Abstract
Neutrophil extracellular traps (NETs) play an important role in innate immunity to microbial infections. NETs have been described in several species, but the molecular details of NET formation and their role in infection has not been addressed, partly because we lack optimal experimental models. Here we describe tools to investigate NET formation in neutrophils isolated from mice. Upon in vitro stimulation of wild-type mouse neutrophils with PMA, we analyzed 3 important steps in the process of NET formation: reactive oxygen species (ROS) production, NET cell death and NET release. As expected, neutrophils from NADPH oxidase-deficient mice failed to produce ROS and did not die nor release NETs upon stimulation. We found that neutrophils from several mouse strains produced NETs with different efficiency and that NET formation correlated with the amount of ROS produced. Activation with Candida albicans also resulted in ROS production and NET cell death. The hyphal form of this fungus induced NETs more effectively than the yeast form. With this work, we provide tools to study in vitro NET assembly in the mouse system.
Key Words: Neutrophils, PMA, Host defense, Fungal/oxidative burst, In vitro assays, Animal models, Knockout mice
Introduction
Neutrophils are professional phagocytes that serve as the first line of defense against invading microorganisms [1]. During maturation in the bone marrow, neutrophils synthesize enzymes and antimicrobial proteins, which are stored in different granules [2]. These are, together with their lobulated nuclei, morphological hallmarks of neutrophils. Mature neutrophils leave the bone marrow and enter the circulation, where they have a short half-life. If recruited to the site of infection, neutrophils kill microorganisms intracellularly by phagocytosis or extracellularly by the release of neutrophil extracellular traps (NETs) [3]. NETs are structures made of chromatin decorated with antimicrobial proteins and have been described in humans, rabbits [3], horses [4], cows [5], fish [6] and mice [7, 8]. NETs are involved in trapping pathogens [3, 9, 10], severe sepsis [11], preeclampsia [12] as well as autoimmune diseases [13] and could potentially serve as a prognostic marker for multitrauma [14]. Recently, other immune cells, such as mast cells [15] and eosinophils [16], were found to release NET-like structures that were collectively termed extracellular traps (ETs) [17].
NETs are released through a novel cell death pathway that depends on the generation of reactive oxygen species (ROS). Upon stimulation, neutrophils produce ROS, such as O–, H2O2 and HOCl [18], which are antimicrobial [19] and important for NET formation [20]. Indeed, neutrophils from patients with chronic granulomatous disease (CGD) lack a functional NADPH oxidase [18] and cannot form NETs [20]. The lack of ROS affects both intracellular and extracellular killing mechanisms of neutrophils [20]. Therefore, these patients suffer from life-threatening microbial infections [21]. During the process of NET formation, ROS production leads to morphological changes like delobulation of the nucleus and disassembly of the nuclear envelope. After the breakdown of the nuclear envelope, the chromatin mixes intracellularly with granular proteins. Finally, the plasma membrane ruptures and NETs are released [20], a process which is now referred to as NETosis [22, 23].
An appropriate animal model would be invaluable for the analysis of NET formation and function. Here we analyze NET formation in neutrophils isolated from mice. In this study, we establish tools to quantify NET release upon stimulation with phorbol ester and Candida albicans. Confirming data in human neutrophils, we show that neutrophils from mice lacking a functional NADPH oxidase (gp91–/–) [24] are unable to make NETs. Using these tools, we compared NET formation in neutrophils from 7 different inbred mouse strains [25] and show that they all release NETs, albeit with different efficiency. NET release in the different mouse strains correlates with ROS production.
Materials and Methods
Materials
RPMI 1640, HEPES solution, phosphate-buffered saline (PBS) as well as Hanks balanced saline solution (HBSS) with and without CaCl2/MgCl2 were obtained from Gibco. Mouse serum was obtained from the animal facility of the Max Planck Institute for Infection Biology. All other chemicals were purchased in analytical grade from Sigma.
Antibodies
Antibodies used were a mouse monoclonal against the H2A/H2B-DNA complex [26], a rabbit polyclonal against MPO (DAKO A0398) and a rat monoclonal against a murine neutrophil surface antigen (Serotec). For FACS analysis, the monoclonal antibody against GR-1, the granulocyte receptor 1 was raised from the hybridoma RB6-8C5 [27].
Mice
SJL/J (SJL), PL/J (PLJ), CZECHII/EiJ (CzechII), C57Bl/6, 129S2, CD1, BALB/c and gp91phox (CYBB)-deficient (B6.129S6-Cybbtm1Din/J) mice were obtained from Charles River Laboratories. All mice were bred under specific pathogen-free conditions in the mouse facility of the Max Planck Institute for Infection Biology. For the isolation of neutrophils, 6- to 10-week-old mice were used. All animal experiments were done according to the German animal protection law.
Isolation of Neutrophils
Bone marrow-derived neutrophils were isolated using modifications of published methods [28]. Briefly, the bone marrow was flushed out of the tibia and the femur using RPMI supplemented with 5% FCS, glutamine and penicillin/streptomycin and passed through a 70-μm cell strainer to obtain a single cell solution. Cells were pelleted and remaining erythrocytes were lysed using red blood cell lysis buffer (Sigma). The entire bone marrow was resuspended in HBSS without CaCl2/MgCl2 (HBSS–). Mature neutrophils were purified by centrifugation for 30 min at 1,500 g on a discontinuous Percoll gradient consisting of 52% (v/v), 69% (v/v) and 78% (v/v) Percoll in PBS. Mature neutrophils were recovered from the interphase between 69% and 78% Percoll. The purity of isolated neutrophils was assessed by measuring the cell size in a CasyTM Counter (Schärfe System). The cell fraction from 6.5 to 8.5 μm was taken as neutrophils and confirmed by analyzing hematoxylin and eosin-stained cytospin preparations. The expression of the granulocyte receptor 1 (GR-1), a surface marker for neutrophils, was measured by FACS analysis in a LSRII (Becton Dickinson).
ROS Measurement
ROS production was measured in a luminol-based chemiluminescence assay [29]. Neutrophils were seeded in a white 96-well plate with HBSS medium containing MgCl2/CaCl2, 50 μM luminol and 1.2 U/ml horseradish peroxidase (Calbiochem). Neutrophils were stimulated either with 100 nM PMA, C. albicans or left untreated. The chemiluminescence resulting from ROS production was measured in a Victor light luminometer (Perkin Elmer).
Cell Death Assay
Neutrophils were seeded in a black 96-well plate with 1× HBSS medium containing MgCl2/CaCl2, 2% DNase-free mouse serum and 1 mM Sytox green (Invitrogen). Cells were stimulated either with 100 nM PMA or C. albicans, left untreated or lysed with 0.2% Triton X-100 as a 100% lysis control. The fluorescence was measured every 10 min in an Askent Fluoroskan (Thermo Scientific) for a period of 16 h. The cells were kept at 37°C and 5% CO2. Every 30 min, 3 μl double-distilled H2O was added to all wells to compensate for evaporation.
Microscopy
For immunofluorescence and scanning electron microscopy (SEM), 5 × 105 neutrophils were seeded on 13-mm glass cover slips treated with 0.001% poly-lysine in HBSS containing MgCl2 / CaCl2 and 2% DNase-free mouse serum. Cells were stimulated either with 100 nM PMA, C. albicans or Listeria monocytogenes and subsequently fixed with 1% PFA at the indicated time points.
For immunofluorescence, specimens were blocked with 3% cold water fish gelatin, 5% donkey serum, 1% BSA and 0.25% Tween 20 in PBS, incubated with primary antibodies, and washed. Primary antibodies were detected with species-specific secondary antibodies. Specimens were analyzed using a DMRB microscope (Leica) equipped with a digital camera (DXM 1200; Nikon) or an SP5 confocal microscope (Leica).
For SEM, samples were postfixed with glutardialdehyde and contrasted using repeated incubations with osmium tetroxide and tannic acid. After dehydration in an ethanol series, samples were critical-point dried and coated with a layer of carbon/platinum [30]. Samples were analyzed at 10 kV in a LEO 1550 field emission SEM (Zeiss SMT).
For transmission electron microscopy (TEM), 2 × 106 neutrophils were seeded into 6-well plates with 1× HBSS containing MgCl2/CaCl2 and 2% DNAse-free mouse serum. After stimulation with 100 nM PMA, cells were fixed with 2.5% glutaraldehyde, contrasted using osmium tetroxide, tannic acid and uranyl acetate, and dehydrated in a graded ethanol series. Cells were detached from the bottom of the culture vessels with styrol and embedded in Polybed (Fluka). Ultrathin sections were cut on an ultramicrotome (Leica), lead citrate contrasted in a TEM stainer (Nanofilm) and analyzed in a LEO 906E transmission EM (Zeiss SMT), equipped with a side-mounted digital camera (Morada; Olympus SIS).
Statistical Analysis
Data of the cell death assay were analyzed with the unpaired Student t test (fig. 5c). To test for monotonous relationship, Spearman's rank correlation test was used (fig. 4c). p < 0.05 was considered statistically significant.
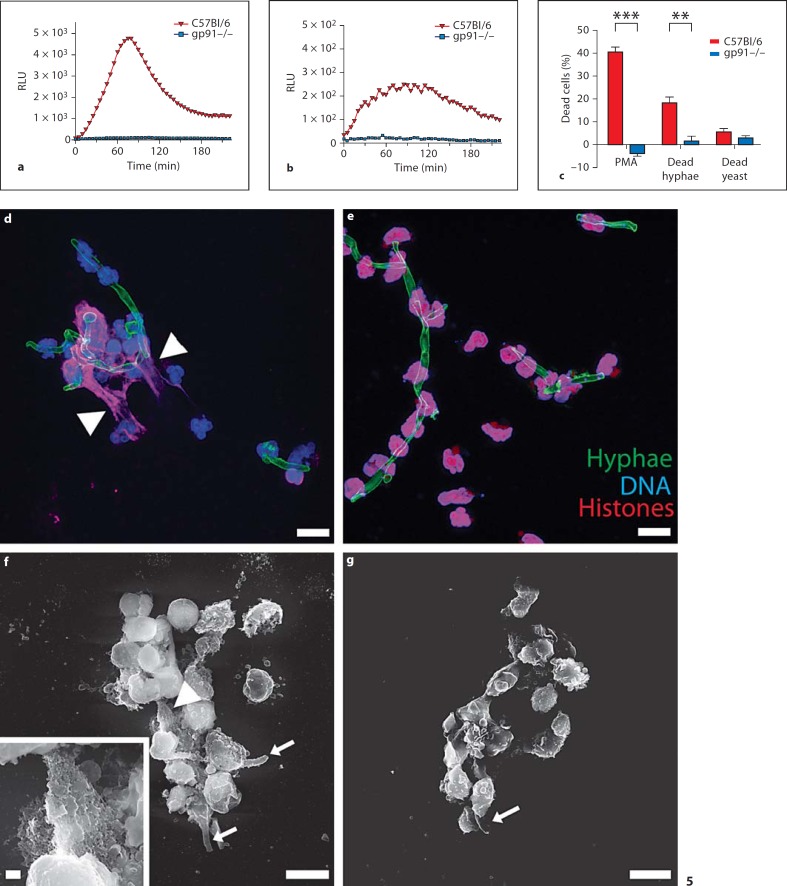
Fig. 5.
Mouse NETs trap C. albicans hyphae. Neutrophils purified from wild-type C57Bl/6 (wild type, red) produce more ROS when stimulated with C. albicans hyphae (a) than with yeast forms (b). gp91–/– mice (KO, blue) were used as negative controls. c Neutrophils purified from wild-type mice (red) underwent NET cell death when stimulated with C.albicans hyphae but not with the yeast form. Neutrophils from KO mice (blue) were used as negative controls and PMA-activated wild-type neutrophils as positive controls. NET cell death was assessed after 10 h (error bars, SEM of at least 4 independent experiments). ** p < 0.01, *** p < 0.001, significantly different from unpaired t test. To analyze NET release, activated wild-type (d) and KO (e) neutrophils were stained for DNA (blue), hyphae (green) and histone/DNA complexes (red) and analyzed by confocal microscopy. Both wild-type and KO neutrophils entwine the hyphae, but only wild-type neutrophils make NETs (arrowheads in d and f indicate NETs). High-resolution SEM analysis of wild-type (f) or KO (g) neutrophils stimulated with C. albicans hyphae (arrows in f and g indicate C. albicans hyphae) for 16 h shows that murine neutrophils make NETs. Scale bars = 10 μm, insert scale bar = 1 μm. RLU = Relative luminescence units.
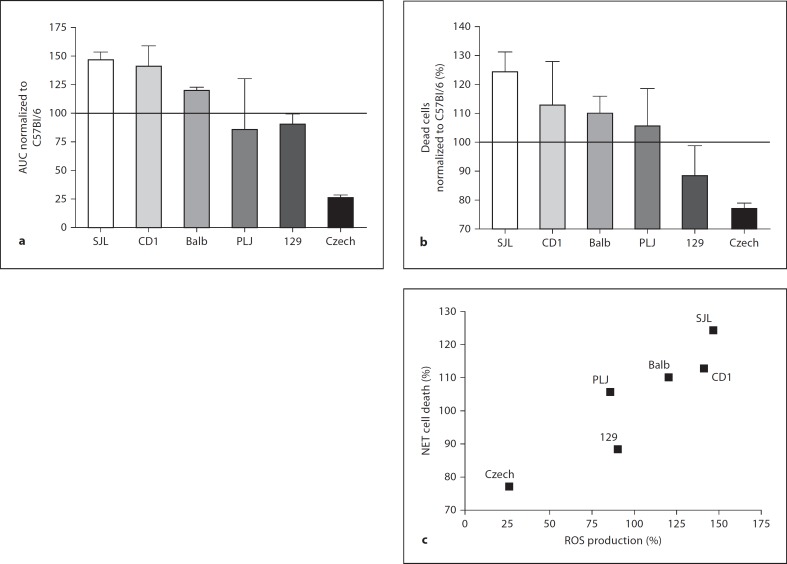
Fig. 4.
ROS production by activated neutrophils correlates with NET cell death in different mouse strains. Neutrophils from the indicated mouse strains were activated with 100 nM PMA for 10 h. a ROS production was measured with a luminometric assay. Total amounts of produced ROS were obtained by analyzing the area under the curve (AUC) and amounts were normalized to the strain C57Bl/6 (= 100). b NET cell death was analyzed 10 h after stimulation with the cell death assay described in figure 1. Values were normalized to the C57Bl/6 strain (= 100; error bars, SEM of at least 3 independent experiments per strain). c Values from the cell death assay and the ROS production were set in relationship and analyzed with a test for monotonous relationships (Spearman). Rank correlation coefficient is 0.9429 with a p value of 0.0167. Neutrophils from all tested strains produce ROS and die. The amount of produced ROS significantly correlates to NET cell death.
Results
Mouse Neutrophils Form NETs in vitro
We isolated mature neutrophils from mouse bone marrow by density centrifugation as described in Materials and Methods. The obtained population contained 85.8 ± 4.2% neutrophils, as measured by cell size analysis in a CasyTM counter and confirmed with FACS analysis using antibodies against GR-1 (online suppl. fig. 1a, b, see www.karger.com/doi/10.1159/000205281). These neutrophils phagocytosed particles (data not shown) and showed the characteristic lobulated nuclei morphology in hematoxylin and eosin stains (online suppl. fig. 1c).
gp91 is a subunit of the NADPH oxidase. In its absence, the enzyme complex is not functional, and mice deficient in gp91 (gp91–/–) are unable to produce ROS. Thus, these neutrophils are used as a model of CGD and, in these experiments, as a negative control. Neutrophils isolated from wild-type, but not from gp91–/– mice, produced ROS upon stimulation with PMA (fig. 1a).
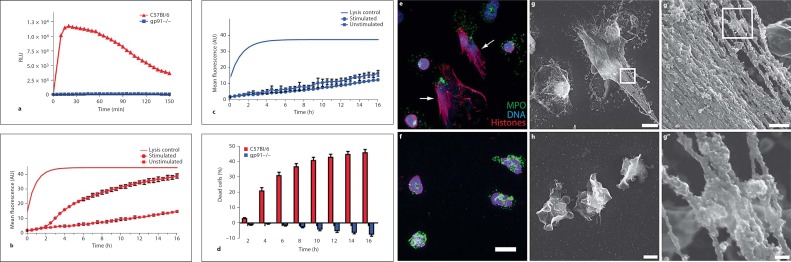
Fig. 1.
Mouse neutrophils release extracellular traps in vitro. Neutrophils were purified from C57Bl/6 (wild type) and gp91–/– mice (KO). a ROS production by wild-type and KO neutrophils activated with 100 nM PMA was measured with a luminometric assay. Wild-type (b) or KO (c) neutrophils were activated with 100 nM PMA and cell death was analyzed as described in Materials and Methods. NET cell death was calculated by subtracting the fluorescence of unstimulated neutrophils from the stimulated neutrophils at each time point and dividing it by the lysis control. d Cumulative data of cell death induced in wild-type and KO neutrophils. Fig. 1. e Wild-type and KO (f) neutrophils were activated for 16 h with 100 nM PMA, stained for DNA (blue), histone/DNA complexes (red) and MPO (green) and visualized by confocal microscopy. Only wild-type neutrophils show NET formation (arrows in e indicate NETs). High-resolution SEM analysis of wild-type (g, g’, g”) and KO (h) neutrophils. Cells were activated for 16 h with 100 nM PMA. Wild-type, but not KO neutrophils show formation of NETs. a–d Data are representative of at least 5 independent experiments [error bars (b, c), SEM of 5 samples]. f Scale bar = 10 μm. g, h Scale bars = 5 μm. g’ Scale bar = 500 nm. g” Scale bar = 100 nm. RLU = Relative luminescence units; AU = arbitrary units.
We established an assay to detect neutrophil cell death (NET cell death). We incubated the cells with Sytox green, a non-cell membrane permeable fluorescent DNA dye. We then monitored fluorescence emission every 10 min for a period of 16 h as a reporter of cell death. Unstimulated neutrophils served as a negative control. Lysis of the culture with the nonionic detergent Triton X-100 allowed us to measure the maximal possible signal. Upon PMA stimulation, wild-type neutrophils underwent cell death (fig. 1b), whereas gp91–/– neutrophils did not (fig. 1c), but rather survived longer than the unstimulated control. To calculate the percentage of cell death, we subtracted the background fluorescence from the sample values at each time point and divided it by the lysis control. We found that 40% of all wild-type neutrophils died upon activation with PMA (fig. 1d). In contrast, neutrophils isolated from gp91–/– mice survived longer than 16 h and activation with PMA increased their survival.
To visualize NET formation in vitro we seeded neutrophils onto cover slips and fixed them 16 h after stimulation. We stained the neutrophils using a DNA dye and antibodies against MPO and the histone/DNA complex. By indirect immunofluorescence microscopy we found that the nuclei of most wild-type neutrophils were delobulated and the DNA as well as MPO signals were co-localized. Additionally, we found MPO colocalizing with extracellular DNA, demonstrating that neutrophils released NETs (fig. 1e, arrows). Under the same conditions we observed some neutrophils with lobulated nuclei, indicating that they were not fully activated. This is consistent with our cell death assay (fig. 1d), since not all neutrophils respond to PMA by making NETs. Stimulated gp91–/– neutrophils flattened, indicating activation, but in contrast to wild-type cells, the nuclei remained lobulated. MPO remained localized to the granules and this distribution was similar to that observed in unactivated wild-type neutrophils (fig. 1f). Using SEM, we confirmed that wild-type neutrophils released NETs (fig. 1g), whereas gp91–/– neutrophils did not (fig. 1h). SEM analysis revealed that stimulated gp91–/– neutrophils had lost their globular shape and firmly attached to the bottom of the culture dish. High-resolution SEM (fig. 1g’, g”) of wild-type neutrophils showed that murine NETs consisted of smooth fibers (diameter of about 15 nm) and globular domains (>30 nm) similar to human NETs [3]. However, in contrast to human NETs, the globular domains attached to the fibers are tightly packed and cover nearly the entire fiber surface (fig. 1g’).
Kinetics of Murine NET Formation
To follow the different stages of NET formation, we stimulated neutrophils with PMA and fixed them after the indicated time points. We analyzed the samples for NET release by staining with the DNA stain Draq5 and antibodies against histone/DNA complexes and MPO (fig. 2a–h). Thirty minutes after stimulation, wild-type and gp91–/– neutrophils attached to the bottom of the culture vessel and flattened (fig. 2a, e). The nuclei were still lobulated and the MPO signal clearly depicts granules. At this time point, the periphery of some nuclei was stained by the antibody against histone/DNA complexes (red). After 4 h of stimulation, granular and nuclear patterns were still unchanged in gp91–/– cells (fig. 2f), and remained in this state during the entire course of the experiment (fig. 2g, h). In contrast, in wild-type neutrophils, we detected overlapping of the granular and the nuclear signal (fig. 2b, white areas), indicating morphological changes. Most nuclei lost their lobules, and many of them showed a homogeneous staining with the antibody against histone/DNA complexes (fig. 2b). After 8 h of stimulation, nearly all wild-type nuclei were round, delobulated and stained by the antibody against histone/DNA complexes. The area of nuclear and granular overlap increased (fig. 2c, white). At 12 h after stimulation, many wild-type neutrophils released NETs (fig. 2d, arrows), while in gp91–/– neutrophils the nuclear and granular morphology remained unchanged (fig. 2h).
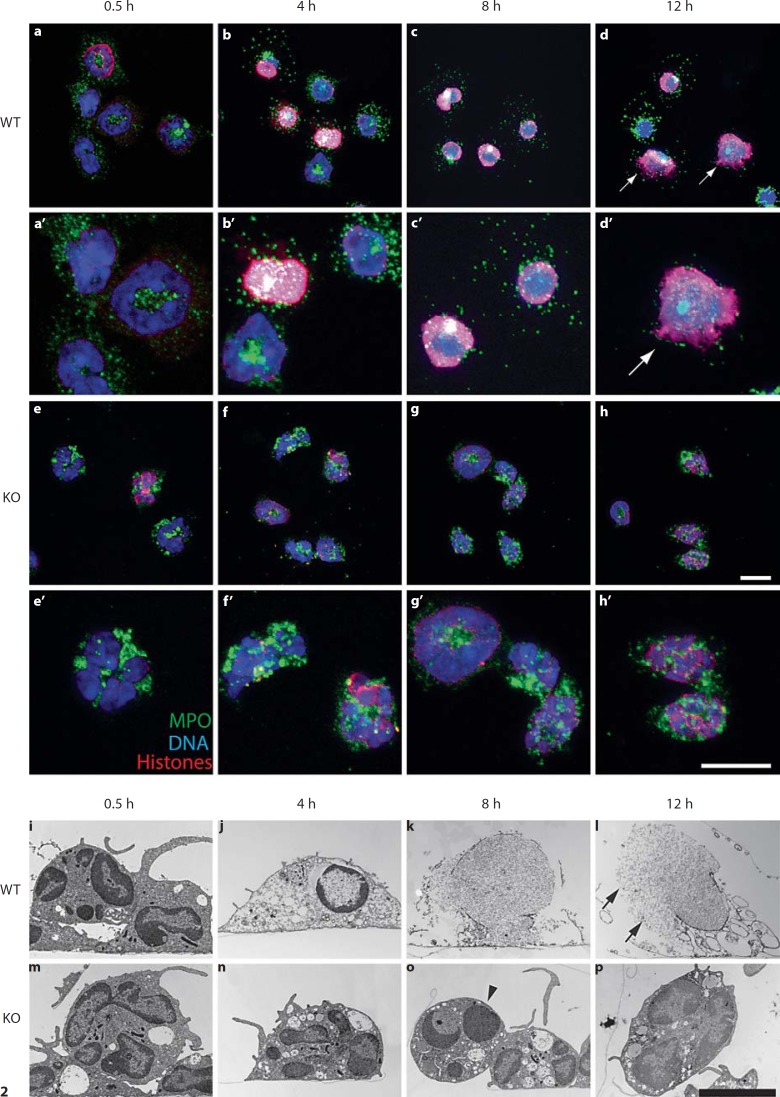
Fig. 2.
Microscopical analysis of NET formation in murine neutrophils. Neutrophils were purified from C57Bl/6 (wild type, WT) or gp91–/– (KO) mice and stimulated with 100 nM PMA for the indicated time. Confocal immunomicroscopy of wild-type (a–d) and KO (e–h) neutrophils stained for DNA (blue), MPO (green) and histone/DNA complexes (red). a’–d’ and e’–h’ represent a higher magnification of the corresponding pictures a–d and e–h, respectively Changes of nuclear morphology were visualized by TEM of wild-type (i–l) and KO (m–p) neutrophils. Both techniques show that 4 h after stimulation, the nuclei of wild-type neutrophils delobulate and there is colocalization of MPO and chromatin. NET formation is observed 12 h after activation (arrows in d and l indicate NETs, arrowhead in o indicates apoptotic cell). Data are representative of at least 3 independent experiments. Scale bars = 10 μm.
We analyzed fine structural modifications during NET formation by TEM. Shortly after stimulation, both wild-type and gp91–/– neutrophils settled down and flattened along the culture vessel bottom (fig. 2i, m). Vacuolization in wild-type neutrophils was as prominent as in gp91–/– neutrophils. Wild-type cells stimulated for 4 h showed delobulated nuclei and more electron-translucent cytoplasm (fig. 2j). Increased vacuolization was the only morphological change in stimulated NADPH oxidase-deficient neutrophils. Moreover, gp91–/– neutrophils kept the lobulated nuclei even after 12 h of stimulation, although some cells underwent apoptosis (fig. 2o, arrowhead). In contrast, 8 h after stimulation, wild-type neutrophils showed a complete loss of cytoplasmic and nuclear organization, evidenced by the disintegration of the nuclear envelope (fig. 2k). After 12 h, many stimulated wild-type neutrophils released NETs (fig. 2l, arrows).
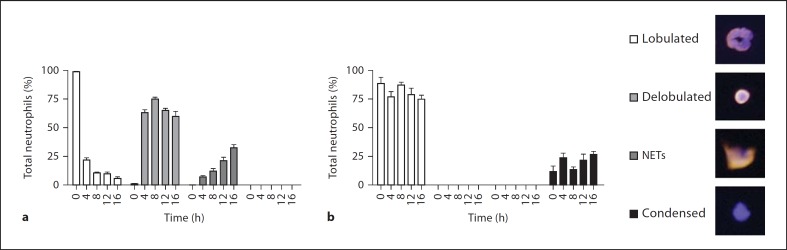
At each time point, we quantified the number of neutrophils that remained unchanged or showed different stages of NET formation, which we defined as (1) delobulated nuclei, (2) released NETs and (3) condensed chromatin which exclusively appeared in gp91–/– neutrophils (fig. 3a, b). After 4 h, we observed that the nuclei of more than 75% of the wild-type neutrophils were delobulated. In this intermediate stage, the nuclei stained homogeneously for histone/DNA complexes. After 16 h of stimulation, 59.8% of the cells remained in this state without releasing NETs. However, the amount of NETs increased continuously to a maximum of 30% after 16 h (fig. 3a; online suppl. fig. 2a). In contrast, the gp91–/– neutrophils neither developed delobulated nuclei nor released NETs. After 4–8 h, only condensed nuclei were present. These condensed nuclei were not positive for histone/DNA complexes and do not represent a stage of NET formation (fig. 3b; online suppl. fig. 2a). This confirmed our findings that neutrophils from gp91–/– mice, which fail to produce ROS upon stimulation, cannot make NETs.
Fig. 3.
Neutrophils were purified from C57Bl/6 (wild type) or gp91–/– (KO) mice and stimulated with 100 nM PMA. To quantify the morphological changes, neutrophils were stained for histone/DNA complexes and DNA at the indicated time points. Wild-type (a) and KO (b) neutrophils were assigned to the different morphological stages (error bars, SEM of 5 independent areas per time point). Data are representative of 3 independent experiments.
Comparison of Different Mouse Strains
We tested the efficiency of NET release with respect to the number of responding cells, the amount of ROS and the kinetics leading to cell death in 7 different inbred mouse strains. The strains were: C57Bl/6, 129S2, BALB/c, PLJ, SJL, CD1 and CzechII. These mouse strains were selected because they are genetically unrelated and commercially available.
We measured ROS levels for 3.5 h and determined the area under the curve. For each experiment, area under the curve values were normalized to C57Bl/6, an inbred strain often used for immunological research (fig. 4a). SJL and CD1 mice produced more ROS (between 130 and 150%) compared to neutrophils from C57Bl/6. ROS production in neutrophils from BALB/c, 129 and PLJ was similar to the standard. Notably, neutrophils from CzechII mice produced only 25% of the amount of ROS produced by C57Bl/6.
NET cell death correlated to the amount of ROS production. We analyzed NET cell death and normalized it to the cell death induced in neutrophils from C57Bl/6 mice. We measured cell death 10 h after stimulation, since NET cell death does not increase significantly after this time point (fig. 1b). On average, 20% more neutrophils from SJL mice and 30% fewer neutrophils from Czech mice died compared to C57Bl/6 (fig. 4b). Neutrophils from CD1, BALB/c, PLJ and 129 mice differed by only 12% compared to our standard. We could not detect any differences in the kinetics of NET formation in the tested strains (data not shown). We analyzed the correlation of ROS production and NET cell death using Spearman's test for monotonous relationships (fig. 4c). The rank correlation coefficient is significant (0.9429; p = 0.0167). In conclusion, all tested strains produce ROS and undergo NET cell death to different extents, and the efficiency of ROS production significantly correlates to NET cell death.
C. albicans Hyphae Induce NETs in vitro
Fungal hyphae cannot be phagocytosed by neutrophils, but they are susceptible to human NETs [10]. We tested whether C. albicans activates neutrophils to form NETs. We coincubated neutrophils with C. albicans yeast or hyphae at a ratio of 1:2. To discriminate between the stimulatory activity of yeast and hyphal forms we used dead C. albicans, since under neutrophil culture conditions C. albicans forms hyphae and overgrows the culture. We found that upon contact with both hyphal and yeast forms, neutrophils produced ROS. Interestingly, the response to hyphae was nearly 20 times stronger (fig. 5a, red) than that to yeast (fig. 5b, red). Under both conditions, ROS production reached a maximum at around 90 min after infection. As expected, neutrophils from gp91–/– mice did not produce ROS under these conditions (fig. 5a, b, blue).
C. albicans also induced NET cell death. Hyphae induced NET cell death 3 times more efficiently than the yeast form (fig. 5c). Notably, C. albicans hyphae induced cell death to nearly half of the efficiency of PMA, the most potent inducer of NET formation. Microscopic analysis showed that murine neutrophils release NETs upon challenge with C. albicans hyphae (fig. 5d). We stained the samples with the DNA dye Draq5 (blue) as well as antibodies against histone/DNA complexes (red) and C. albicans (green). NETs surrounding hyphae were clearly visible (fig. 5d, arrowheads). As expected, gp91–/– neutrophils did not form NETs (fig. 5e). Using SEM, we showed that both wild-type and gp91–/– neutrophils entwined C. albicans hyphae (fig. 5f, g, arrows indicate C. albicans), but only wild-type cells released NETs (fig. 5f, insert). Thus, hyphae-induced ROS production leads to NETosis in murine neutrophils and finally to NET release in vitro.
Murine NETs Trap L. monocytogenes
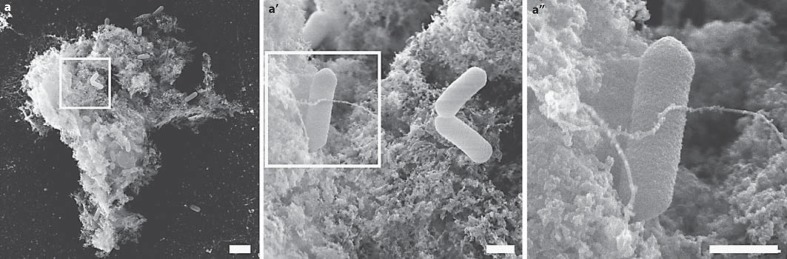
Using human neutrophils, it was shown that NETs not only trap fungi but also bacteria. We coincubated L. monocytogenes at a multiplicity of infection of 1 for 16 h with neutrophils purified from C57Bl/6 mice. SEM analysis revealed that mouse neutrophils released NETs and trapped L. monocytogenes (fig. 6a). High-resolution SEM analysis showed that the structure of the released NETs are similar to NETs induced with PMA or C. albicans (fig. 6a’, a”).
Fig. 6.
Mouse NETs trap L. monocytogenes. Neutrophils purified from C57Bl/6 mice were stimulated with L. monocytogenes (multiplicity of infection = 1) for 16 h. a–a” SEM shows that L. monocytogenes is trapped in NETs. a Scale bar = 2 μm. a’, a” Scale bars = 300 nm.
Discussion
Despite all advances in the analysis of NET formation and their function [8, 31, 32], good tools to study murine NET release in vitro have not been described. Compared to the mouse system, research with human neutrophils is relatively facile because they represent 65–75% of all peripheral blood leukocytes. The murine model, which is more amenable for experimentation, is complicated by the fact that in blood of this animal only 10–25% of leukocytes are neutrophils [33, 34]. Bone marrow contains large quantities of naive and mature neutrophils that can be isolated by a Percoll gradient purification [28, 35]. The transcriptional profiles of blood and bone marrow neutrophils have a Pearson correlation coefficient (γ) of 0.95, indicating that the developmental distance is very low [36]. Nevertheless, blood neutrophils might be significantly more mature than bone marrow neutrophils. We cannot exclude that these small differences may lead to a faster or more efficient release of NETs in neutrophils isolated from peripheral blood.
The 3 tools described here analyze 3 different steps in NET formation: (1) the quantification of ROS production, a very early event in NET formation, (2) the cell death assay detecting the last step prior to NET formation when the cell membrane ruptures and (3) the visualization of NET release by microscopy. In our studies, ROS production correlates with the cell death assay and NET release as determined by microscopy. Additionally, the specificity of our cell death assay was controlled by testing gp91–/– neutrophils. These results are consistent with data showing that human neutrophils isolated from CGD patients do not die upon PMA stimulation nor show release of NETs. These data indicate that the cell death assay allows the evaluation of NETs independently of microscopy. Thus, the cell death assay provides a screening method for analyzing mouse neutrophil extracellular trap assembly in vitro.
Using these assays, we analyzed 7 different mouse strains in their ability to release NETs upon stimulation. We found only minor differences in ROS production, NET cell death and NET formation (data not shown), showing that different inbred mice strains used in immunological research are capable of releasing NETs. Interestingly, we found a good correlation between the amount of ROS produced and NET cell death. In neutrophils, ROS are an essential component for NET formation, although their direct role remains to be determined.
We found that ROS production and NET formation can be triggered by PMA as well as by pathogenic fungi (C. albicans). Moreover, the amount of ROS production in murine neutrophils correlated with the concentration of PMA and the growth form of the fungi. Furthermore, we demonstrate that murine NETs trap L. monocytogenes. It is known that C. albicans, especially the hyphal form, stimulates a neutrophil respiratory burst [37]. We found that hyphae rather than yeast are a potent activator of neutrophils in terms of ROS production and NET release. Neutrophils recognize yeast forms as well, but the provided stimulus does not result in an oxidative burst and NET release comparable to hyphae. This is consistent with the notion that neutrophils can engulf yeast forms but not hyphae, which are too large. Our data indicate that NETs could therefore serve as extracellular control mechanism specific for hyphae, the growth form essential for invasion and dissemination of C. albicans [38].
Changes of the nucleus are very prominent during NET formation. The naive nucleus is well organized with clearly defined eu- and heterochromatin structured in several lobules. After initiation of NET formation, the nuclear structure gradually disassembles cumulating in the breakdown of the nuclear envelope. Concurrently, the chromatin decondenses, resulting in a fainter fluorescence signal with DNA dyes and a reduced electron density in TEM micrographs. This process is specific for the cell death program leading to NET formation. In contrast, during apoptosis, the chromatin is highly condensed and packed into membrane-bound apoptotic bodies. In apoptotic cells, the condensed chromatin produces a strong fluorescence signal after binding of DNA dyes and in TEM micrographs, these areas are very electron dense [20].
The degree of chromatin condensation seems to be reflected by the staining pattern of the murine monoclonal antibody directed against the subnucleosomal complex composed of H2A, H2B and DNA [26]. With this antibody, nuclei from naive murine neutrophils stain only at the periphery. When the nuclei decondensed and delobulated upon initiation of NET formation, the fluorescence pattern changed. We observed a homogenous staining of the entire nucleus. In contrast, delobulated but condensed nuclei from apoptotic neutrophils did not stain with this antibody. This indicates that the epitope recognized by the antibody is only accessible in decondensed chromatin, consistent with a strong homogenous staining of NETs.
Although the overall process is similar, NET formation in human and murine neutrophils differs in several details. In both species, NETs are the result of a series of morphological changes neutrophils undergo after activation and ROS production. The nucleus delobulates, rounds up and decondenses, the nuclear envelope desintegrates, nucleoplasm and cytoplasm mix, and finally the cell membrane ruptures, releasing the mixture of nuclear, granular and cytoplasmic components to form NETs. In human neutrophils, it takes 3–4 h until about 80% of the cells have undergone NETosis [20]. In contrast, murine neutrophils require about 16 h until approximately 30% of the stimulated mouse neutrophils have released NETs. This indicates that NET formation by mouse neutrophils takes longer and is less efficient than by human neutrophils. Unequal requirements of mouse and human neutrophils in ex vivo experiments might cause these differences. However, the role of NETs in bacterial infections of the mouse is clearly established [7, 8].
In immunofluorescence microscopy, NETs released from human neutrophils appear as outspread and web-like structures often extending over several cell diameters [3, 20]. In contrast, mouse NETs show a more compact structure and the majority are in close contact to the remnants of the neutrophils they originated from. High resolution field emission scanning microscopy revealed that similar to human NETs, murine NETs are composed of smooth stretches of about 15 nm diameter to which globular domains are attached. In contrast to human NETs, though, the packing density of the globular domains is higher, so almost no free stretches can be found. This finding is consistent with a more compact appearance of murine NETs in fluorescence microscopy.
At present, receptors and signaling pathways leading to NET release are still largely unknown. Using the assays described in this work on mouse strains deficient for signaling molecules may allow to unravel the cascade leading to NET formation. The cell death assay in particular provides the capability to perform high-throughput screens of receptors, signaling pathways and microorganisms leading to NET release.
Supplementary Material
Supplementary data
Acknowledgment
We thank Dr. U. Klemm for providing and organizing the different mouse strains and K. Metzler for critical reading of the manuscript. We thank Dr. S. Rupp for the C. albicans strain SC 5314 and Dr T. Joeris for providing L. monocytogenes, for critical reading of the manuscript and fruitful discussions.
References
- 1.Nathan C. Neutrophils and immunity: challenges and opportunities. Nat Rev. 2006;6:173–182. doi: 10.1038/nri1785. [DOI] [PubMed] [Google Scholar]
- 2.Faurschou M, Borregaard N. Neutrophil granules and secretory vesicles in inflammation. Microbes Infect. 2003;5:1317–1327. doi: 10.1016/j.micinf.2003.09.008. [DOI] [PubMed] [Google Scholar]
- 3.Brinkmann V, Reichard U, Goosmann C, Fauler B, Uhlemann Y, Weiss DS, Weinrauch Y, Zychlinsky A. Neutrophil extracellular traps kill bacteria. Science. 2004;303:1532–1535. doi: 10.1126/science.1092385. [DOI] [PubMed] [Google Scholar]
- 4.Alghamdi AS, Foster DN. Seminal DNase frees spermatozoa entangled in neutrophil extracellular traps. Biol Reprod. 2005;73:1174–1181. doi: 10.1095/biolreprod.105.045666. [DOI] [PubMed] [Google Scholar]
- 5.Lippolis JD, Reinhardt TA, Goff JP, Horst RL. Neutrophil extracellular trap formation by bovine neutrophils is not inhibited by milk. Vet Immunol Immunopathol. 2006;113:248–255. doi: 10.1016/j.vetimm.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 6.Palic D, Andreasen CB, Ostojic J, Tell RM, Roth JA. Zebrafish (Danio rerio) whole kidney assays to measure neutrophil extracellular trap release and degranulation of primary granules. J Immunol Methods. 2007;319:87–97. doi: 10.1016/j.jim.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 7.Beiter K, Wartha F, Albiger B, Normark S, Zychlinsky A, Henriques-Normark B. An endonuclease allows streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr Biol. 2006;16:401–407. doi: 10.1016/j.cub.2006.01.056. [DOI] [PubMed] [Google Scholar]
- 8.Buchanan JT, Simpson AJ, Aziz RK, Liu GY, Kristian SA, Kotb M, Feramisco J, Nizet V. DNase expression allows the pathogen Group A Streptococcus to escape killing in neutrophil extracellular traps. Curr Biol. 2006;16:396–400. doi: 10.1016/j.cub.2005.12.039. [DOI] [PubMed] [Google Scholar]
- 9.Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, Sagay SA, Egah DZ, Iya D, Afolabi BB, Baker M, Ford K, Ford R, Roux KH, Keller TC., 3rd Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in plasmodium falciparum infected children under six years of age. Malar J. 2008;7:41. doi: 10.1186/1475-2875-7-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Urban CF, Reichard U, Brinkmann V, Zychlinsky A. Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms. Cell Microbiol. 2006;8:668–676. doi: 10.1111/j.1462-5822.2005.00659.x. [DOI] [PubMed] [Google Scholar]
- 11.Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med. 2007;13:463–469. doi: 10.1038/nm1565. [DOI] [PubMed] [Google Scholar]
- 12.Gupta AK, Hasler P, Holzgreve W, Gebhardt S, Hahn S. Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia. Hum Immunol. 2005;66:1146–1154. doi: 10.1016/j.humimm.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 13.Fairhurst AM, Wandstrat AE, Wakeland EK. Systemic lupus erythematosus: multiple immunological phenotypes in a complex genetic disease. Adv Immunol. 2006;92:1–69. doi: 10.1016/S0065-2776(06)92001-X. [DOI] [PubMed] [Google Scholar]
- 14.Margraf S, Logters T, Reipen J, Altrichter J, Scholz M, Windolf J. Neutrophil-derived circulating free DNA (cf-DNA/NETs): a potential prognostic marker for posttraumatic development of inflammatory second hit and sepsis. Shock. 2008;30:352–358. doi: 10.1097/SHK.0b013e31816a6bb1. [DOI] [PubMed] [Google Scholar]
- 15.von Kockritz-Blickwede M, Goldmann O, Thulin P, Heinemann K, Norrby-Teglund A, Rohde M, Medina E. Phagocytosis-independent antimicrobial activity of mast cells by means of extracellular trap formation. Blood. 2008;111:3070–3080. doi: 10.1182/blood-2007-07-104018. [DOI] [PubMed] [Google Scholar]
- 16.Yousefi S, Gold JA, Andina N, Lee JJ, Kelly AM, Kozlowski E, Schmid I, Straumann A, Reichenbach J, Gleich GJ, Simon HU. Catapult-like release of mitochondrial DNA by eosinophils contributes to antibacterial defense. Nat Med. 2008;14:949–953. doi: 10.1038/nm.1855. [DOI] [PubMed] [Google Scholar]
- 17.Wartha F, Henriques-Normark B. ETosis: a novel cell death pathway. Sci Signal. 2008;1:pe25. doi: 10.1126/stke.121pe25. [DOI] [PubMed] [Google Scholar]
- 18.Babior BM. The respiratory burst of phagocytes. J Clin Invest. 1984;73:599–601. doi: 10.1172/JCI111249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lambeth JD. Nox enzymes and the biology of reactive oxygen. Nat Rev. 2004;4:181–189. doi: 10.1038/nri1312. [DOI] [PubMed] [Google Scholar]
- 20.Fuchs TA, Abed U, Goosmann C, Hurwitz R, Schulze I, Wahn V, Weinrauch Y, Brinkmann V, Zychlinsky A. Novel cell death program leads to neutrophil extracellular traps. J Cell Biol. 2007;176:231–241. doi: 10.1083/jcb.200606027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Heyworth PG, Cross AR, Curnutte JT. Chronic granulomatous disease. Curr Opin Immunol. 2003;15:578–584. doi: 10.1016/s0952-7915(03)00109-2. [DOI] [PubMed] [Google Scholar]
- 22.Steinberg BE, Grinstein S. Unconventional roles of the NADPH oxidase: signaling, ion homeostasis, and cell death. Sci STKE. 2007;379:pe11. doi: 10.1126/stke.3792007pe11. [DOI] [PubMed] [Google Scholar]
- 23.Brinkmann V, Zychlinsky A. Beneficial suicide: why neutrophils die to make nets. Nat Rev Microbiol. 2007;5:577–582. doi: 10.1038/nrmicro1710. [DOI] [PubMed] [Google Scholar]
- 24.Pollock JD, Williams DA, Gifford MA, Li LL, Du X, Fisherman J, Orkin SH, Doerschuk CM, Dinauer MC. Mouse model of x-linked chronic granulomatous disease, an inherited defect in phagocyte superoxide production. Nat Genet. 1995;9:202–209. doi: 10.1038/ng0295-202. [DOI] [PubMed] [Google Scholar]
- 25.Beck JA, Lloyd S, Hafezparast M, Lennon-Pierce M, Eppig JT, Festing MF, Fisher EM. Genealogies of mouse inbred strains. Nat Genet. 2000;24:23–25. doi: 10.1038/71641. [DOI] [PubMed] [Google Scholar]
- 26.Losman MJ, Fasy TM, Novick KE, Monestier M. Monoclonal autoantibodies to subnucleosomes from a MRL/Mp(–)+/+ mouse: oligoclonality of the antibody response and recognition of a determinant composed of histones H2A, H2B, and DNA. J Immunol. 1992;148:1561–1569. [PubMed] [Google Scholar]
- 27.Tepper RI, Coffman RL, Leder P. An eosinophil-dependent mechanism for the antitumor effect of interleukin-4. Science. 1992;257:548–551. doi: 10.1126/science.1636093. [DOI] [PubMed] [Google Scholar]
- 28.Allport JR, Lim YC, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, Vestweber D, Luscinskas FW. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J Leukoc Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- 29.Liu L, Dahlgren C, Elwing H, Lundqvist H. A simple chemiluminescence assay for the determination of reactive oxygen species produced by human neutrophils. J Immunol Methods. 1996;192:173–178. doi: 10.1016/0022-1759(96)00049-x. [DOI] [PubMed] [Google Scholar]
- 30.Goosmann C, Abed UA, Brinkmann V. Infection at the cellular level. Methods Cell Biol. 2008;88:477–496. doi: 10.1016/S0091-679X(08)00424-X. [DOI] [PubMed] [Google Scholar]
- 31.Wartha F, Beiter K, Albiger B, Fernebro J, Zychlinsky A, Normark S, Henriques-Normark B. Capsule and d-alanylated lipoteichoic acids protect streptococcus pneumoniae against neutrophil extracellular traps. Cell Microbiol. 2007;9:1162–1171. doi: 10.1111/j.1462-5822.2006.00857.x. [DOI] [PubMed] [Google Scholar]
- 32.Wartha F, Beiter K, Normark S, Henriques-Normark B. Neutrophil extracellular traps: casting the net over pathogenesis. Curr Opin Microbiol. 2007;10:52–56. doi: 10.1016/j.mib.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 33.Heinecke H. Das Blutbild der Maus (eine Übersicht) I. Das normale quantitative und qualitative weiβe Blutbild. Zschr Versuchstierk. 1961;1:16–37. [Google Scholar]
- 34.Russell ES, Neufeld EF, Higgins CT. Comparison of normal blood picture of young adults from 18 inbred strains of mice. Proc Soc Exp Biol Med. 1951;78:761–766. doi: 10.3181/00379727-78-19210. [DOI] [PubMed] [Google Scholar]
- 35.Boxio R, Bossenmeyer-Pourie C, Steinckwich N, Dournon C, Nusse O. Mouse bone marrow contains large numbers of functionally competent neutrophils. J Leukoc Biol. 2004;75:604–611. doi: 10.1189/jlb.0703340. [DOI] [PubMed] [Google Scholar]
- 36.Theilgaard-Monch K, Jacobsen LC, Borup R, Rasmussen T, Bjerregaard MD, Nielsen FC, Cowland JB, Borregaard N. The transcriptional program of terminal granulocytic differentiation. Blood. 2005;105:1785–1796. doi: 10.1182/blood-2004-08-3346. [DOI] [PubMed] [Google Scholar]
- 37.Lyman CA, Simons ER, Melnick DA, Diamond RD. Unopsonized candida albicans hyphae stimulate a neutrophil respiratory burst and a cytosolic calcium flux without membrane depolarization. J Infect Dis. 1987;156:770–776. doi: 10.1093/infdis/156.5.770. [DOI] [PubMed] [Google Scholar]
- 38.Gow NA, Brown AJ, Odds FC. Fungal morphogenesis and host invasion. Curr Opin Microbiol. 2002;5:366–371. doi: 10.1016/s1369-5274(02)00338-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data