).
In our in vitro study, MTT assay was used to determine the cytotoxicity of the vitexin on the cells. This test develops a quantitative colorimetric assay via tetrazolium salt of mammalian cell survival and proliferation. It detects living cells. The viability depends on the degree of active cells (Riss et al., 2016). In vitro cytotoxicity methods are important test methods in assessing the biocompatibility of a new formulation. Once the effects of the formulations on the cell growth rates in cell cultures have been investigated, clearence of toxicity profiles provides both time and economic advantages. In addition, the cytotoxicity assay of the formulations prepared is of vital importance to ensure that the experimental animals do not present any risk or that the acute toxicity response does not appear (Li et al., 2015). In this study, NIH-3T3 mouse fibroblast cells were selected because fibroblasts are found in the matrix and connective tissue of the body and are widely used to define cellular cytotoxicity and genotoxicity of many formulations (Coradeghini et al., 2013, Ridolfi et al., 2011). The concentrations used in our cytotoxicity study were selected depending on the studies performed in the literature (Zhou et al., 2009, Zhou et al., 2013). Our results showed that the IC50 value for vitexin was 90 μg/mL which indicates the low cytotoxicity of vitexin. Wound healing assay is a standard in vitro technique used for the determination of cell migration. In this assay, a cell-free area is created in a unified single layer by removal of cells in the respective region. The important data obtained from the wound healing assay is the rate of gap closure, which is a measure of the speed of the collective movement of the cells (Jonkman et al., 2014). Moreover, rapid and functional wound closure is the primary target in wound treatment. The wound healing assay is easily used for applications such as small molecule screening and drug discovery (Süntar et al., 2013, Hulkower and Herber, 2011, Yarrow et al., 2004). Quantification of fibroblast proliferation, migration and collagen synthesis in fibroblast cell cultures were first proposed by Graham et al. (1984) as a method for testing in vitro wound healing activity. This approach has been successfully applied to investigate the development and mechanism of action of new drugs using fibroblast cells with different origins (Korkmaz et al., 2000, Agar et al., 2015). In this study, it was observed that the cell migration was relatively faster in formulation containing vitexin compared to vitexin-free chitosan and control groups. Also, there was no visually obvious difference between vitexin-free chitosan and vitexin formulation groups. The difference in cell migration in HaCaT cells is better understood when evaluated in terms of cells.
In conclusion, our test substance chitosan-based gel formulation containing vitexin significantly accelerated wound healing both in vivo and in vitro. It is thought that anti-oxidant, anti-inflammatory, epithelial tissue regeneration and cell migration enhancing effects of vitexin play a positive role on wound healing. Chitosan also has similar effects on wound healing, which demonstrated synergistic activity with vitexin. Authors believe that usage of chitosan-based gel formulation containing vitexin may raise a new perspective in wound treatment and provide an advantage to modern medicine since the exist topical wound healing medications are limited in markets and also may cause allergic skin reactions. However, further investigation may be required for clinical use.
Declarations of Competing Interest
None
Acknowledgements
This study was financially supported by Anadolu University Scientific Research Projects Unit (Project no: 1605S310); Anadolu University, Eskisehir, Turkey.
Footnotes
Peer review under responsibility of King Saud University.
Contributor Information
Nurcan Bektas, Email: nurcanbektas@anadolu.edu.tr.
Behiye Şenel, Email: behiyek@anadolu.edu.tr.
Evrim Yenilmez, Email: evrimakyil@anadolu.edu.tr.
Orhan Özatik, Email: orhan.ozatik@ksbu.edu.tr.
Rana Arslan, Email: rbeis@anadolu.edu.tr.
References
- Abbas O.L., Özatik O., Gönen Z.B., Ögüt S., Entok E., Özatik F.Y., Musmul A. Prevention of Burn Wound Progression by Mesenchymal Stem Cell Transplantation: Deeper Insights Into Underlying Mechanisms. Ann Plast Surg. 2018;81(6):715–724. doi: 10.1097/SAP.0000000000001620. https://doi:10.1097/SAP.0000000000001620 [DOI] [PubMed] [Google Scholar]
- Agar O.T., Dikmen M., Ozturk N., Yilmaz M.A., Temel H., Turkmenoglu F.P. Comparative studies on phenolic composition, antioxidant, wound healing and cytotoxic activities of selected Achillea L. species growing in Turkey. Molecules. 2015;20:17976–18000. doi: 10.3390/molecules201017976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkol, E. K., Koca, U., Pesin, I., Yilmazer, D., 2011. Evaluation of the wound healing potential of Achillea biebersteinii Afan.(Asteraceae) by in vivo excision and incision models. Evidence-Based Complementary and Alternat Med. http://dx.doi.org/10.1093/ecam/nep039 [DOI] [PMC free article] [PubMed]
- Aoyagi S., Onishi H., Machida Y. Novel chitosan wound dressing loaded with minocycline for the treatment of severe burn wounds. Int. J. Pharm. 2007;330(1–2):138–145. doi: 10.1016/j.ijpharm.2006.09.016. [DOI] [PubMed] [Google Scholar]
- Aslam M.S., Ahmad M.S., Mamat A.S. Pharmacological potential of vitexin. IRJPS. 2015;2(2):114–122. [Google Scholar]
- Borghi S.M., Carvalho T.T., Staurengo-Ferrari L., Hohmann M.S., Pinge-Filho P., Casagrande R., Verri W.A., Jr Vitexin inhibits inflammatory pain in mice by targeting TRPV1, oxidative stress, and cytokines. J. Nat. Prod. 2013;76(6):1141–1149. doi: 10.1021/np400222v. [DOI] [PubMed] [Google Scholar]
- Che, X., Wang, X., Zhang, J., Peng, C., Zhen, Y., Shao, X., ... & Dong, L., 2016. Vitexin exerts cardioprotective effect on chronic myocardial ischemia/reperfusion injury in rats via inhibiting myocardial apoptosis and lipid peroxidation. Am. J. Transl. Res. 8(8), 3319-28 eCollection 2016. PMID: 27648122 [PMC free article] [PubMed]
- Chen G.L., Fan M.X., Wu J.L., Li N., Guo M.Q. Antioxidant and anti-inflammatory properties of flavonoids from lotus plumule. Food. Chem. 2019;277:706–712. doi: 10.1016/j.foodchem.2018.11.040. [DOI] [PubMed] [Google Scholar]
- Coradeghini R., Gioria S., García C.P., Nativo P., Franchini F., Gilliland D., Rossi F. Size-dependent toxicity and cell interaction mechanisms of gold nanoparticles on mouse fibroblasts. Toxicol. Lett. 2013;217(3):205–216. doi: 10.1016/j.toxlet.2012.11.022. [DOI] [PubMed] [Google Scholar]
- Değim Z., Çelebi N., Alemdaroğlu C., Deveci M., Öztürk S., Özoğul C. Evaluation of chitosan gel containing liposome-loaded epidermal growth factor on burn wound healing. Int Wound J. 2011;8:343–354. doi: 10.1111/j.1742-481X.2011.00795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demirezer O. Medikal & Nobel Kitapevi; Ankara: 2007. Tedavide Kullanılan Bitkiler. [Google Scholar]
- Dreifke M.B., Jayasuriya A.A., Jayasuriya A.C. Current wound healing procedures and potential care. Mater. Sci. Eng. C. 2015;48:651–662. doi: 10.1016/j.msec.2014.12.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Działo M., Mierziak J., Korzun U., Preisner M., Szopa J., Kulma A. The potential of plant phenolics in prevention and therapy of skin disorders. Int. J. Mol. Sci. 2016;17(2):160. doi: 10.3390/ijms17020160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fouda M.M., Wittke R., Knittel D., Schollmeyer E. Use of chitosan/polyamine biopolymers based cotton as a model system to prepare antimicrobial wound dressing. Int. J. Diabetes. Mellit. 2009;1(1):61–64. [Google Scholar]
- Gencer S., Cebeci A., Irmak-Yazicioglu M.B. Silencing of the MMP-3 Gene by siRNA transfection in gastric cancer AGS cells. J. Gastrointestin. Liver. Dis. 2010;20:19–26. PMID: 21451793. [PubMed] [Google Scholar]
- Gilani A.H., Khan A.U., Ghayur M.N., Ali S.F., Herzig J.W. Antispasmodic effects of Rooibos tea (Aspalathus linearis) is mediated predominantly through K+-channel activation. Basic. Clin. Pharmacol. Toxicol. 2006;99(5):365–373. doi: 10.1111/j.1742-7843.2006.pto_507.x. [DOI] [PubMed] [Google Scholar]
- Gonzalez A.C.D.O., Costa T.F., Andrade Z.D.A., Medrado A.R.A.P. Wound healing-A literature review. Anais brasileiros de dermatologia. 2016;91(5):614–620. doi: 10.1590/abd1806-4841.20164741. https://10.1590/abd1806-4841.20164741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gökbulut A., Özhan O., Karacaoğlu M., Şarer E. Radical scavenging activity and vitexin content of Vitex agnus-castus leaves and fruits. FABAD J. Pharm. Sci. 2010;35:85–91. [Google Scholar]
- Graham M.F., Diegelmann R.F., Cohen I.K. An in vitro model of fibroplasia: Simultaneous quantification of fibroblast proliferation, migration, and collagen synthesis. Proc. Soc. Exp. Biol. Med. 1984;176:302–308. doi: 10.3181/00379727-176-41875. [DOI] [PubMed] [Google Scholar]
- Hadagali M.D., Chua L.S. The anti-inflammatory and wound healing properties of honey. Eur. Food. Res. Technol. 2014;239(6):1003–1014. [Google Scholar]
- Hebling J., Bianchi L., Basso F.G., Scheffel D.L., Soares D.G., Carrilho M.R., Pashley D.H., Tjäderhane L., de Souza Costa C.A. Cytotoxicity of dimethyl sulfoxide (DMSO) in direct contact with odontoblast-like cells. Dent Mater. 2015;31(4):399–405. doi: 10.1016/j.dental.2015.01.007. http://doi:10.1016/j.dental.2015.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoemann C.D., Sun J., Legare A., McKee M.D., Buschmann M.D. Tissue engineering of cartilage using an injectable and adhesive chitosan-based cell-delivery vehicle. Osteoarthr. Cartil. 2005;13(4):318–329. doi: 10.1016/j.joca.2004.12.001. [DOI] [PubMed] [Google Scholar]
- http-1 https://www.cellbiolabs.com/wound-healing-assays (accessed 25 July 2019)
- Hulkower K.I., Herber R.L. Cell migration and invasion assays as tools for drug discovery. Pharmaceutics. 2011;3(1):107–124. doi: 10.3390/pharmaceutics3010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- İşeri S.Ö., Düşünceli F., Erzik C., Uslu B., Arbak S., Yeğen B.Ç. Oxytocin or social housing alleviates local burn injury in rats. J Surg Res. 2010;162(1):122–131. doi: 10.1016/j.jss.2009.02.018. [DOI] [PubMed] [Google Scholar]
- Jonkman J.E., Cathcart J.A., Xu F., Bartolini M.E., Amon J.E., Stevens K.M., Colarusso P. An introduction to the wound healing assay using live-cell microscopy. Cell. Adh. Migr. 2014;8(5):440–451. doi: 10.4161/cam.36224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kant V., Gopal A., Pathak N.N., Kumar P., Tandan S.K., Kumar D. Antioxidant and anti-inflammatory potential of curcumin accelerated the cutaneous wound healing in streptozotocin-induced diabetic rats. Int. Immunopharmacol. 2014;20(2):322–330. doi: 10.1016/j.intimp.2014.03.009. [DOI] [PubMed] [Google Scholar]
- Kim J.H., Lee B.C., Kim J.H., Sim G.S., Lee D.H., Lee K.E., Pyo H.B. The isolation and antioxidative effects of vitexin fromAcer palmatum. Arch. Pharm. Res. 2005;28(2):195. doi: 10.1007/BF02977715. [DOI] [PubMed] [Google Scholar]
- Korkmaz S., Zeytinoglu H., Zeytinoglu M., Aydın S., Ozturk Y., Baser K.H.C. Testing the wound-healing activity in T15 fibroblast culture: A morphometric analysis. Altern. Lab. Anim. 2000;28:41–51. doi: 10.1177/026119290002800107. [DOI] [PubMed] [Google Scholar]
- Lodhi S., Singhai A.K. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua Linn. on streptozotocin induced diabetic rats Asian Pac. J Trop Med. 2013;13;6(4):253–259. doi: 10.1016/S1995-7645(13)60053-X. [DOI] [PubMed] [Google Scholar]
- Lodhi S., Jain A.P., Rai G., Yadav A.K. Preliminary investigation for wound healing and anti-inflammatory effects of Bambusa vulgaris leaves in rats. J Ayurveda Integr Med. 2016;7(1):14–22. doi: 10.1016/j.jaim.2015.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W., Zhou J., Xu Y. Study of the in vitro cytotoxicity testing of medical devices. Biomed. Rep. 2015;3(5):617–620. doi: 10.3892/br.2015.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Jornet P., Camacho-Alonso F., Gómez-Garcia F., Molina Minano F., Cañas X., Serafín A., Vicente-Ortega V. Effects of potassium apigenin and verbena extract on the wound healing process of SKH-1 mouse skin. Int. Wound. J. 2014;11(5):489–495. doi: 10.1111/j.1742-481X.2012.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maver T., Maver U., Stana Kleinschek K., Smrke D.M., Kreft S. A review of herbal medicines in wound healing. Int. J. Dermatol. 2015;54(7):740–751. doi: 10.1111/ijd.12766. [DOI] [PubMed] [Google Scholar]
- Mekonnen A., Sidamo T., Asres K., Engidawork E. In vivo wound healing activity and phytochemical screening of the crude extract and various fractions of Kalanchoe petitiana A. Rich (Crassulaceae) leaves in mice. J. Ethnopharmacol. 2013;145(2):638–646. doi: 10.1016/j.jep.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Nguyen V.L., Truong C.T., Nguyen B.C.Q., Van Vo T.N., Dao T.T., Nguyen V.D., Bui C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PloS. one. 2017;12(10):e0185674. doi: 10.1371/journal.pone.0185674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikfarjam B.A., Hajiali F., Adineh M., Nassiri-Asl M. Anti-inflammatory effects of quercetin and vitexin on activated human peripheral blood neutrophils:the effects of quercetin and vitexin on human neutrophils. Journal of Pharmacopuncture. 2017;20(2):127. doi: 10.3831/KPI.2017.20.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Özkay Ü.D., Can Ö.D. Anti-nociceptive effect of vitexin mediated by the opioid system in mice. Pharmacol. Biochem. Behav. 2013;109:23–30. doi: 10.1016/j.pbb.2013.04.014. [DOI] [PubMed] [Google Scholar]
- Periayah M.H., Halim A.S., Saad A.Z.M. Chitosan: A promising marine polysaccharide for biomedical research. Pharmacogn. Rev. 2016;10(19):39. doi: 10.4103/0973-7847.176545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiao Y., Bai X.F., Du Y.G. Chitosan oligosaccharides protect mice from LPS challenge by attenuation of inflammation and oxidative stress. Int. Immunopharmacol. 2011;11(1):121–127. doi: 10.1016/j.intimp.2010.10.016. [DOI] [PubMed] [Google Scholar]
- Ridolfi D.M, Marcato P.D., Machado D., Silva R.A., Justo G.Z., Durán N. In vitro cytotoxicity assays of solid lipid nanoparticles in epithelial and dermal cells. J. Phys.: Conf. Ser. 2011;304:012032. [Google Scholar]
- Riss, T.L., Moravec, R.A., Niles, A L., Duellman, S., Benink, H A., Worzella, T.J., Minor, L., 2016. Cell viability assays. In Assay Guidance Manual [Internet]. Eli Lilly & Company and the National Center for Advancing Translational Sciences.
- Rosa S.I.G., Rios-Santos F., Balogun S.O., de Oliveira Martins D.T. Vitexin reduces neutrophil migration to inflammatory focus by down-regulating pro-inflammatory mediators via inhibition of p38, ERK1/2 and JNK pathway. Phytomedicine. 2016;23(1):9–17. doi: 10.1016/j.phymed.2015.11.003. [DOI] [PubMed] [Google Scholar]
- Ruel-Gariepy E., Leclair G., Hildgen P., Gupta A., Leroux J.C. Thermosensitive chitosan-based hydrogel containing liposomes for the delivery of hydrophilic molecules. Journal of J Control Release. 2002;82(2–3):373–383. doi: 10.1016/s0168-3659(02)00146-3. [DOI] [PubMed] [Google Scholar]
- Süntar I., Küpeli Akkol E., Keles H., Yesilada E., Sarker S.D. Exploration of the wound healing potential of Helichrysum graveolens (Bieb.) Sweet: isolation of apigenin as an active component. J. Ethnopharmacol. 2013;26;149(1):103–110. doi: 10.1016/j.jep.2013.06.006. [DOI] [PubMed] [Google Scholar]
- Yarrow J.C., Perlman Z.E., Westwood N.J., Mitchison T.J. A high-throughput cell migration assay using scratch wound healing, a comparison of image-based readout methods. BMC biotechnology. 2004;4(1):21. doi: 10.1186/1472-6750-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yenilmez E., Başaran E., Arslan R., Berkman M.S., Güven U.M., Bayçu C., Yazan Y. Chitosan gel formulations containing egg yolk oil and epidermal growth factor for dermal burn treatment. Pharmazie. 2015;70(2):67–73. [PubMed] [Google Scholar]
- Zhou J., Hu H., Long J., Wan F., Li L., Zhang S., Chen Y. Vitexin 6, a novel lignan, induces autophagy and apoptosis by activating the Jun N-terminal kinase pathway. Anti-cancer drugs. 2013;24(9):928–936. doi: 10.1097/CAD.0b013e328364e8d3. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Liu Y.E., Cao J., Zeng G., Shen C., Li Y., Shi Y.E. Vitexins, nature-derived lignan compounds, induce apoptosis and suppress tumor growth. Clin Cancer Res. 2009;15(16):5161–5169. doi: 10.1158/1078-0432.CCR-09-0661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q., Mao L.N., Liu C.P., Sun Y.H., Jiang B., Zhang W., Li J.X. Antinociceptive effects of vitexin in a mouse model of postoperative pain. Sci. Rep. 2016;6:19266. doi: 10.1038/srep19266. [DOI] [PMC free article] [PubMed] [Google Scholar]