Abstract
Purpose
To compare the functional outcomes and astigmatic tolerability after implantation of multifocal intraocular lenses (IOLs) with a +2.5, +3.0, and +3.75 diopter (D) addition power.
Methods
This study included 122 eyes of 61 patients who had bilateral cataract extraction and implantation of diffractive aspheric multifocal acrylic IOLs with +2.5 D (+2.5 group), +3.0 D (+3.0 group), and +3.75 D (+3.75 group) addition powers. 1-year after surgery, distance corrected near (DNVA) and intermediate (DIVA) visual acuities at 32, 40, 45, 50, 55, and 60 cm; and contrast sensitivity measurements under photopic, mesopic and mesopic with glare conditions; spherical and astigmatic defocus testing; distance-intermediate-near vision patient satisfaction levels; spectacle dependance; patient-reported outcomes were assessed binocularly.
Results
The +2.50 D group had better DIVA than both +3.0 group and +3.75 groups at 45 cm, 50 cm, 55 cm, and 60 cm (p < 0.05). The +3.75 group had better DNVA than both +2.5 and +3.0 IOL groups at 32 cm (p < 0.05). There was no significant difference in mean contrast values at all frequencies between three IOL groups (p > 0.05). The +2.50 D group showed better astigmatic tolerability than +3.00 group (at 2.00 D) and +3.75 group (at 1.50 D, and at 2.00 D) (p < 0.05).
Conclusion
Multifocal IOLs with +2.5 add power have better intermediate vision, but worse near vision compared to multifocal IOLs with +3.00 D and +3.75 D add power. Multifocal IOLs with +2.50 D add power tend to have better astigmatic defocus tolerability than multifocal IOLs with +3.00 D and +3.75 D add powers.
Keywords: Astigmatism, Cataract, Intraocular lens, Phacoemulsification, Presbyopia
Introduction
Multifocal intraocular lenses (IOLs) were introduced to free patients from spectacles after cataract surgery,1, 2 Despite lower levels of satisfaction with the earlier models,3 recent multifocal IOLs are reported to have satisfactory outcomes.2 Yet, the development of the multifocal IOLs still continues and different technologies for IOL design are being introduced to address issues related to contrast sensitivity, night vision, unwanted visual phenomena, astigmatism correction, and good vision at different distances such as intermediate vision.1, 2 Therefore, the evaluation of such parameters and quality of vision are important to better inform the surgery candidate and to select the most suitable IOL.4
Previous studies reported that multifocal IOLs with diffractive technology may provide better visual acuity with less unwanted visual phenomena.5, 6, 7, 8 Also, there are multifocal IOLs with different addition power with a claim of better near or intermediate vision, and with less visual phenomena.9, 10 Around 5 years ago a multifocal IOL was introduced with a decrease of the addition power from +3.0 D to +2.5 D moving to provide a better intermediate vision.
The aim of the current study was to evaluate and compare the visual acuity at different distances and spherical and astigmatic defocus curves, the quality of vision, and the clinical outcomes of 3 multifocal IOLs with an hybrid diffractive-refractive design, with different addition powers.
Methods
This non-randomised comparative prospective study comprised patients having bilateral cataract extraction with implantation of 3 different hybrid diffractive-refractive aspheric acrylic multifocal IOLs. Institutional review board approval was obtained and this study was approved by the university ethics committee. The study was performed in compliance with good clinical practice guidelines and the ethical principles of the Declaration of Helsinki. All patients provided informed consent before study participation and surgery.
Eligible patients were at least 21 years of age and had a preoperative corrected distance visual acuity (CDVA) worse than 0.2 logMAR (Snellen equivalent 20/32); preoperative corneal astigmatism of 1.0 D or less; clear intraocular media other than cataract; and a need for correction with an IOL with a power from 15.0 to 26.0 D.
Preoperative exclusion criteria included any previous eye surgery, significant corneal or ocular surface disorders and significant intraoperative complication during cataract surgery.
Intraocular lenses
The multifocal acrylic IOLs, AcrySof IQ ReSTOR SV25T0 with +2.50 D addition power at the IOL plane (+2.50 D group), AcrySof IQ ReSTOR SN6AD1 with +3.0 D addition power at the IOL plane (+3.0 D group), and Reviol MF613 with +3.75 D addition power at the IOL plane (+3.75 D group) were used in this study. The characteristics of the each multifocal IOL is given in Table 1.
Table 1.
Characteristics of intraocular lenses (IOLs) implanted in the study.
| Restor +2.5 | Restor +3.0 | Reviol +3.75 | |
|---|---|---|---|
| Material | Hydrophobic acrylic | Hydrophobic acrylic | Hydrophilic Acrylic with hydrophic surface |
| Optic design | Aspheric multifocal | Aspheric multifocal | Aspheric multifocal |
| Diameter (Optic/Total) | 6.0/13.0 mm | 6.0/13.0 mm | 6.0/13.0 mm |
| Lens design | Single-piece bifocal hybrid diffractive/refractive | Single-piece bifocal hybrid diffractive/refractive | Single-piece bifocal diffractive |
| Diffractive rings | 7 | 9 | 29 |
| Addition at the IOL plane | +2.50 | +3.00 | +3.75 |
| Multifocality | Central 3.6 mm Apodized Diffractive | Central 3.6 mm Apodized Diffractive | Full diffractive, not apodized |
| SA for a 6 mm pupil diameter (mm) | −0.200 | −0.200 | −0.165 |
| Light distribution%far/%near | 50/50 | 50/50 | 60/40 |
| Blue light filter | Yes | Yes | No |
SA = spherical aberration, PCO = posterior capsule opacification.
Surgical technique
The same surgeon (O.M.) performed all procedures using the 2 different phaco machines (Infiniti Vision Systems, Alcon Laboratories, Inc, or Pentasys, Fritz Ruck Ophthalmologische Systeme Gmbh), with the modified crater and split technique.11, 12, 13 The eye with the more advanced cataract had IOL implantation first; the second eye had implantation of the same IOL model 7 to 30 days after the first surgery.
Examinations
All evaluations in this study were performed at least 1-year after second IOL implantation. Manifest refraction, binocular uncorrected distance visual acuity (UDVA), and binocular corrected distance visual acuity (CDVA) – using the Early Treatment Diabetic Retinopathy Study (ETDRS) charts and transformed into logMAR – were measured.
Distance corrected near (DCNVA) and intermediate visual acuities (DCIVA) were tested under photopic conditions using a handheld 100% contrast Snellen chart set at 32 cm (near visual acuity, NVA), 40 cm, 45 cm, 50 cm, 55 cm, and 60 cm (intermediate visual acuity, IVA) on the near-point rod.
Defocus was tested using a phoropter and a 100% contrast Snellen chart under photopic conditions with manifest refraction designated to the zero baseline. A defocus of −4.00 D spherical correction from the CDVA (manifest refraction) was set. Negative spherical power was decreased in 0.50 D increments, with logMAR acuity recorded at each change in correction until only manifest refraction remained. Then, a defocus of +1.00 D spherical correction from the manifest refraction was set and the logMAR acuity recorded. Positive spherical power was decreased in 0.50 D increments, with logMAR acuity recorded at each change in correction until only manifest refraction remained.
Astigmatic defocus was simulated by adding 0.50 D, 1.00 D, 1.50 D, and 2.00 D cylindrical lenses at the 180-degree meridian at the spectacle plane with keeping the spherical equivalent less than ±0.50 D, after full correction of the manifest refraction. Only against-the-rule (ATR) astimatism was induced, because most eyes with senile cataract have this type of astigmatism.
Contrast sensitivity was measured using CSV 1000E (Vector Vision) with and without glare at photopic and mesopic levels.
The adverse events, clinical and IOL observations, intraocular pressure (IOP), surgical complications, and presence or absence of posterior capsule opacification (PCO) were recorded.
Patient reported outcomes
Patients were asked to grade the satisfaction of distant, intermediate, near vision (uncorrected) at home, under daylight/streetlight, and at night/at twilight on a scale of 1 to 4 based on the following subcategories: 1: fair; 2: mediocre; 3: good, 4: very good. Also, patients were asked to grade the frequency of spectacle wear based on the following subcategories: 0% (never), between 1% and 25% of time, between 26% and 50% of time, between 51% and 75% of time, and between 76% and 100% of time. The following visual disturbances were assessed by a patient questionnaire: ghost image, double vision, halos, glare, discoloration, distorted near or far vision, blurred near or far vision. Patients were asked to rate the effect of each phenomenon on a scale of 0 to 4 based on the following subcategories: 0: none; 1: mild; 2: moderate; 3: severe, 4: incapacitating. All patient-reported outcomes were based on patients’ uncorrected vision except the frequency of spectacle wear.
Statistical analysis
The normality was checked using the Kolmogorov-Smirnov and Shapiro-Wilk tests. Within-group categorical scale results were analyzed using the Wilcoxon signed-rank test and are reported as the point percentage change within the respective categories. All analyses included all patients except the defocus curve evaluation, which was based on a best-case cohort. Kruskall-Wallis test was used to test the differences between groups and Mann-Whitney test was used for between-groups comparions with Bonferroni correction for multiple comparisons.
Results
Patient demographics
The study included 122 eyes of 61 patients (33 female, 28 male) who underwent bilateral implantation of diffractive multifocal IOL. The demographic data of the patients is given in Table 2. The mean age of the patients at the time of surgery was 60.3 ± 8.4 (44 to 85) years. No patient was lost to follow-up or excluded.
Table 2.
Demographics, follow-up, and pre- and postoperative refractive data.
| +2.5 D IOL | +3.0 D IOL | +3.75 D IOL | P | |
|---|---|---|---|---|
| Patients/eyes | 20/40 | 20/40 | 21/42 | – |
| Mean age (years) | 58.1 ± 9.1 (42–72) | 59.7 ± 8.7 (44–77) | 63.8 ± 7.6 (53–81) | 0.112 |
| Sex | 9F, 11 M | 11F, 9 M | 13F, 8 M | |
| Pre-op K (D) | 42.9 ± 1.2 | 43.12 ± 1.32 | 43.06 ± 1.24 | 0.854 |
| Post-op SE (D) | 0.22 ± 0.39 | 0.18 ± 0.41 | 0.008 ± 0.44 | 0.892 |
| Post-op C (D) | 0.37 ± 0.33 | 0.38 ± 0.38 | 0.38 ± 0.29 | 0.911 |
IOL = intraocular lens.
P = Wilcoxon signed ranks test.
Visual acuity
The mean UDVA, and CDVA are given in Table 3. There was no statistically significant difference in UDVA and CDVA between three groups (p > 0.05). The mean distance corrected near-intermediate visual acuities at different distances are given in Fig. 1. The +2.50 group had better IVA than both +3.0 group (at 50 cm, and 55 cm), and +3.75 group (at 45 cm, 50 cm, 55 cm, and 60 cm). The +3.0 group had better NVA than +2.50 group at 32 cm, and had better IVA than +3.75 group (at 50 cm, and 55 cm). The +3.75 D group had better NVA than both +2.50 group (at 32 cm, and at 40 cm) and +3.0 group at 32 cm (p < 0.05).
Table 3.
Comparison of visual acuity among 3 different multifocal IOL groups.
| +2.5 D IOL | +3.0 D IOL | +3.75 D IOL | P | P2.5-3.0 | P2.5-3.75 | P3.0-3.75 | |
|---|---|---|---|---|---|---|---|
| UDVA | 0.01 ± 0.04 (−0.08 to 0.10) |
0.04 ± 0.07 (0.0 to 0.18) |
0.03 ± 0.04 (−0.08 to 0.10) |
0.434 | 0.214 | 0.156 | 0.176 |
| CDVA | 0.01 ± 0.01 (−0.08 to 0.10) |
0.01 ± 0.02 (−0.08 to 0.10) |
0.02 ± 0.02 (−0.08 to 0.10) |
0.786 | 0.414 | 0.456 | 0.463 |
IOL = intraocular lens, UDVA = uncorrected distance visual acuity, CDVA = corrected-distance visual acuity, D = diopters.
P = Wilcoxon signed ranks test.
P2.5-3.0 = statistical significance of difference between +2.5 D IOL and +3.0 D IOL groups, Mann-Whitney U test; P2.5-3.75 = statistical significance of difference between +3.0 D IOL and +3.75 D IOL groups, Mann-Whitney U test; P3.0-3.75 = statistical significance of difference between +3.0 D IOL and +3.75 D IOL groups, Mann-Whitney U test.
Fig. 1.
The mean distance corrected near-intermediate visual acuities at different distances.
Defocus curves
The spherical defocus curves of the three different IOLs are given in Fig. 2. The mean binocular defocus curves showed that all three IOLs produced the full range of vision from near to far. However, the +2.50 group curve showed a mean binocular intermediate visual acuity of better than both +3.00 group (from −1.00 D to −1.50 D) and +3.75 group (from −1.00 D to −2.00 D), and the +3.0 group curve showed a mean binocular intermediate visual acuity better than +3.75 group (from −1.50 D to −2.0 D). The +3.75 group showed a better near visual acuity than +2.50 group (from-2.50 to −4.00 D), and +3.00 group (from-3.00 to −4.00 D) (p < 0.05).
Fig. 2.
The spherical defocus curves of the three different IOLs.
The ATR astigmatic defocus results of three different IOLs are given in Fig. 3. The +2.50 D group showed better ATR astigmatic tolerability than +3.00 group (at 2.00 D) and +3.75 group (at 1.50 D, and at 2.00 D) (p < 0.05).
Fig. 3.
The astigmatic (against-the-rule) defocus curves of the three different IOLs.
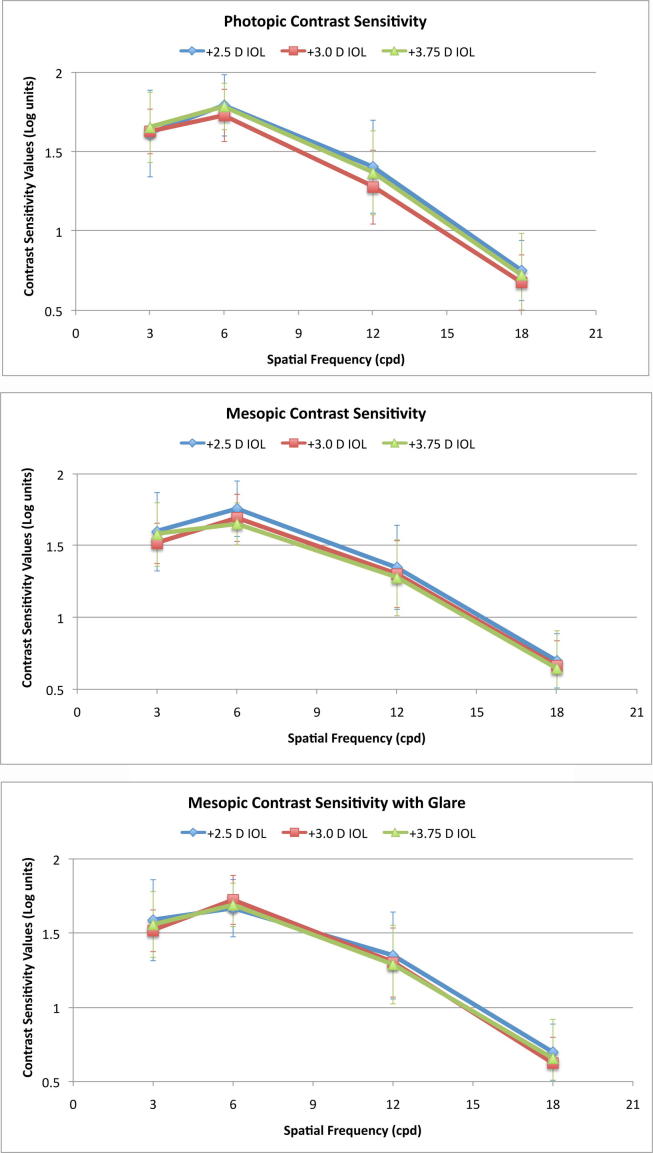
Contrast sensitivity
The contrast sensitivity results of three multifocal IOL groups under photopic, mesopic, and mesopic with glare conditions are given in Fig. 4. There was no significant difference in mean photopic, mesopic, and mesopic with glare logarithmic contrast values at all frequencies (3, 6, 12, and 18 cyles per degree) between three multifocal IOL groups (p > 0.05).
Fig. 4.
Contrast sentitivty (CS) measurements of three different IOL groups under photopic (upper), mesopic (middle), and mesopic with glare (lower) conditions. Statistical differences (P-values, Kruskall-Wallis test) between three IOL groups for photopic CS were 3 cpd = 0.902, 6 cpd = 0.273, 12 cpd = 0.053, 18 cpd = 0.212; for mesopic CS were 3 cpd = 0.663, 6 cpd = 0.143, 12 cpd = 0.476, 18 cpd = 0.663; and for mesopic with glare CS were 3 cpd = 0.792, 6 cpd = 0.392, 12 cpd = 0.541, 18 cpd = 0.448.
Patient-reported outcomes
The mean distance, intermediate, and near vision satisfaction levels are given in Fig. 5. The +2.50 group had the best levels for distance vision satisfaction at all situations followed by +3.0 group, and +3.75 group. However, there was no statistically significant difference in satisfaction levels between multifocal IOL groups for the distant vision satisfaction (p > 0.05). The +2.50 group had the best levels for intermediate vision satisfaction at home and at daylight, followed by +3.00 group; but the +3.75 group had better satisfaction at night/twilight. The +2.50 group had the worst levels for near vision satisfaction at all situations, and the +3.75 IOL had best near vision satisfaction at all situations. The +3.75 group had statistically significantly better near vision satisfaction than those of +2.50 group, and +3.00 group at night/twilight.
Fig. 5.
The distance, intermediate, and near vision satisfaction levels of three different IOLs under three different illumination circumstances (home, daylight/street, night/twilight).
All patients reported spectacle independence for distance vision. The spectacle dependance for near vision levels of three different IOL groups are given in Fig. 6. Ten (67%) of 15 patients in +2.50 group reported they use spectacles, and 2 patients in +2.50 group reported that they wear spectacles for near %50 to %75 of times. Four (27%) patients in group +3.00 reported spectacle dependance for near 0% to 25% of time. All patients in +3.75 group reported spectacle independence all time. Fourteen of 15 patients in +2.50 group, and 9 of 15 patients in +3.00 group reported that their near vision got worse and their spectacle independence increased by time between 1 to 6 months during the follow-up. All patients in +2.50 group, and +3.00 group reported that they need to adjust light during near vision.
Fig. 6.
Spectacle dependance levels of three different IOLs.
The mean visual disturbance rate reported by patients in three different IOL groups are given in Fig. 7. There were no statistically significant differences in ghost image, double vision, glare, discoloration, distortion, and blur between groups (p > 0.05). The +2.50 group reported significantly less halos and glares than those of +3.00 and +3.75 groups. Of the all scores, they were of mild severity.
Fig. 7.
The visual disturbance rate of three different IOLs.
Safety
No other complication was reported in any of the groups. All eyes were well centered, and none was tilted. 2 eyes of 2 patient developed grade 1 PCO, and 1 eye of 1 patient developed grade 3 PCO that underwent neodymium: yytrium argon (Nd:YAG Laser) capsulotomy in +3.75 group. 1 eye of 1 patient developed grade 1 PCO in +3.00 D IOL group.
Discussion
The current study showed that eyes with different types of multifocal IOLs have good distance, intermediate, and near vision that these IOLs designed to provide. All 3 IOL groups in this study provided good results for distance vision, whereas +2.50 IOL group provided better intermediate vision between 45 to 55 cm and defocus curve than the +3.00, and +3.75 groups. On the other hand as expected, the +3.75 group provided the best near vision, followed by +3.00 IOL and +2.50 groups.
Previous studies,10, 14, 15 including a randomized prospective study by Maxwell et al.14 compared the visual acuity results of multifocal IOLs with +3.00 D add with +4.00 D add and found similar near vision but better intermediate vision with +3.00 D add multifocal IOL compared to those with +4.00 D add. However, the distance of near vision target was different for each of the multifocal IOL in these studies.10, 14, 15
In an investigator-initiated study, Gundersen and Potvin16 compared the visual performance of hybrid multifocal IOLs with +2.50 D add power and +3.00 D add power and found that intermediate vision was not statistically significant between groups. These results are relatively in compliance with our study but different study designs and different near target distances in studies may explain the differences between our study and previous ones.4, 10, 15, 16, 17
Although the distance visual acuity results and satisfaction levels were not significantly different between IOL groups in our study, the +2.50 and +3.00 groups provided best distance satisfaction levels particularly under low light conditions. This may be explained by hybrid design of these IOLs that allows them to act more like a monofocal IOL under low light conditions.
The +2.50 group provided best intermediate vision defocus curve, followed by +3.00 and +3.75 groups. However, the satisfaction levels of +3.75 group for intermediate vision was significantly better than the +2.50 and +3.00 groups under low light conditions which can be explained by full diffractive design of this IOL that still provides some intermediate vision under low light conditions. Conversely, the hybrid IOL design allows more light energy focused for distance with larger pupils and may not provide enough intermediate vision under low light.5, 18
The satisfaction of multifocal IOL significantly depends on spectacle independence and the average adult reading distance is 33 to 40 cm.1, 2 The +3.75 group and +3.00 group had significantly better near vision and better near vision satisfaction than +2.50 group. Ten (66%) of 15 patients with 2.50 group, and 3 (8%) of 15 patients with +3.00 group had spectacle dependance for near vision at some point, but mostly under 50% of time. This may imply that patients that are planned to be implanted with multifocal IOL with +2.50 D addition should be warned for the possibility of needs for near glasses, or a distance adjustment for a functional near vision. Also, as it is the case with the intermediate vision, the satisfaction levels for near vision with hybrid IOL designs were lower than that of full diffractive IOLs under low light conditions, possibly because of hybrid design that acts like a monofocal IOL under low light conditions.5, 18 One should always keep in mind that the reading distance preference can affect the results of near vision satisfaction.
Our study showed that +2.50 group had better astigmatism tolerability (higher than 1.0 D of against-the-rule astigmatism) for distance vision than +3.00, and +3.75 groups. Compared to monofocal IOLs, multifocal IOLs inherently split the light and they are more sensitive to the postoperative astigmatism. It was suggested that postoperative astigmatism may significantly affect the visual quality of the eyes with multifocal diffractive IOLs.1, 2 In a previous study, Hayashi et al.17 compared the against-the-rule astigmatic tolerability of +3.00 hybrid multifocal IOL with +4.00 hybrid multifocal IOL and monofocal IOL, and found better astigmatism tolerability with the difference increasing with the amount of astigmatism. The differences in the number of the diffractive rings, lens design, and add power may possibly change the effects of postoperative astigmatism on vision in eyes with multifocal IOLs. Our results may imply that lower number of diffractive rings may have better astigmatic tolerability.
In this study, we were not able to find any statistically significant difference in photopic, and mesopic contrast sensitivity with glare and without glare between the 3 IOL groups. Although there is variability among different studies, previous studies also could not find a significant difference in contrast sensitivity between +3.00 and +4.00 addition power.4, 10, 19 The hybrid multifocal IOL design (the +2.50 D and +3.00 D IOLs) acts more like a monofocal and could be expected to have a better contrast sensitivity under mesopic conditions.18 On the other hand, according to the manifacturer, the multifocal IOL with +3.75 D add power has smoothened ridges at the diffractive ring transitions that were designed to increase retinal image quality.
The rate of perception of visual disturbances were relatively little but slightly different between the 3 IOL groups in our study. The halo perception was higher in +3.75 group, than +3.00 D group, and +3.00 group more than +2.50 group but was only statistically significant between +3.75 group and +2.50 groups. This may be explained by the halo size that depends on the add power of the near focus.10, 20 On the other hand, there was no significant difference in glare between the 3 IOL groups. Previous studies reported different incidences and severities of visual disturbances, partly because of different patient groups, different IOLs, and different measurement techniques.
In terms of complications there was no significant differences between 3 IOL groups. There were some weaknesses and limitations of the study. In the measurements of the near and intermediate vision, the same optotypes were used for each distance examined and visual acuities without correction were not shown.
The number of the eyes in each group was limited and higher number of eyes could give better outcomes for subtle differences. Also, subjective tests such as visual acuity and contrast sensitivity measurements that were used in our study, as well as patient reported satisfaction levels and visual disturbances might have been affected by the patient’s psychophysical situation, education, occupation, socioeconomic status, and more so expectations.
Conclusions
Our study shows that different multifocal IOLs with different addition power have superiority to each other. Multifocal IOLs with +2.50 D add power tend to have better intermediate vision than multifocal IOLs with +3.00 D and +3.75 D add powers. Patients with +2.50 D add power multifocal IOLs would need spectacles for near vision more than multifocal IOLs with +3.00 D and +3.75 D add powers. Hybrid design multifocal IOLs tend to have worse intermediate and near vision at low light levels. Multifocal IOLs with +2.50 D add power tend to have better astigmatic defocus tolerability than multifocal IOLs with +3.00 D and +3.75 D add powers. Therefore multifocal IOL selection should be based on patients needs and preferences. Preoperative information is important for realistic patient experience and satisfaction. Possibly, furhter studies with higher number of patients that evaluate the differences in objective measurements are needed to better elucidate the differences between multifocal IOLs.
Funding information
This study was not funded.
Declaration of interest
The authors have no financial interest in any of the issues contained in this article and have no proprietary interest in the development of marketing of or materials used in this study.
The manuscript was presented as a free paper at the 33rd Congress of the European Society of Cataract and Refractive Surgeons (ESCRS) on the 8th of September, 2015 in Barcelona, Spain.
Footnotes
Peer review under responsibility of Saudi Ophthalmological Society, King Saud University.
References
- 1.Cochener B., Lafuma A., Khoshnood B., Courouve L., Berdeaux G. Comparison of outcomes with multifocal intraocular lenses: a meta-anaylsis. Clin Ophthalmol. 2011;5:45–56. doi: 10.2147/OPTH.S14325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Braga-Mele R., Chang D., Dewey S. Multifocal intraocular lenses: relative indications and contraindications for implantation. J Cataract Refract Surg. 2014;40(2):313–322. doi: 10.1016/j.jcrs.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 3.Olson R.J., Werner L., Mamalis N., Cionni R. New intraocular lens technology. Am J Ophthalmol. 2005;140(4):709–716. doi: 10.1016/j.ajo.2005.03.061. [DOI] [PubMed] [Google Scholar]
- 4.Cillino G., Casuccio A., Pasti M., Bono V., Mencucci R., Cillino S. Working-age cataract patients: visual results, reading performance, and quality of life with three diffractive multifocal intraocular lenses. Ophthalmology. 2014;121(1):34–44. doi: 10.1016/j.ophtha.2013.06.034. [DOI] [PubMed] [Google Scholar]
- 5.Artigas J.M., Menezo J.L., Peris C., Felipe A., Díaz-Llopis M. Image quality with multifocal intraocular lenses and the effect of pupil size: comparison of refractive and hybrid refractive-diffractive designs. J Cataract Refract Surg. 2007;33(12):2111–2117. doi: 10.1016/j.jcrs.2007.07.035. [DOI] [PubMed] [Google Scholar]
- 6.Xu X., Zhu M.M., Zou H.D. Refractive versus diffractive multifocal intraocular lenses in cataract surgery: a meta-analysis of randomized controlled trials. J Refract Surg. 2014;30(9):634–644. doi: 10.3928/1081597X-20140814-04. [DOI] [PubMed] [Google Scholar]
- 7.Gil M.A., Varón C., Cardona G., Vega F., Buil J.A. Comparison of far and near contrast sensitivity in patients symmetrically implanted with multifocal and monofocal IOLs. Eur J Ophthalmol. 2014;24(1):44–52. doi: 10.5301/ejo.5000335. [DOI] [PubMed] [Google Scholar]
- 8.Dyrda A., Martínez-Palmer A., Martín-Moral D. Clinical results of diffractive, refractive, hybrid multifocal, and monofocal intraocular Lenses. J Ophthalmol. 2018;25(2018):8285637. doi: 10.1155/2018/8285637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pepose J.S., Wang D., Altmann G.E. Comparison of through-focus image sharpness across five presbyopia-correcting intraocular lenses. Am J Ophthalmol. 2012;154:20–28. doi: 10.1016/j.ajo.2012.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Petermeier K., Messias A., Gekeler F. Effect of +3.00 diopter and +4.00 diopter additions in multifocal intraocular lenses on defocus profiles, patient satisfaction, and contrast sensitivity. J Cataract Refract Surg. 2011;37(4):720–726. doi: 10.1016/j.jcrs.2010.11.027. [DOI] [PubMed] [Google Scholar]
- 11.Aslan B.S., Muftuoglu O., Gayretli D. Crater and split technique for phacoemulsification: modification of the crater-and-chop technique. J Cataract Refract Surg. 2012;38:1526–1530. doi: 10.1016/j.jcrs.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 12.Mackool R.J. Personal phacoemulsification technique. In: Buratto L., Werner L., Zanini M., Apple D., editors. Phacoemulsification; principles and techniques. 2nd ed. Thorofare; NJ, Slack: 2003. pp. 363–376. [Google Scholar]
- 13.Tello A. Crater and split: not a new technique. J Cataract Refract Surg. 2013;39(2):305. doi: 10.1016/j.jcrs.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 14.Maxwell W.A., Cionni R.J., Lehmann R.P., Modi S.S. Functional outcomes after bilateral implantation of apodized diffractive aspheric acrylic intraocular lenses with a +3.0 or +4.0 diopter addition power; randomized multicenter clinical study. J Cataract Refract Surg. 2009;35(12):2054–2061. doi: 10.1016/j.jcrs.2009.06.041. [DOI] [PubMed] [Google Scholar]
- 15.Alfonso J.F., Fernandez-Vega L., Puchades C., Montés-Micó R. Intermediate visual function with different multifocal intraocular lens models. J Cataract Refract Surg. 2010;36(5):733–739. doi: 10.1016/j.jcrs.2009.11.018. [DOI] [PubMed] [Google Scholar]
- 16.Gundersen K.G., Potvin R. Comparative visual performance with monofocal and multifocal intraocular lenses. Clin Ophthalmol. 2013;7:1979–1985. doi: 10.2147/OPTH.S52922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hayashi K., Manabe S., Yoshida M., Hayashi H. Effect of astigmatism on visual acuity in eyes with a diffractive multifocal intraocular lens. J Cataract Refract Surg. 2010;36(8):1323–1329. doi: 10.1016/j.jcrs.2010.02.016. [DOI] [PubMed] [Google Scholar]
- 18.Davison J.A., Simpson M.J. History and development of the apodized diffractive intraocular lens. J Cataract Refract Surg. 2006;32(5):849–858. doi: 10.1016/j.jcrs.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Santhiago M.R., Wilson S.E., Netto M.V. Visual performance of an apodized diffractive multifocal intraocular lens with +3.00 D addition: 1-year follow-up. J Refract Surg. 2011;27(12):899–906. doi: 10.3928/1081597X-20110816-01. [DOI] [PubMed] [Google Scholar]
- 20.Pieh S., Lackner B., Hanselmayer G. Halo size under distance and near conditions in refractive multifocal intraocular lenses. Br J Ophthalmol. 2001;85(7):816–821. doi: 10.1136/bjo.85.7.816. [DOI] [PMC free article] [PubMed] [Google Scholar]