Abstract
Background
Abnormal P‐wave indices (PWIs)—reflecting underlying left atrial abnormality—are associated with increased risk of stroke independent of atrial fibrillation. We assessed whether abnormal PWIs are associated with incident dementia and greater cognitive decline, independent of atrial fibrillation and ischemic stroke.
Methods and Results
We included 13 714 participants (mean age, 57±6 years; 56% women; 23% black) who were followed for dementia through the end of 2015. (Abnormal P‐wave terminal force in lead V1, ≥4000 μV×ms), abnormal P‐wave axis (>75° or <0°), prolonged P‐wave duration (>120 ms), and advanced interatrial block were determined from ECGs at visits 2 to 4. Dementia was adjudicated by an expert panel using data from cognitive tests and hospitalization International Classification of Diseases codes. Cognitive function was measured longitudinally using 3 neuropsychological tests. Cox proportional hazards models were used to assess the association between time‐dependent abnormal PWIs with incident dementia. Linear regression models were used to evaluate PWIs with cognitive function over time. At the conclusion of the study, 19%, 16%, 28%, and 1.9% of participants had abnormal P‐wave terminal force in lead V1, abnormal P‐wave axis, prolonged P‐wave duration, and advanced interatrial block, respectively. During mean follow‐up of 18 years, there were 1390 (10%) dementia cases. All abnormal PWIs except advanced interatrial block were associated with an increased risk of dementia even after adjustment for incident atrial fibrillation and stroke: multivariable hazard ratio of abnormal P wave terminal force in lead V1=1.60, 95% CI, 1.41 to 2.83; abnormal P‐wave axis, hazard ratio =1.36, 95% CI, 1.17 to 2.57; prolonged P‐wave duration, hazard ratio=1.60, 95% CI, 1.42 to 1.80. Only abnormal P‐wave terminal force in lead V1 was associated with greater decline in global cognition.
Conclusions
Abnormal PWIs are independently associated with an increased risk of dementia. This novel finding should be replicated in other cohorts and the underlying mechanisms should be evaluated.
Keywords: atrium, cognitive impairment, dementia, electrocardiography
Subject Categories: Cognitive Impairment, Electrophysiology, Cardiovascular Disease
Clinical Perspective
What Is New?
In this large community‐based cohort study with 25 years of follow‐up, we observed that ECG markers of atrial abnormality are associated with an increased risk of dementia and greater cognitive decline independent of incident atrial fibrillation and stroke.
Our observation suggests that atrial cardiomyopathy is independently associated with increased risk of dementia and greater cognitive decline.
What Are the Clinical Implications?
More research is warranted to define the underlying mechanisms to facilitate discovery of novel approaches to prevent dementia.
Introduction
Atrial fibrillation (AF) is the most common sustained cardiac arrhythmia and is associated with elevated risks of ischemic stroke, cognitive decline, and dementia.1, 2 Recent evidence indicates that electrocardiographic and echocardiographic markers of left atrial abnormality or cardiomyopathy are associated with ischemic stroke independent of AF.3, 4, 5, 6, 7, 8 However, it is unclear whether these electrocardiographic markers are also associated with greater cognitive decline and increased risk of dementia.
Abnormal atrial conduction measured through analysis of P‐wave morphology—P wave indices (PWIs)—has been associated with atrial remodeling9, 10, 11, 12, 13 and stroke.3, 4, 7, 13, 14, 15, 16, 17 An abnormal P‐wave terminal force in lead V1 (aPTFV1) is a manifestation of atrial pathology including fibrosis, atrial dilatation, and elevated filling pressures.18, 19, 20 Other ECG markers of atrial remodeling include prolonged P‐wave duration (PPWD), advanced interatrial block (aIAB), and abnormal P‐wave axis (aPWA).21, 22 ECG indices of atrial cardiomyopathy have been associated with diastolic dysfunction, risk of AF, hypertension, and other cardiovascular disease but it is unknown whether these abnormal indices are associated with cognitive function. We hypothesized that abnormal PWIs are associated with greater cognitive decline and increased risk of dementia independent of AF and ischemic stroke. We tested this hypothesis in the ARIC‐NCS (Atherosclerosis Risk in Communities Neurocognitive Study), a community‐based cohort study.
Methods
Data Availability
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC policies. Data are maintained by ARIC through the University of North Carolina Collaborative Studies Coordinating Center. The ARIC study data can be accessed by contacting the ARIC Coordinating Center (aricpub@unc.edu).
Study Population
The ARIC Study is a community‐based, prospective cohort study of individuals from 4 US communities: Forsyth County, North Carolina; Jackson, Mississippi; Minneapolis suburbs, Minnesota; and Washington County, Maryland. The study population consists of 15 792 mostly black and white men and women, who were 45 to 64 years of age at the initial visit (1987–1989).23 The ARIC field centers in all 4 communities selected participants by probability sampling; the Mississippi field center recruited only black people, the other sites recruited according to the racial composition of the communities (15% black in the North Carolina site, predominantly white in Minnesota and Maryland). After the first visit, there were 5 additional exams: visit 2 (1990–1992), visit 3 (1993–1995), visit 4 (1996–1998), visit 5 (2011–2013), and visit 6 (2016–2017). Additionally, ARIC participants have received annual follow‐up calls (semi‐annual after 2012), with response rates of ≥90% among survivors. Cognitive function was evaluated at visit 2, visit 4, and visit 5. The study was approved by each participating institution's institutional review board, and all participants provided written informed consent.
Cognitive scores were first measured at visit 2, which serves as the baseline visit for this analysis. Of the 14 348 participants who attended visit 2, we excluded those with missing PWIs (n=377), prevalent dementia (n=3), missing baseline cognitive scores (n=141), nonwhite and nonblack participants in all field centers, and blacks in the Minnesota and Maryland sites because of small numbers (n=91), no follow‐up beyond baseline (n=3), and missing covariates (n=19). After exclusions, our final sample size included 13 714 participants. A flow diagram of the study population is provided in Figure 1.
Figure 1.
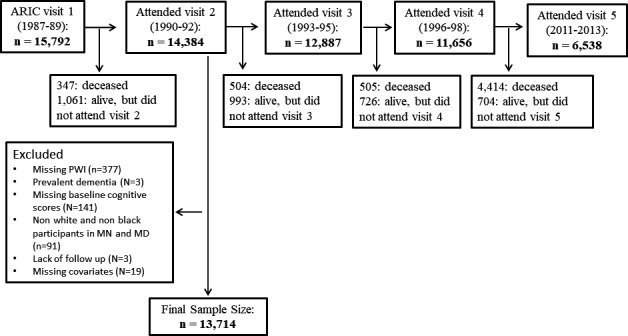
Flow diagram of study participants. ARIC indicates Atherosclerosis Risk in Communities; PWI, P‐wave indices.
Assessment of PWIs
PWIs were measured from resting 12‐lead ECGs in sinus rhythm at visit 2, visit 4, and visit 5. The ECGs obtained during all study visits were recorded on MAC PC Personal Cardiographs (Marquette Electronics Inc, Milwaukee, WI) and processed at the EPICARE center (Wake Forest University, Winston‐Salem, NC) and EPICORE Center (University of Alberta, Edmonton, Alberta, Canada).13 Our study focused on 4 different PWIs. An abnormal PTFV1 is a marker of left atrial pathology, which reflects a delayed electrical activation of the left atrium. It is calculated as the product of the duration (ms) and the absolute value of the depth (μV) of the downward deflection (terminal portion) of the P wave in lead V1. A value ≥4000 μV×ms was considered abnormal. P‐wave duration reflects the time that it takes for atrial depolarization and is measured from the end of the onset of the P wave to the return to baseline where the PR interval segment starts. PPWD was defined as >120 ms. The axis of the P wave describes the direction of atrial depolarization and is measured by calculating the net deflection of the P wave on each of the 6 limb leads of the ECG. It is a computer‐generated parameter reported routinely on all ECGs. A P‐wave axis >75° or <0° was considered abnormal. Finally, an aIAB defined as an interatrial conduction block in the Bachman's bundle causing a superior activation of the left atrium was detected by P‐wave prolongation and a biphasic P wave in leads III and aVF (augmented Vector Foot) or a notched P wave in lead II. Each individual abnormal PWI was defined by the first ECG that showed the abnormal PWI and the participant was considered to have that abnormal PWI for the duration of the study period.
Ascertainment of Dementia
Dementia was adjudicated by an expert panel using a predetermined algorithm incorporating data from the cognitive evaluations at visits 2, 4, and 5. All surviving participants were invited to an in‐person assessment at visit 524 when a full neuropsychological assessment was performed; other data sources included informant interviews, hospital discharge codes, or diagnostic codes from death certificates, as previously described.24 The algorithm was based on the National Institute on Aging‐Alzheimer's Association working group formulations of dementia25 and DSM‐5.26
Cognitive Testing
We used 3 neuropsychological tests (performed at visit 2, 4, and 5) to assess cognitive function: the Delayed Word Recall test, the Digit Symbol Substitution test of the Wechsler Adult Intelligence Scale‐Revised, and the Word Fluency test. Protocols for the tests were standardized, and trained examiners administered the tests in a fixed order during 1 session in a quiet room.
The Delayed Word Recall test assesses short‐term memory and verbal learning. It was administered by giving participants 10 nouns with which they had to make sentences and recall the words after 5 minutes. A point was given for each noun that was correctly recalled for a maximum of 10 points.27 The Digit Symbol Substitution test is a paper and pencil test designed to assess executive function, processing speed, attention, and working memory.28 The test consisted of a grid of numbers and matching symbols where participants had to match the symbols to the numbers. The score was the number of correct number‐symbol matches made in 90 seconds. Finally, the Word Fluency test tests executive function and expressive language by asking participants to generate as many words as they can in 60 seconds that start with the letters S, A, and F. The score was the number of words generated. Additionally, the 3 test scores were summed to create a global score of cognitive function, consistent with previously published ARIC neurocognitive articles.29
Covariates
Detailed procedures on covariate measures have been previously published.23 Covariates were ascertained at every ARIC visit. Body mass index was calculated by dividing weight in kilograms by height in meters squared. Blood pressure was measured after 5 minutes of rest twice and the average of the last 2 measurements was used for analysis. Participants were considered diabetic if their fasting glucose level was ≥126 mg/dL or nonfasting level ≥200 mg/dL, if they were on any antidiabetic medication, or had a self‐reported prior physician‐reported diagnosis of diabetes mellitus. Smoking status was reported by participants.23 Fasting blood samples were obtained at each visit for enzymatic determination of lipid panel. APOE genotyping was performed based on blood sample using the TaqMan assay (Applied Biosystems, Foster City, CA). Annual follow‐up telephone calls (semi‐annual after 2012) were placed to cohort participants to identify hospitalizations and deaths. In addition, local hospitals were surveyed for potential cardiovascular events and hospital discharge records were gathered from all hospitalizations, and the cohort was linked to State and National Death Indices. Heart failure was determined at each visit if participants were on heart failure medications in the previous 2 weeks and by hospitalization International Classification of Diseases, Ninth Revision (ICD‐9) discharge code 428.15 Baseline coronary heart disease was defined as self‐reported prior revascularization, a diagnosis of myocardial infarction or indication of a prior myocardial infarction by ECG. Incident coronary heart disease was ascertained by the ARIC Morbidity and Mortality Classification Committee using data from follow‐up calls, hospitalization records, and death certificates. AF was ascertained by study visit ECGs and hospitalization records.30 All ECGs were transmitted to the ARIC reading center for automatic coding and evaluation by a cardiologist. A hospital discharge ICD‐9 code 427.31 or 427.32 was considered AF. An AF discharge code occurring simultaneously with discharge codes containing heart revascularization surgery or other cardiac surgery involving heart valves or septa was not considered an AF event.31 To identify incident stroke, hospital reports were reviewed for discharge diagnosis including a cerebrovascular disease code (ICD‐9 codes 430–438), mention of a cerebrovascular procedure in the summary, or if the computed tomographic or magnetic resonance imaging report showed evidence of cerebrovascular disease. Medical records for potential stroke events were forwarded to a nurse abstractor at a central ARIC study office who abstracted each record for number, type, and severity of neurological deficits and supporting angiographic, computed tomographic, magnetic resonance imaging, spinal tap, or autopsy evidence. The ARIC Study adapted National Survey of Stroke criteria for its stroke definition.32 A computerized algorithm and physician reviewer independently confirmed the diagnosis of stroke, with disagreements adjudicated by a second physician reviewer.13, 33 All covariates were updated over time except for sex, race, field center, education, occupation, and APOE, which were assessed at baseline.
Statistical Analysis
The association of abnormal PWIs with dementia incidence was assessed using a Cox proportional hazards model, allowing for time‐dependent PWIs and time‐varying covariates. Follow‐up time was from visit 2 (1990–1992) until dementia, death, loss to follow‐up, or administrative censoring on December 31, 2015, whichever occurred first. Three models were performed from data measured at visits 2, 3, and 4. Model 1 included age, sex, and race/field center (5 levels). Model 2 included APOE genotype (0, 1, or 2 alleles), education (high school graduate versus not), occupation (categorical), and the time‐varying covariates of age (continuous), smoking (current versus not current), body mass index (continuous), systolic and diastolic blood pressure (continuous), antihypertensive medication use (yes/no), total cholesterol (continuous), diabetes mellitus (yes/no), coronary heart disease (yes/no), heart failure (yes/no), and stroke (yes/no) in addition to the variables mentioned in model 1. Model 2 adjusted for potential confounders for the association between abnormal PWIs and dementia. Additionally, model 3 adjusted for time‐dependent stroke and AF, which were ascertained through 2015. The purpose of model 3 was to determine whether the association of abnormal PWIs with dementia was explained by incident AF and incident stroke (AF and stroke being in the causal pathway between abnormal PWIs and dementia). The proportional hazard assumption was checked using Schoenfield residuals and via inspection of log(−log(survival) curves, and there was no evidence of violation. We tested race and sex interactions by incorporating a multiplicative term between each time‐dependent continuous PWI and race or sex, adjusting for model 2. To visualize the association of abnormal PWIs and dementia, we calculated the cumulative risk of incident dementia by time‐dependent PWIs status, taking into account the competing risk of death.34 We used competing‐risks survival regression based on Fine and Gray's proportional subhazards model to calculate survival functions. We explored the association between continuous PWIs and dementia using restricted cubic splines.
To assess the association of abnormal PWIs and cognitive function over time, we used PWIs, covariates, and cognitive scores measured at visits 2, 4, and 5 to correspond to the dates of cognitive testing. We calculated z scores ([test score−mean score]/SD) of the 3 neuropsychological tests and the combined global test at each of the 3 visits. To test the association between abnormal PWIs and the rate of cognitive decline, we used linear regression models fit with generalized estimating equations to evaluate associations with cognitive performance trajectories using robust variance and an unstructured correlation matrix. There was an ≈6‐year difference between measures from visit 2 to visit 4, and an ≈14‐year difference between measures from visit 4 to visit 5. To account for the different slopes of decline during the 20 years of total follow‐up, models included time modeled using a linear spline with a knot at 6 years (visit 4) and interaction terms between time and abnormal PWIs status. The association between abnormal PWIs and cognitive scores was assessed using the same covariates from the 3 models listed above for dementia. Interactions between follow‐up time and covariates were included. Separate models were run for each cognitive test (Delayed Word Recall test, Digit Symbol Substitution test, and Word Fluency test) and the global cognitive score. Of note, to account for attrition during follow‐up, we conducted the analysis using inverse probability of attrition weighting.29, 35 Weights for each individual were calculated at visits 4 and 5, and were the inverse of the estimated probabilities of (1) being alive at time of the follow‐up visit, and (2) attending the visit, conditional on being alive at the time of the examination, and the final weights were stabilized by the baseline variables of age, sex, race/center, education, and APOE genotype. These weights were incorporated into the linear regression models.
To account for potential floor effects in the cognitive scores, we conducted a sensitivity analysis in which we excluded participants who had baseline scores in the bottom 5% of race and sex‐specific scores.
All statistical analyses were performed with SAS v 9.4 (SAS Inc, Cary, NC) or STATA 14.0 (StataCorp LP, College Station, TX). A 2‐sided P value of <0.05 was considered statistically significant.
Results
The study sample consisted of 13 714 participants, 56% females, and 23% were black. The mean age at baseline was 57±6 years. At baseline (visit 2), 3688 (27%) participants had at least 1 abnormal PWI (Table 1); 8% had aPTFV1, 7% aPWA, 15% PPWD, and 0.8% aIAB. Baseline characteristics of participants by PWIs are shown in Table 1. Participants with abnormal PWIs had higher prevalence of comorbidities at baseline such as diabetes mellitus, coronary heart disease, heart failure, and stroke than participants with normal PWIs. The proportion of participants with abnormal PWIs who developed AF and stroke was also higher than those with normal PWI. In addition, participants with abnormal PWIs had lower cognitive z scores at baseline than those with normal PWI. At the end of the ECG follow‐up including visits 2 to 4, 19%, 15%, 28%, and 1.9% of participants had aPTFV1, aPWA, PPWD, and aIAB, respectively.
Table 1.
Participant Characteristics by Baseline PWI, Atherosclerosis Risk in Communities Study Visit 2, 1990–1992
| Characteristics | Normal P Wave (n=10 026) | Abnormal PTFV1 (n=1160)a | PPWD (n=2097)a | aPWA (n=1008)a | aIAB (n=108)a |
|---|---|---|---|---|---|
| Age, y | 56.6 (6) | 58.8 (6) | 57.9 (6) | 57.9 (6) | 61.6 (5) |
| Female sex | 5862 (58%) | 553 (48%) | 897 (43%) | 558 (55%) | 35 (32%) |
| Black race | 2061 (21%) | 441 (38%) | 731 (35%) | 221 (22%) | 31 (29%) |
| Education < High school | 1963 (20%) | 367 (32%) | 543 (25%) | 213 (21%) | 32 (30%) |
| APOE ε4, 2 alleles | 239 (2%) | 39 (3%) | 59 (3%) | 34 (3%) | 0 |
| APOE ε4, 1 allele | 2671 (27%) | 333 (29%) | 590 (28%) | 285 (28%) | 24 (22%) |
| Current smoker | 2150 (21%) | 329 (28%) | 406 (19%) | 311 (31%) | 20 (19%) |
| Body mass index, kg/m2 | 27.8 (5) | 29.0 (6) | 29.9 (6) | 25.1 (5) | 30.2 (5) |
| Systolic BP, mm Hg | 121 (18) | 129 (22) | 126 (19) | 120 (19) | 132 (22) |
| Diastolic BP, mm Hg | 72 (10) | 74 (12) | 74 (11) | 70 (10) | 74 (11) |
| Hypertensive medication use | 2838 (28%) | 610 (53%) | 1021 (49%) | 267 (26%) | 69 (63%) |
| Diabetes mellitus | 1342 (13%) | 279 (24%) | 403 (19%) | 106 (11%) | 23 (21%) |
| Coronary heart disease | 425 (4%) | 176 (15%) | 189 (9%) | 63 (6%) | 18 (17%) |
| Heart failure | 368 (4%) | 116 (10%) | 162 (8%) | 44 (4%) | 13 (12%) |
| Stroke | 124 (1%) | 50 (4%) | 51 (2%) | 18 (2%) | 10 (9%) |
| Incident atrial fibrillation through 2015 | 1755 (18%) | 334 (29%) | 558 (27%) | 218 (22%) | 44 (41%) |
| Incident stroke through 2015 | 776 (8%) | 158 (14%) | 220 (10%) | 100 (10%) | 16 (15%) |
| Mean cognitive score (SD) | |||||
| Global z score | 0.1 (1.0) | −0.4 (1.0) | −0.2 (1.0) | 0.0 (1.0) | −0.5 (1.0) |
| DWRT, number of words | 6.7 (1.5) | 6.3 (1.5) | 6.4 (1.5) | 6.6 (1.5) | 6.0 (1.6) |
| DWRT, z score | 0.1 (1.0) | −0.2 (1.0) | −0.2 (1.0) | 0.0 (1.0) | −0.4 (1.1) |
| DSST, number of symbols | 46.0 (14) | 38.6 (14) | 41.1 (15) | 44.1 (15) | 37.8 (14) |
| DSST, z score | 0.1 (1.0) | −0.4 (1.0) | −0.2 (1.0) | 0.0 (1.0) | −0.5 (1.0) |
| WFT, number of words | 33.7 (12) | 30.9 (13) | 31.6 (12) | 33.3 (12) | 30.5 (12) |
| WFT, z score | 0.0 (1.0) | −0.2 (1.0) | −0.1 (1.0) | 0.0 (1.0) | −0.2 (0.9) |
Values are mean (SD) or number (%). aIAB indicates advanced interatrial block; aPWA, abnormal P‐wave axis; BP, blood pressure; DSST, Digit Symbol Substitution test; DWRT, Delayed Word Recall test; PPWD, prolonged P‐wave duration; PTFV1, the terminal force of the P wave in ECG lead V1; PWI, P‐wave indices; WFT, word fluency test.
Participants may have more than 1 abnormal P‐wave measure and therefore would be included in more than 1 column.
Abnormal PWIs and Incident Dementia
During a mean follow‐up of 18 years, there were 1390 (10%) cases of incident dementia. The incidence rate of dementia was 5.76 per 1000 person‐years in participants without any abnormal PWI and ranged from 6.4 per 1000 person‐years in those with aIAB to 8.4 per 1000 person‐years in those with aPTFV1 (Table 2). We examined the associations between continuous measures of PWIs and incident dementia using a restricted cubic spline. Figure 2A shows that as PTFVI increased from 2000 to 5000 μV×ms, the risk of dementia increased linearly. In Figure 2B, the risk of dementia increased linearly with increasing P‐wave duration from 110 to 130 ms. Finally, in Figure 2C, dementia risk increased linearly as P‐wave axis increased above 75° and decreased below 0°.
Table 2.
Association of PWI With Incident Dementia, Atherosclerosis Risk in Communities Study, 1990–2015
| PTFV1 | No aPTFV1 (n=11 063) | aPTFV1 (n=2651) | P Value |
|---|---|---|---|
| # Dementia events | 1066 | 324 | |
| Person‐years | 202 973 | 38 486 | |
| Incidence rate (95% CI)a | 5.3 (4.9–5.6) | 8.4 (7.5–9.4) | |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 1.70 (1.50–1.93) | <0.0001 |
| Model 2 | 1 (REF) | 1.65 (1.45–1.87) | <0.0001 |
| Model 3 | 1 (REF) | 1.60 (1.41–2.83) | <0.0001 |
| PPWD | No PPWD (n=9865) | PPWD (n=3849) | P Value |
|---|---|---|---|
| No. dementia events | 934 | 456 | |
| Person‐years | 179 657 | 60 972 | |
| Incidence rate (95% CI)a | 5.2 (4.9–5.5) | 7.5 (6.8–8.2) | |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 1.66 (1.48–1.86) | <0.0001 |
| Model 2 | 1 (REF) | 1.64 (1.46–1.84) | <0.0001 |
| Model 3 | 1 (REF) | 1.60 (1.42–1.80) | <0.0001 |
| PWA | No aPWA (n=11 589) | aPWA (n=2125) | P Value |
|---|---|---|---|
| No. dementia events | 1164 | 226 | |
| Person‐years | 210 701 | 32 497 | |
| Incidence rate (95% CI)a | 5.5 (5.2–5.8) | 7.0 (6.1–7.9) | |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 1.49 (1.29–1.72) | <0.0001 |
| Model 2 | 1 (REF) | 1.40 (1.21–1.62) | <0.0001 |
| Model 3 | 1 (REF) | 1.36 (1.17–2.57) | <0.0001 |
| aIAB | No aIAB (n=13 456) | aIAB (n=258) | P Value |
|---|---|---|---|
| No. dementia events | 1368 | 22 | |
| Person‐years | 243 984 | 3425 | |
| Incidence rate (95% CI)a | 5.6 (5.3–5.9) | 6.4 (4.1–9.5) | |
| Hazard ratio (95% CI) | |||
| Model 1 | 1 (REF) | 1.01 (0.66–1.55) | 0.95 |
| Model 2 | 1 (REF) | 0.98 (0.64–1.50) | 0.94 |
| Model 3 | 1 (REF) | 0.93 (0.61–1.42) | 0.72 |
Model 1 is adjusted for age, sex and race/field center. Model 2: Model 1 and additionally adjusted for education, occupation, apolipoprotein E, smoking, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication, total cholesterol, diabetes mellitus, prevalent coronary heart disease, heart failure, and stroke. Model 3: Model 2 and additionally adjusted for time‐dependent incident stroke and AF. All covariates except sex, race‐field center, education, occupation, and apolipoprotein E are time‐varying variables. aIAB indicates advanced interatrial block; aPWA, abnormal P‐wave axis; PPWD, prolonged P‐wave duration; PTFV1, the terminal force of the P wave in ECG lead V1.
Incidence rate is per 1000 person‐years.
Figure 2.
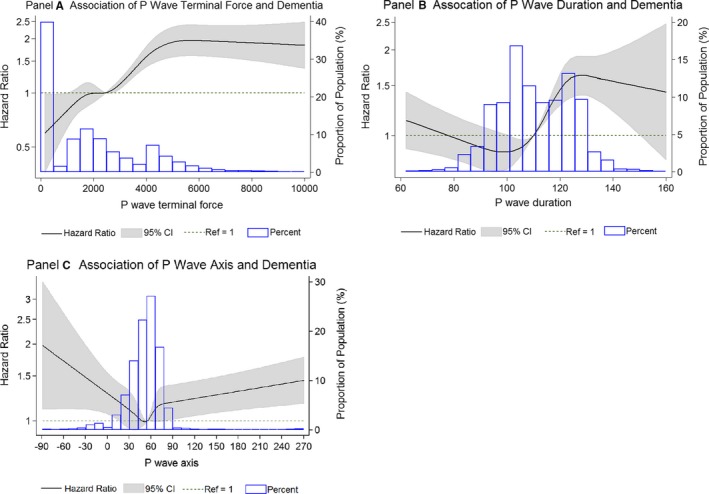
Association between time‐dependent P‐wave indices and incident dementia, Atherosclerosis Risk in Communities Study, 1990–2015. The solid line depicts the hazard ratio, the gray area represents the 95% CI, and the histogram illustrates the distribution of P‐wave indices in the ARIC population. The hazard ratio crosses the dotted reference line at the median P‐wave value. The model is adjusted for age, race, and sex. A, Association between time‐dependent terminal force of the P‐wave in ECG lead V1 and incident dementia. B, Association between time‐dependent P‐wave duration and incident dementia. C, Association between time‐dependent P‐wave axis and incident dementia. ARIC indicates Atherosclerosis Risk in Communities.
All PWIs except aIAB were significantly associated with higher risk of dementia after multivariable adjustment (Table 2). Abnormal PTFV1 was associated with a 70% higher risk of dementia after adjustment for age, sex, and race/field center. The association remained significant even after adjusting for time‐dependent incident stroke and AF in model 3 (hazard ratio, 1.60; 95% CI, 1.42–2.83). The presence of PPWD was associated with a 1.6‐fold higher risk of dementia with no change after adjusting for other covariates including incident stroke and AF (95% CI 1.41–2.83). aPWA was associated with a 49% higher risk of incident dementia in model 1 and 36% higher risk after adjusting for other covariates, including incident stroke and AF (hazard ratio 1.36, 95% CI 1.17–2.57). aIAB was not significantly associated with incident dementia (hazard ratio 0.93, 95% CI 0.61–1.42). Figure 3 depicts the cumulative risk of incident dementia by time‐dependent PWIs, adjusting for the competing risk of death. There were no significant interactions by race or sex between PWIs and dementia.
Figure 3.
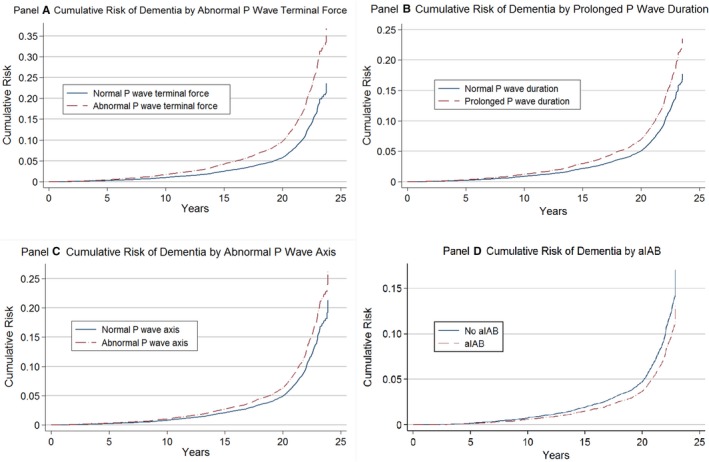
Cumulative risk of incident dementia by time‐dependent P‐wave indices, adjusting for the competing risk of death, Atherosclerosis Risk in Communities Study, 1990–2015. A, Cumulative risk of incident dementia by terminal force of the P wave in ECG lead V1. B, Cumulative risk of incident dementia by P‐wave duration. C, Cumulative risk of incident dementia by P‐wave axis. D, Cummulative Risk of Dementia by aIAB. aIAB indicates advanced interatrial block.
PWIs and Cognitive Scores
The association of abnormal PWIs with the rate of cognitive change over 20 years is shown in Table 3. An abnormal PTFV1 was associated with greater decline in Word Fluency test and global z score in the fully adjusted model. A negative estimate indicates a greater cognitive decline over the 20‐year period in those with aPWI compared with those with normal PWI. Participants with aPTFV1 had an additional 0.07 (95% CI, 0.01–0.13) decline in global z score over 20 years as compared with participants with a normal PTFV1. PPWD, aPWA, and aIAB were not associated with greater cognitive decline over 20 years. In a sensitivity analysis excluding participants who scored at the bottom 5% in their baseline cognitive tests, our results remained essentially unchanged. Finally, we did not observe any interactions by sex or race in the association between PWIs and change in cognitive function.
Table 3.
Additional Adjusted 20‐Year Cognitive Change Associated With Abnormal PWI, Atherosclerosis Risk in Communities Study, 1990–2015
| Cognitive Tests | Normal PTFV1 | Additional Cognitive Change in z Score (95% CI) | |||||
|---|---|---|---|---|---|---|---|
| Abnormal PTFV1 | |||||||
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| DWRT z score | 0 (Ref) | −0.06 (−0.17 to 0.04) | 0.23 | −0.09 (−0.19 to 0.02) | 0.10 | −0.08 (−0.19 to 0.02) | 0.11 |
| DSST z score | 0 (Ref) | −0.01 (−0.05 to 0.03) | 0.67 | −0.03 (−0.08 to 0.01) | 0.13 | −0.03 (−0.08 to 0.01) | 0.13 |
| WFT z score | 0 (Ref) | −0.06 (−0.12 to 0.01) | 0.07 | −0.07 (−0.13 to −0.01) | 0.03 | −0.07 (−0.13 to −0.01) | 0.03 |
| Global z score | 0 (Ref) | −0.04 (−0.11 to 0.03) | 0.22 | −0.07 (−0.13 to −0.01) | 0.04 | −0.07 (−0.13 to −0.01) | 0.04 |
| Normal PWD | PPWD | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| DWRT z score | 0 (Ref) | −0.02 (−0.10 to 0.06) | 0.55 | −0.02 (−0.10 to 0.06) | 0.61 | −0.02 (−0.10 to 0.06) | 0.71 |
| DSST z score | 0 (Ref) | −0.01 (−0.05 to 0.03) | 0.65 | −0.01 (−0.04 to 0.03) | 0.82 | −0.01 (−0.04 to 0.04) | 0.94 |
| WFT z score | 0 (Ref) | −0.03 (−0.08 to 0.02) | 0.22 | −0.01 (−0.06 to 0.03) | 0.56 | −0.01 (−0.06 to 0.04) | 0.67 |
| Global z score | 0 (Ref) | −0.03 (−0.08 to 0.02) | 0.24 | −0.02 (−0.07 to 0.03) | 0.51 | −0.01 (−0.06 to 0.04) | 0.65 |
| Normal PWA | Abnormal PWA | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| DWRT z score | 0 (Ref) | 0.00 (−0.10 to 0.10) | 0.99 | 0.01 (−0.09 to 0.10) | 0.89 | 0.01 (−0.09 to 0.10) | 0.92 |
| DSST z score | 0 (Ref) | 0.04 (−0.01 to 0.09) | 0.12 | 0.03 (−0.01 to 0.08) | 0.15 | 0.03 (−0.01 to 0.08) | 0.16 |
| WFT z score | 0 (Ref) | −0.01 (−0.06 to 0.06) | 0.88 | −0.03 (−0.08 to 0.03) | 0.39 | −0.02 (−0.08 to 0.03) | 0.41 |
| Global z score | 0 (Ref) | 0.03 (−0.03 to 0.10) | 0.28 | 0.03 (−0.03 to 0.09) | 0.36 | 0.03 (−0.03 to 0.09) | 0.36 |
| No aIAB | aIAB | ||||||
|---|---|---|---|---|---|---|---|
| Model 1 | P Value | Model 2 | P Value | Model 3 | P Value | ||
| DWRT z score | 0 (Ref) | −0.05 (−0.36 to 0.26) | 0.74 | −0.09 (−0.39 to 0.21) | 0.54 | −0.08 (−0.38 to 0.21) | 0.58 |
| DSST z score | 0 (Ref) | 0.15 (−0.01 to 0.29) | 0.06 | 0.10 (−0.04 to 0.23) | 0.15 | 0.10 (−0.03 to 0.24) | 0.13 |
| WFT z score | 0 (Ref) | 0.11 (−0.08 to 0.30) | 0.25 | 0.08 (−0.09 to 0.26) | 0.35 | 0.09 (−0.09 to 0.27) | 0.33 |
| Global z score | 0 (Ref) | 0.12 (−0.08 to 0.31) | 0.23 | 0.07 (−0.11 to 0.25) | 0.45 | 0.08 (−0.11 to 0.26) | 0.41 |
Model 1=adjusted for age (centered at 60 years), sex, race/center, time as a linear spline with a knot at 6 years, age by time spline terms, sex by time spline terms, and race/center by time spline terms. Model 2=Model 1+education, occupation, apolipoprotein E, the time‐dependent variables of smoking, body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication, diabetes mellitus, prevalent coronary heart disease, prevalent heart failure, prevalent stroke, plus all these variables by spline terms. Model 3=Model 2 and additionally adjusted for time‐dependent incident stroke and AF. Covariates are updated at visits except for: sex, race, center, education and occupation are from visit 1. A negative estimate indicates a greater cognitive decline in those with aPWI compared with those with normal PWI. This analysis incorporates inverse probability of attrition weights to account for attrition. A negative estimate indicates a greater cognitive decline over the 20‐year period in those with aPWI compared with those with normal PWI. AF indicates atrial fibrillation; aIAB, advanced interatrial block; aPWI, abnormal PWI; DWR, Delayed Word Recall (test of memory); DSS, Digit Symbol Substitution (test of executive function); PPWD, prolonged P‐wave duration; PTFV1, the terminal force of the P wave in ECG lead V1; PWA, P‐wave axis; WF, Word Fluency (test of verbal fluency).
Discussion
In this large community‐based cohort study with 25 years of follow‐up, we observed that ECG markers of atrial abnormality—aPTFV1, PPWD, and aPWA—were associated with increased risk of dementia. In addition, aPTFV1 was associated with greater decline in global cognitive and verbal function. Of note, these associations were independent of incident AF or stroke. Collectively, our findings suggest that atrial abnormality or cardiomyopathy is independently associated with increased risk of dementia and greater cognitive decline.
Markers of atrial abnormality or cardiomyopathy on ECG—including aPTFV1 and aPWA—have been associated with stroke in the absence of AF.3, 13, 36, 37 Furthermore, left atrial enlargement assessed by echocardiogram or cardiac magnetic resonance imaging has been associated with greater cognitive decline including language, delayed memory, global score, and mini‐mental exam in patients without a history of stroke, AF, or diagnosis of dementia.38, 39 Several potential mechanisms can explain our observations. First, we have previously shown that aPWA is associated with an increased risk of cardioembolic stroke, independent of AF.13 Furthermore, subclinical cerebral infarcts are an important mechanism underlying the association between AF and greater cognitive decline.40 Therefore, subclinical cerebral infarcts could potentially explain the association between abnormal PWIs and increased risk of dementia. A study based on the Cardiovascular Health Study reported that in patients without AF, abnormal PTFV1 was associated with worsening leukoaraiosis and incident infarcts detected by magnetic resonance imaging.7 Second, decreased brain perfusion from impaired left atrial function and lower cardiac output may result in chronic subcortical ischemia. Left atrial enlargement has been associated with cognitive dysfunction even in the absence of stroke, AF, or dementia.38, 39 Third, shared vascular risk factors (eg, diabetes mellitus, hypertension, elevated brain natriuretic peptide, and heart failure) may be important mechanisms underlying the abnormal PWI–dementia association, although we adjusted for these risk factors.41, 42 It is conceivable that the risk factors driving aPWI including cardiometabolic conditions such as hypertension and diabetes mellitus lead to lower cognitive scores at baseline, which precede the development of dementia. Finally, there is emerging literature to suggest that in patients with neurodegenerative diseases such as Parkinson's disease,43 central autonomic issues may contribute to rhythm disturbances, raising the possibility of reverse causation. However, this is unlikely given the longitudinal analysis with aPWIs preceding development of dementia. Moreover, aPWIs are not rhythm disturbances, but rather ECG surrogates of underlying atrial abnormality.
Our findings have some clinical and public health implications: More research is warranted to identify novel approaches to prevent the development of atrial abnormality as a strategy to prevent dementia. In addition, there are ongoing clinical trials (ARCADIA [Atrial Cardiopathy and Antithrombotic Drugs in Prevention of Cryptogenic Stroke]) (NCT03192215) to test whether anticoagulation can prevent ischemic stroke in patients with atrial abnormality in the absence of AF. In a similar vein, clinical trials are warranted to determine whether anticoagulation can prevent dementia in patients with atrial abnormality and without AF.
Two observations are worth noting. First, aIAB was not found to be significantly associated with an increased risk of dementia. There were only 258 participants with aIAB, of whom 22 developed dementia; therefore, we may have been underpowered to detect any association with this marker. Second, cognitive decline is a precursor to dementia, but in our study abnormal PWIs (except for aPTFV1) were associated with increased risk of dementia but not with greater cognitive decline. A similar finding was also noted when we reported that left ventricular hypertrophy was associated with higher risk of dementia but not with cognitive decline.44 It is possible that atrial abnormality may put patients at risk for sudden events with a large drop in cognition (eg, because of stroke or cerebral infarct) but not a progressive decline.
The principal strengths of our study include the large sample of blacks and whites, long follow‐up, time which is important given the long natural history of dementia; numerous dementia cases; comprehensive cognitive tests; and an extensive list of covariates using standardized procedures. Some limitations, however, should be noted. First, an inherent challenge to accurately quantifying long‐term risk factor associations in observational studies is that ill participants are less likely to return for study visits. Thus, attrition is an important concern in any long‐term observational study. To address this concern, in our analysis we implemented inverse probability of attrition weights to account for attrition. Second, not all participants attended visit 5, when dementia was ascertained. Thus, we may have underascertained dementia. However, even in those who did not attend visit 5 because of nonparticipation or death, dementia diagnoses were identified through ICD‐9 hospital discharge diagnostic codes. Also, for all dementia cases, the dates of onset are uncertain. Third, the 3 cognitive scores measured in ARIC cannot be considered a true measure of global cognitive function because they do not assess all cognitive domains exhaustively. Furthermore, AF was ascertained using study ECGs and hospital discharge codes; thus, we may have underascertained AF. However, AF incidence in the ARIC study is consistent with other population‐based studies, and utilizing hospital discharge records for the purposes of AF detection has been previously validated.45, 46 Finally, as with any observational study, despite extensive adjustments residual confounding may exist.
In conclusion, our report from a large population‐based cohort study provides evidence that ECG markers of atrial abnormality or cardiomyopathy—including aPTFV1, aPWA, and PPWD—are associated with increased risk of dementia independent of stroke and AF. These findings should be replicated in other independent cohorts and efforts should be made to elucidate the underlying mechanisms.
Sources of Funding
The Atherosclerosis Risk in Communities study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute, National Institutes of Health, Department of Health and Human Services, under Contract numbers (HHSN268201700001l, HHSN268201700002l, HHSN2682017000003l, HHSN2682017000005l, and HHSB2682017000004l). Dr Chen is supported by R01HL41288 and R01 HL126637.
Disclosures
None.
Acknowledgments
The authors thank the staff and participants of the ARIC study for their important contributions.
(J Am Heart Assoc. 2019;8:e014553 DOI: 10.1161/JAHA.119.014553.)
References
- 1. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation: a major contributor to stroke in the elderly. The Framingham Study. Arch Intern Med. 1987;147:1561–1564. [PubMed] [Google Scholar]
- 2. Wolf PA, Abbott RD, Kannel WB. Atrial fibrillation as an independent risk factor for stroke: the Framingham Study. Stroke. 1991;22:983–988. PMID: 1866765. [DOI] [PubMed] [Google Scholar]
- 3. Kamel H, Soliman EZ, Heckbert SR, Kronmal RA, Longstreth WT Jr, Nazarian S, Okin PM. P‐wave morphology and the risk of incident ischemic stroke in the Multi‐Ethnic Study of Atherosclerosis. Stroke. 2014;45:2786–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Soliman EZ, Prineas RJ, Case LD, Zhang ZM, Goff DC Jr. Ethnic distribution of ECG predictors of atrial fibrillation and its impact on understanding the ethnic distribution of ischemic stroke in the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:1204–1211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Barnes ME, Miyasaka Y, Seward JB, Gersh BJ, Rosales AG, Bailey KR, Petty GW, Wiebers DO, Tsang TS. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. [DOI] [PubMed] [Google Scholar]
- 6. Benjamin EJ, D'Agostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death. The Framingham Heart Study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 7. Kamel H, Bartz TM, Longstreth WT Jr, Okin PM, Thacker EL, Patton KK, Stein PK, Gottesman RF, Heckbert SR, Kronmal RA, Elkind MS, Soliman EZ. Association between left atrial abnormality on ECG and vascular brain injury on MRI in the Cardiovascular Health Study. Stroke. 2015;46:711–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Russo C, Jin Z, Liu R, Iwata S, Tugcu A, Yoshita M, Homma S, Elkind MS, Rundek T, Decarli C, Wright CB, Sacco RL, Di Tullio MR. LA volumes and reservoir function are associated with subclinical cerebrovascular disease: the CABL (Cardiovascular Abnormalities and Brain Lesions) study. JACC Cardiovasc Imaging. 2013;6:313–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ariyarajah V, Mercado K, Apiyasawat S, Puri P, Spodick DH. Correlation of left atrial size with p‐wave duration in interatrial block. Chest. 2005;128:2615–2618. [DOI] [PubMed] [Google Scholar]
- 10. Goyal SB, Spodick DH. Electromechanical dysfunction of the left atrium associated with interatrial block. Am Heart J. 2001;142:823–827. [DOI] [PubMed] [Google Scholar]
- 11. Jin L, Weisse AB, Hernandez F, Jordan T. Significance of electrocardiographic isolated abnormal terminal P‐wave force (left atrial abnormality). An echocardiographic and clinical correlation. Arch Intern Med. 1988;148:1545–1549. [PubMed] [Google Scholar]
- 12. Tsao CW, Josephson ME, Hauser TH, O'Halloran TD, Agarwal A, Manning WJ, Yeon SB. Accuracy of electrocardiographic criteria for atrial enlargement: validation with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2008;10:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Maheshwari A, Norby FL, Soliman EZ, Koene RJ, Rooney MR, O'Neal WT, Alonso A, Chen LY. Abnormal P‐wave axis and ischemic stroke: the ARIC Study (Atherosclerosis Risk in Communities). Stroke. 2017;48:2060–2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kamel H, Hunter M, Moon YP, Yaghi S, Cheung K, Di Tullio MR, Okin PM, Sacco RL, Soliman EZ, Elkind MS. Electrocardiographic left atrial abnormality and risk of stroke: Northern Manhattan Study. Stroke. 2015;46:3208–3212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Loehr LR, Rosamond WD, Chang PP, Folsom AR, Chambless LE. Heart failure incidence and survival (from the Atherosclerosis Risk in Communities study). Am J Cardiol. 2008;101:1016–1022. [DOI] [PubMed] [Google Scholar]
- 16. Lorbar M, Levrault R, Phadke JG, Spodick DH. Interatrial block as a predictor of embolic stroke. Am J Cardiol. 2005;95:667–668. [DOI] [PubMed] [Google Scholar]
- 17. O'Neal WT, Kamel H, Zhang ZM, Chen LY, Alonso A, Soliman EZ. Advanced interatrial block and ischemic stroke: the Atherosclerosis Risk in Communities Study. Neurology. 2016;87:352–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Alpert MA, Munuswamy K. Electrocardiographic diagnosis of left atrial enlargement. Arch Intern Med. 1989;149:1161–1165. [PubMed] [Google Scholar]
- 19. Morris JJ Jr, Estes EH Jr, Whalen RE, Thompson HK Jr, McIntosh HD. P‐wave analysis in valvular heart disease. Circulation. 1964;29:242–252. [DOI] [PubMed] [Google Scholar]
- 20. Scott CC, Leier CV, Kilman JW, Vasko JS, Unverferth DV. The effect of left atrial histology and dimension on P wave morphology. J Electrocardiol. 1983;16:363–366. [DOI] [PubMed] [Google Scholar]
- 21. Hancock EW, Deal BJ, Mirvis DM, Okin P, Kligfield P, Gettes LS, Bailey JJ, Childers R, Gorgels A, Josephson M, Kors JA, Macfarlane P, Mason JW, Pahlm O, Rautaharju PM, Surawicz B, van Herpen G, Wagner GS, Wellens H; American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; American College of Cardiology Foundation; Heart Rhythm Society . AHA/ACCF/HRS recommendations for the standardization and interpretation of the electrocardiogram: part V: electrocardiogram changes associated with cardiac chamber hypertrophy: a scientific statement from the American Heart Association Electrocardiography and Arrhythmias Committee, Council on Clinical Cardiology; the American College of Cardiology Foundation; and the Heart Rhythm Society: endorsed by the International Society for Computerized Electrocardiology. Circulation. 2009;119:e251–e261. [DOI] [PubMed] [Google Scholar]
- 22. Ariyarajah V, Prajapat L, Kumar KK, Barac I, Apiyasawat S, Spodick DH. Quantitative estimation of left atrial linear dimension on a transthoracic echocardiogram using an electrocardiographic formulaic assessment. Am J Cardiol. 2007;100:894–898. [DOI] [PubMed] [Google Scholar]
- 23. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC Investigators. Am J Epidemiol. 1989;129:687–702. [PubMed] [Google Scholar]
- 24. Knopman DS, Gottesman RF, Sharrett AR, Wruck LM, Windham BG, Coker L, Schneider AL, Hengrui S, Alonso A, Coresh J, Albert MS, Mosley TH Jr. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC‐NCS). Alzheimers Dement (Amst). 2016;2:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr, Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Association AP . DSM_5: Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC: Psychiatric Association; 2013. [Google Scholar]
- 27. Knopman DS, Ryberg S. A verbal memory test with high predictive accuracy for dementia of the Alzheimer type. Arch Neurol. 1989;46:141–145. [DOI] [PubMed] [Google Scholar]
- 28. Rosano C, Perera S, Inzitari M, Newman AB, Longstreth WT, Studenski S. Digit Symbol Substitution test and future clinical and subclinical disorders of cognition, mobility and mood in older adults. Age Ageing. 2016;45:688–695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gottesman RF, Rawlings AM, Sharrett AR, Albert M, Alonso A, Bandeen‐Roche K, Coker LH, Coresh J, Couper DJ, Griswold ME, Heiss G, Knopman DS, Patel MD, Penman AD, Power MC, Selnes OA, Schneider AL, Wagenknecht LE, Windham BG, Wruck LM, Mosley TH. Impact of differential attrition on the association of education with cognitive change over 20 years of follow‐up: the ARIC Neurocognitive Study. Am J Epidemiol. 2014;179:956–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. White AD, Folsom AR, Chambless LE, Sharret AR, Yang K, Conwill D, Higgins M, Williams OD, Tyroler HA. Community surveillance of coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study: methods and initial two years’ experience. J Clin Epidemiol. 1996;49:223–233. [DOI] [PubMed] [Google Scholar]
- 31. Alonso A, Agarwal SK, Soliman EZ, Ambrose M, Chamberlain AM, Prineas RJ, Folsom AR. Incidence of atrial fibrillation in whites and African‐Americans: the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2009;158:111–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Robins M, Weinfeld FD. The National Survey of Stroke. Study design and methodology. Stroke. 1981;12:I7–I11. [PubMed] [Google Scholar]
- 33. Rosamond WD, Folsom AR, Chambless LE, Wang CH, McGovern PG, Howard G, Copper LS, Shahar E. Stroke incidence and survival among middle‐aged adults: 9‐year follow‐up of the Atherosclerosis Risk in Communities (ARIC) cohort. Stroke. 1999;30:736–743. [DOI] [PubMed] [Google Scholar]
- 34. Satagopan JM, Ben‐Porat L, Berwick M, Robson M, Kutler D, Auerbach AD. A note on competing risks in survival data analysis. Br J Cancer. 2004;91:1229–1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Weuve J, Tchetgen Tchetgen EJ, Glymour MM, Beck TL, Aggarwal NT, Wilson RS, Evans DA, Mendes de Leon CF. Accounting for bias due to selective attrition: the example of smoking and cognitive decline. Epidemiology. 2012;23:119–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in the Atherosclerosis Risk in Communities study. Ann Neurol. 2015;78:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. He J, Tse G, Korantzopoulos P, Letsas KP, Ali‐Hasan‐Al‐Saegh S, Kamel H, Li G, Lip GYH, Liu T. P‐wave indices and risk of ischemic stroke: a systematic review and meta‐analysis. Stroke. 2017;48:2066–2072. [DOI] [PubMed] [Google Scholar]
- 38. Karadag B, Ozyigit T, Ozben B, Kayaoglu S, Altuntas Y. Relationship between left atrial volume index and cognitive decline in elderly patients with sinus rhythm. J Clin Neurosci. 2013;20:1074–1078. [DOI] [PubMed] [Google Scholar]
- 39. Alosco ML, Gunstad J, Jerskey BA, Clark US, Hassenstab JJ, Xu X, Poppas A, Cohen RA, Sweet LH. Left atrial size is independently associated with cognitive function. Int J Neurosci. 2013;123:544–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Chen LY, Lopez FL, Gottesman RF, Huxley RR, Agarwal SK, Loehr L, Mosley T, Alonso A. Atrial fibrillation and cognitive decline‐the role of subclinical cerebral infarcts: the Atherosclerosis Risk in Communities study. Stroke. 2014;45:2568–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chen LY, Soliman EZ. P wave indices‐advancing our understanding of atrial fibrillation‐related cardiovascular outcomes. Front Cardiovasc Med. 2019;6:53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ferguson IT, Elbejjani M, Sabayan B, Jacobs DR Jr, Meirelles O, Sanchez OA, Tracy R, Bryan N, Launer LJ. N‐terminal pro‐brain natriuretic peptide and associations with brain magnetic resonance imaging (MRI) features in middle age: the CARDIA brain MRI study. Front Neurol. 2018;9:307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Scorza FA, Fiorini AC, Scorza CA, Finsterer J. Cardiac abnormalities in Parkinson's disease and Parkinsonism. J Clin Neurosci. 2018;53:1–5. [DOI] [PubMed] [Google Scholar]
- 44. Norby FL, Chen LY, Soliman EZ, Gottesman RF, Mosley TH, Alonso A. Association of left ventricular hypertrophy with cognitive decline and dementia risk over 20 years: the Atherosclerosis Risk In Communities‐Neurocognitive Study (ARIC‐NCS). Am Heart J. 2018;204:58–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Benjamin EJ, Levy D, Vaziri SM, D'Agostino RB, Belanger AJ, Wolf PA. Independent risk factors for atrial fibrillation in a population‐based cohort. The Framingham Heart Study. JAMA. 1994;271:840–844. [PubMed] [Google Scholar]
- 46. Psaty BM, Manolio TA, Kuller LH, Kronmal RA, Cushman M, Fried LP, White R, Furberg CD, Rautaharju PM. Incidence of and risk factors for atrial fibrillation in older adults. Circulation. 1997;96:2455–2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data, analytic methods, and study materials will be made available to other researchers for purposes of reproducing the results or replicating the procedure in accordance with ARIC policies. Data are maintained by ARIC through the University of North Carolina Collaborative Studies Coordinating Center. The ARIC study data can be accessed by contacting the ARIC Coordinating Center (aricpub@unc.edu).


