Abstract
Background
Previous studies show that patients with primary aldosteronism are associated with higher risk of congestive heart failure (CHF). However, the effect of target treatment to the incidental CHF has not been elucidated. We aimed to investigate the risk of new‐onset CHF in patients with aldosterone‐producing adenomas (APAs) and explore the effect of adrenalectomy on new onset of CHF.
Methods and Results
From 1997 to 2009, 688 APA were identified and matched with essential hypertension controls. The risks of developing incidental CHF (hazard ratio, 0.49; 95% CI, 0.31–0.75; P=0.001) and mortality (hazard ratio, 0.29; 95% CI, 0.20–0.44; P<0.001) were significantly lower in the APA group after targeted treatment. A total of 605 patients with APAs who underwent adrenalectomy lowered the risks of CHF (subdistribution hazard ratio, 0.55; 95% CI, 0.34–0.90; P=0.017) and mortality (adjusted hazard ratio, 0.27; 95% CI, 0.16–0.44; P<0.001) compared with essential hypertension controls.
Conclusions
In conclusion, for patients with APAs, adrenalectomy can be associated with lower risk of incidental CHF and all‐cause mortality in a long‐term follow‐up.
Keywords: adrenalectomy, aldosterone‐producing adenomas, cardiovascular disease, congestive heart failure, essential hypertension, primary aldosteronism
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
Patients with primary aldosteronism have a higher risk of congestive heart failure.
Adrenalectomy is the treatment of choice for patients with aldosterone‐producing adenomas, but its association with future congestive heart failure is unknown.
We found that the risks of incident congestive heart failure and mortality were significantly lower in the group of patients with aldosterone‐producing adenomas undergoing adrenalectomy compared with control patients with essential hypertension.
What Are the Clinical Implications?
These findings highlight the importance of reaching an accurate diagnosis of primary aldosteronism and aldosterone‐producing adenomas as adrenalectomy appears to be associated with improved outcomes in these patients.
Introduction
Primary aldosteronism (PA) is a major cause of secondary hypertension caused by autonomous aldosterone secretion, which leads to hypertension and hypokalemia and is independent of renin.1, 2, 3 It affects 5% to 13% of patients with hypertension, which consequently makes it the leading cause of secondary hypertension.4 Previous studies have indicated that patients with PA have an increased prevalence of cardiovascular events,5, 6 cardiomegaly,7 and left ventricular dimension index.8 Moreover, long‐term exposure to high aldosterone levels, in addition to high blood pressure, may eventually lead to cardiovascular and renal structural and functional damages,9 including marked left ventricular (LV) hypertrophy, increasing collagen deposition in myocardium, and renal hyperfiltration, and proteinuria.6, 10, 11, 12, 13 In addition, aldosterone excess could produce a substantially increased risk of cardiovascular atherosclerosis via proinflammatory and profibrotic effects on the vascular wall and is associated more with metabolic syndrome.14, 15, 16, 17, 18, 19 PA traditionally can exist at least in 2 heterogeneous forms, that is, aldosterone‐producing adenoma (APA; where excessive aldosterone secretion is associated with an unilateral adrenal mass [lateralized PA]), or idiopathic bilateral hyperaldosteronism (where excessive aldosterone secretion is associated with bilateral adrenal hyperplasia).4
Adrenalectomy has been advocated as the treatment of choice for APAs once the differential diagnosis is confirmed (with adrenal venous sampling, postural stimulation test, or NP59‐SPECT), and the patient's anesthetic risk is acceptable. Adrenalectomy can significantly correct hypertension, hypokalemia, and biochemical abnormalities and even regress increased LV masses in patients with APAs. The treatment of idiopathic bilateral hyperaldosteronism is a mineralocorticoid receptor antagonist (MRA), plus other antihypertensives if and as indicated.20
Congestive heart failure (CHF) is a more concerning public health problem than is currently acknowledged.21 However, clinical evidence to investigate an association between APAs and new‐onset CHF is lacking, especially on a population scale, so exploring the CHF risk after the target treatment is warranted.
Therefore, we conducted this study to investigate the risks of CHF and mortality in patients with PA with APAs, as compared with their propensity score matched essential hypertension (EH) controls, thus to establish evidence of the benefits of adrenalectomy for a lower risk of incidental CHF.
Material and Methods
Data Availability Statement
We are unable to make our data, analytic methods, and study materials available to other researchers because of the following limitation: The Taiwanese Government allows researchers to analyze the National Health Insurance (NHI) data only from locations within Taiwan. Currently, the government owns 2 centers that store NHI data for academic use: the data science center of health and welfare (https://dep.mohw.gov.tw/DOS/np-2497-113.html) and the information integration and application service center of the NHI Administration (https://www.nhi.gov.tw/Content_List.aspx?n=468220093AFEB6DF&topn=CA428784F9ED78C9). Researchers in Taiwan are required to submit their research plans and institutional review board approvals for the proposed studies and to pay for using such data. The amount of payment depends on the volume of data retrieved.
Data Source
This population‐based retrospective cohort study is based on data from the NHI Research Database dating between 1997 and 2009. The NHI Research Database has been implemented in Taiwan since 1995 and is one of the largest and most comprehensive population‐based health insurance databases in the world; it has been used extensively in various studies.22, 23, 24 The NHI covers almost all of the 23.7 million people living in Taiwan and contains medical information regarding ambulatory visits, hospital admissions, prescriptions, interventional procedures, disease profiles, and vital status.25, 26 The NHI Administration is the only healthcare costs payer of the NHI; it routinely checks for data accuracy and reliability.27, 28 To avoid rejection of claims reimbursement from NHI Administration, physicians in Taiwan usually follow clinical guidelines. Since diagnostic tests for PA are costly and intrusive, Taiwanese physicians follow the Aldosteronism Society's clinical practice guidelines—the Taiwan PA consensus29—and focus on performing lateralization tests on patients found to have a high possibility of PA after a confirmation test.
Study and Control Cohorts
This study included patients diagnosed as having PA (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD‐9‐CM] 255.1) (see Data S1, Tables S1 and S2).22, 23, 24, 25, 26, 27, 28, 30, 31, 32, 33 The administrative data on diagnoses and MRA prescriptions for patients identified with PA in Taiwan had been well audited because of efficient NHI Administration reimbursement system.22 PA was diagnosed by combining ICD‐9‐CM 255.1 and the prescription of an MRA within 1 year before and after the diagnosis. The accuracy of PA diagnoses has been validated with a sensitivity of 0.89 and specificity of 0.8.22 We also developed additional algorithms to ascertain comorbid conditions among patients with PA. Except for a couple of comorbid conditions, for which confirmation required more detailed ICD‐9‐CM coding, we used only the first 3 digits of the ICD‐9‐CM codes to identify comorbid conditions. This rule also tended to yield a lower rate of type II errors in identifying comorbidities.34, 35 While reviewing NHI data, the identification of a specific comorbid condition was based on the criterion that there was at least 1 inpatient NHI record or 3 outpatient records within 1 year before the initial PA diagnosis.22, 23, 25, 28, 36
In this nested propensity score–matched case, patients with EH were recruited from those with diagnoses of hypertension, and received antihypertensive agents (from the Anatomical Therapeutic Chemical Classification) after exclusion of patients with secondary hypertension. The Figure shows the flow diagram of selecting our study subjects.
Figure 1.
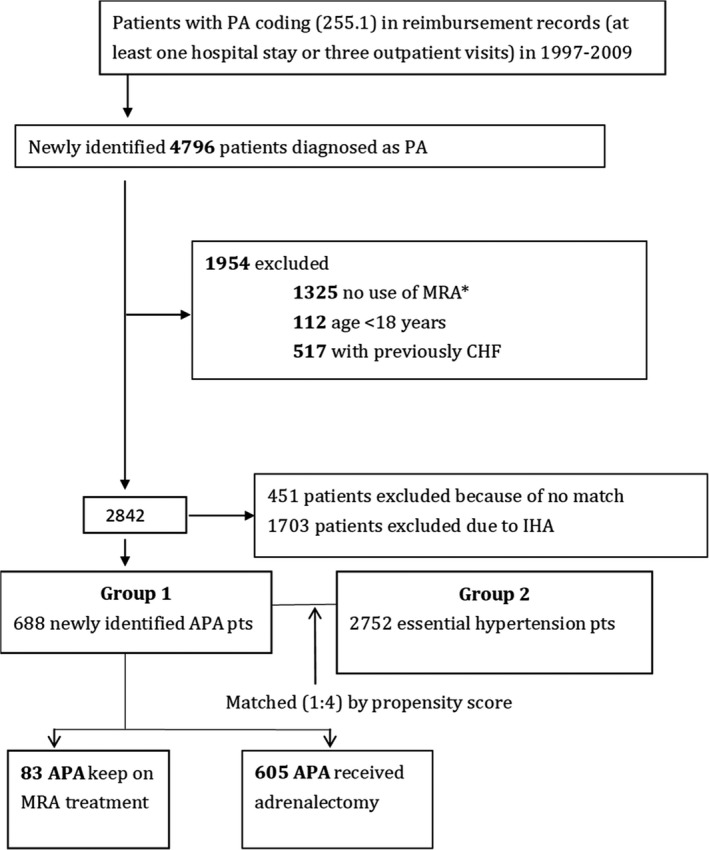
Flow diagram of selecting study subjects. Our study enrolled patients with PA, which was diagnosed by combining ICD‐9‐CM 255.1 and use of MRA 1 year before and after the diagnosis. We also excluded patients with documented congestive heart failure (CHF). APA indicates aldosterone‐producing adenoma; IHA, Idiopathic bilateral hyperaldosteronism; MRA, mineralocorticoid receptor antagonist; PA, primary aldosteronism.
Ethics Statement
Our study followed all applicable institutional and governmental regulations concerning the ethical use of data from human subjects. Informed consent was waived because patients were anonymous in the present analysis, and there was neither a breach of privacy nor interference with clinical decisions related to patient care. This study was exempt from a full ethical review by the Institutional Review Board of National Taiwan University Hospital (201301017RINC).
Outcome Measures
All 688 patients with PA and 2752 matched patients with EH were followed until the events, defined as either death of the study subjects or to the end of 2010. The diagnosis of CHF is compounded by the typical reliance on the first listed diagnosis, which was well validated using the ICD‐9 code from a population‐based surveillance program.21
Statistical Analysis
Baseline characteristics of the study population were described using frequencies, with percentages for categorical variables. Given the differences in baseline characteristics and risks between study and control cohorts, we tried to match each patient in the APA cohort with 4 patients in the respective EH cohort with 2 sets of similar propensity scores based on nearest‐neighbor matching without replacement, and using a caliper width equal to 0.1 of the SD from the propensity score. We constructed the propensity score by all the factors listed in Table 1.
Table 1.
Baseline Characteristics of Study Population With APA
| Variables | Matched APA/EH | |||
|---|---|---|---|---|
| EH (n=2752) | APA (n=688) | P Value | SD | |
| Propensity score | −2.52±0.46 | −2.52±0.46 | 0.998 | 0.000 |
| Sex | ||||
| Women, n (%) | 1592 (57.85) | 399 (57.99) | 0.966 | −0.003 |
| Men, n (%) | 1160 (42.15) | 289 (42.01) | −0.003 | |
| Age | 46.93±13.27 | 47.04±11.06 | 0.510 | −0.009 |
| Urbanization level | ||||
| Urban, n (%) | 1281 (46.55) | 326 (47.38) | 0.287 | −0.009 |
| Suburban, n (%) | 726 (26.38) | 195 (28.34) | −0.032 | |
| Rural, n (%) | 745 (27.07) | 167 (24.27) | 0.060 | |
| Monthly income, n (%) | ||||
| <NT$19 100, n (%) | 1655 (60.14) | 422 (61.34) | 0.845 | −0.012 |
| NT$19 100–NT$41 999, n (%) | 905 (32.89) | 220 (31.98) | 0.014 | |
| ≥NT$42 000, n (%) | 192 (6.98) | 46 (6.69) | 0.011 | |
| Preexisting comorbidity | ||||
| Cerebrovascular disease, n (%) | 136 (4.94) | 34 (4.94) | 0.999 | 0.000 |
| CKD, n (%) | 21 (0.76) | 5 (0.73) | 0.999 | −0.004 |
| COPD, n (%) | 57 (2.07) | 14 (2.03) | 0.999 | −0.003 |
| Coronary artery disease, n (%) | 16 (0.58) | 4 (0.58) | 0.999 | 0.000 |
| Dementia, n (%) | 12 (0.44) | 0 (0.00) | 0.141 | −0.094 |
| Diabetes mellitus, n (%) | 236 (8.58) | 66 (9.59) | 0.407 | 0.035 |
| Hemiplegia, n (%) | 15 (0.55) | 5 (0.73) | 0.576 | 0.023 |
| Liver disease, n (%) | 117 (4.25) | 31 (4.51) | 0.753 | 0.012 |
| Peptic ulcer, n (%) | 155 (5.63) | 42 (6.10) | 0.646 | 0.020 |
| Peripheral vascular disease, n (%) | 11 (0.40) | 3 (0.44) | 0.999 | 0.006 |
| RA, n (%) | 10 (0.36) | 1 (0.15) | 0.704 | −0.043 |
| Solid tumor, n (%) | 55 (2.00) | 14 (2.03) | 0.999 | 0.003 |
| SLE, n (%) | 6 (0.22) | 2 (0.29) | 0.664 | 0.014 |
| Atrial fibrillation, n (%) | 18 (0.65) | 0 (0.00) | 0.034 | −0.115 |
| Dyslipidemia, n (%) | 320 (11.63) | 80 (11.63) | 0.999 | 0.000 |
| Parkinson disease, n (%) | 8 (0.29) | 1 (0.15) | 0.698 | −0.031 |
| Medication for hypertension | ||||
| α‐Blocker, n (%) | 129 (4.69) | 43 (6.25) | 0.097 | 0.069 |
| ACEI or ARB, n (%) | 1087 (39.50) | 274 (39.83) | 0.896 | 0.007 |
| β‐Blocker, n (%) | 1322 (48.04) | 348 (50.58) | 0.233 | 0.051 |
| Calcium‐channel blocker, n (%) | 1857 (67.48) | 465 (67.59) | 0.999 | 0.002 |
| Diuretic, n (%) | 1005 (36.52) | 250 (36.34) | 0.965 | −0.004 |
| Other medication | ||||
| Aspirin, n (%) | 135 (4.91) | 41 (5.96) | 0.287 | 0.047 |
| Clopidogrel, n (%) | 56 (2.03) | 12 (1.74) | 0.759 | −0.021 |
| Ticlopidine, n (%) | 23 (0.84) | 2 (0.29) | 0.206 | −0.073 |
| Warfarin, n (%) | 22 (0.80) | 4 (0.58) | 0.805 | −0.026 |
| PPI, n (%) | 76 (2.76) | 16 (2.33) | 0.598 | −0.028 |
| H2 blocker, n (%) | 198 (7.19) | 53 (7.70) | 0.624 | 0.019 |
| Statin, n (%) | 192 (6.98) | 46 (6.69) | 0.867 | −0.012 |
| NSAID, n (%) | 1264 (45.93) | 319 (46.37) | 0.864 | 0.009 |
| Steroid, n (%) | 224 (8.14) | 49 (7.12) | 0.430 | −0.038 |
| SSRI, n (%) | 45 (1.64) | 10 (1.45) | 0.866 | −0.015 |
| Nitrate, n (%) | 5 (0.18) | 0 (0.00) | 0.590 | −0.060 |
| Outcome of interests | ||||
| CHF, n (%) | 172 (6.25) | 23 (3.34) | 0.002 | −0.136 |
| Mortality, n (%) | 312 (11.34) | 25 (3.63) | <0.001 | −0.296 |
ACEI indicates angiotensin‐converting enzyme inhibitor; APA, aldosterone‐producing adenoma; ARB, angiotensin receptor blocker; CHF, congestive heart failure; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; EH, essential hypertension; H2 blocker, histamine‐2 receptor antagonist; NSAID, nonsteroidal anti‐inflammatory drug; NT$, New Taiwan dollar; PA, primary aldosteronism; PPI, proton pump inhibitor; RA, rheumatoid arthritis; SLE, systemic lupus erythematosus; SSRI, selective serotonin reuptake inhibitor.
Cox regression models with time‐varying covariates account for the influences on risk of CHF or death together with the matched set. Time‐varying covariates took the value 0 before the start of an MRA or surgical treatment and could switch to 1 at the start of treatment for CHF, and mortalities occurring before the end of 2010 were properly identified. Date of censoring was defined as the earliest of the date of death of study subjects during follow‐up, date of last withdrawal from NHI or date of follow‐up termination, whichever comes the earliest. Because of higher mortality rates in male patients and elderly patients, a competing‐risk regression, using the Fine and Gray model by considering the subdistribution hazard ratio (sHR), was also performed.29, 37 The incidence density rates of end points were calculated using rate per 1000 people‐years. Cox proportional hazards regression, estimated hazard ratio, and 95% CI to plot hazards of PA to EH. All analyses were performed with R software, version 2.8.1 (Free Software Foundation, Inc., Boston, MA).
Results
Characteristics of the Study Population
Baseline characteristics are presented in Table 1, and there were 688 patients with newly diagnosed APAs. In both APA and EH groups, dyslipidemia was the most common underlying disease, followed by diabetes mellitus; in addition, calcium‐channel blockers were the most commonly used antihypertension medication. (Data S1 also provides detailed information regarding comparison of PA and EH; see Tables S3 and S4.)
After an average of >5‐year follow‐up (5.2±3.5 years), 23 (3.34%) patients in the APA group and 172 (6.24%) patients in the EH group had new‐onset (incidental) CHF. There is no statistical difference in onset of atrial fibrillation between nonmatched groups; however, after propensity score matching, the statistical difference of preexisting atrial fibrillation between matched groups was significant (0.65% versus 0.00%; P=0.034). Patients in APA group after matching had lower prevalence of atrial fibrillation.
The Comparative Incidence of Incidental CHF and All‐Cause Mortality in Patients With APA Versus EH
After targeted treatment (including either adrenalectomy or MRA therapy), the incidence of incidental CHF was 5.3 per 1000 person‐years in the APA group, whereas the incidence of incidental CHF in the EH controls (versus the APA group) was 11.1 per 1000 person‐years.
The mortality incidence was 5.7 per 1000 person‐years in the APA group, but 19.1 per 1000 person‐years in the EH control group.
Regarding the comparison of the APA versus its propensity scored–matched EH controls (Table 2), as most patients underwent adrenalectomy (605/688=87.9%) in the APA group, the incidence of CHF and mortality was lower relative to the EH group: CHF (adjusted hazard ratio, 0.49; 95% CI; 0.31–0.75; P=0.001); mortality (adjusted hazard ratio, 0.29; 95% CI, 0.20–0.44; P<0.001; Table 2).
Table 2.
Incidence Rate of CHF, Mortality and HRs (APA and EH)
| Variables | Events | Person‐Years | Incidence Ratea | Events | Person‐Years | Incidence Ratea |
|---|---|---|---|---|---|---|
| EH | APA | |||||
| CHF | 172 | 15 486 | 11.1 | 23 | 4327.9 | 5.3 |
| Mortality | 312 | 16 372 | 19.1 | 25 | 4423.3 | 5.7 |
| Comparison of CHF and Mortality Risks in APA vs EH Patients | ||||||
|---|---|---|---|---|---|---|
| HR | P Value | Adjusted HR (95% CI) | P Value | HR Competing With Mortality (95% CI) | P Value | |
| CHF | 0.49 (0.32–0.76) | 0.001 | 0.49 (0.31–0.75) | 0.001 | 0.51 (0.33–0.79) | 0.002 |
| Mortality | 0.30 (0.20–0.45) | <0.001 | 0.29 (0.20–0.44) | <0.001 | ||
APA indicates aldosterone producing adenoma; CHF, congestive heart failure; EH, essential hypertension; HR, hazard ratio; PA, primary aldosteronism.
Per 1000 person‐years.
Risk of CHF and All‐Cause Mortality After Adrenalectomy
Considering adrenalectomy as a time‐varying factor, risks of developing CHF (sHR, 0.55; 95% CI; 0.34–0.90; P=0.017) or mortality (sHR, 0.27; 95% CI, 0.16–0.44; P<0.001) were significantly lower in the patients with APAs who underwent adrenalectomy, as compared with their respective EH controls. After adjusting mortality as a competing risk, adrenalectomy was associated with the lower risk of incident CHF (sHR, 0.56; 95% CI, 0.34–0.91; P=0.019; Table 3) in the patients with APAs who underwent adrenalectomy. However, there was no statistically significant difference of the risks of developing CHF (sHR, 0.99; 95% CI, 0.82–1.19; P=0.903) or mortality (sHR, 1.08; 95% CI, 0.94–1.23; P=0.262) in the patients with APAs with an MRA and without adrenalectomy, as compared with their respective EH controls.
Table 3.
Risk of CHF and Mortality of Patients With APA Undergoing Adrenalectomy
| Variables | Crude | Adjusta | Competeb | |||
|---|---|---|---|---|---|---|
| Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | Hazard Ratio (95% CI) | P Value | |
| APA patients with adrenalectomy (N=605) | ||||||
| CHF | 0.55 (0.34–0.89) | 0.016 | 0.55 (0.34–0.90) | 0.017 | 0.56 (0.34–0.91) | 0.019 |
| Mortality | 0.26 (0.16–0.44) | <0.001 | 0.27 (0.16–0.44) | <0.001 | NA | NA |
| APA patients with MRA, without adrenalectomy (N=83) | ||||||
| CHF | 0.99 (0.82–1.19) | 0.928 | 0.99 (0.82–1.19) | 0.903 | 0.99 (0.82–1.20) | 0.930 |
| Mortality | 1.00 (0.94–1.23) | 0.266 | 1.08 (0.94–1.23) | 0.262 | NA | NA |
APA indicates aldosterone producing adenoma; CHF, congestive heart failure; NA, not applicable.
After adjusting propensity score matching, expressed as adjusted hazard ratio.
Taking mortality as a competing risk and expressed as subdistribution hazard ratio.
Discussion
Main Findings
This large, long‐term, real‐world study had several significant findings: The patients with APAs who underwent adrenalectomy were associated with lower risks of incidental CHF and mortality than EH controls after targeted treatment. However, there was no statistically significant difference in the risks of developing CHF in the patients with APAs with an MRA and without adrenalectomy, as compared with their respective EH controls. These results suggest that adrenalectomy was associated with a lower risk of incidental CHF as well as mortality in real‐world practice.
Current Therapeutic Practice Among Patients With APA
Although a significant number of patients with PA with APAs can be cured by surgical adrenalectomy, many patients are treated medically with an MRA.37, 38
The benefits of adrenalectomy on our patients are obvious and associated with a lower risk of CHF (sHR, 0.56) and mortality (adjusted hazard ratio, 0.27) in patients with APAs. Therefore, accurate diagnosis (confirmation of the PA diagnosis, differential diagnosis of idiopathic bilateral hyperaldosteronism versus APA subtype, and lateralization of the APA lesion if any) and timely adrenalectomy is suggested in treating patients with lateralized PA, and individual therapeutic strategies could then be planned.
Lower Risk of New‐Onset CHF and All‐Causes Mortality in Patients With APAs Undergoing Adrenalectomy
In this study, we found that adrenalectomy was associated with the significantly lower risk of new‐onset CHF and mortality in patients with APAs. Similar findings of lower rates of cardiovascular events have been reported in several previous studies39, 40, 41, 42 however, there was no associated evidence of lower rates of new‐onset CHF in those studies. Catena et al39 followed 54 patients with PA for 12 years, 24 of whom received adrenalectomy. They found that rates of myocardial infarction, stroke, revascularization procedures, and sustained arrhythmia were comparable with those of EH. Rossi et al40 showed that the percentage of changes of LV mass index in short‐ and long‐term follow‐ups were more prominent in patients undergoing adrenalectomy. Another testament demonstrating the benefit of adrenalectomy is the work of Strauch et al43: surgical adrenalectomy in 29 patients with APAs/PA with 7.4 years follow‐up, compared with conservative treatment of patients with PA, was associated with a significant reduction of blood pressure and improved arterial stiffness parameters. As a result, the regression of risk of cardiovascular complications after adrenalectomy could work to remedy the association between aldosterone and LV hypertrophy.
To our knowledge, our study is the first to show that adrenalectomy in patients with APAs was associated with not only a reduction of 73% chance of mortality but also a reduction of 45% chance of new‐onset CHF. This significant effect of adrenalectomy on lowering all‐cause mortality and new‐onset CHF is important for urging clinicians to reach an accurate diagnosis of PA and prompt differential diagnosis of its APA versus an idiopathic bilateral hyperaldosteronism subdiagnosis, to provide the most effective individualized therapeutic options for the patients with PA.
Possible mechanisms of the superior effect of adrenalectomy in preventing CHF can be explained by the following factors:
Hypertension is one of the most important factors contributing to CHF. Not only could patients with APAs have improved or normalized blood pressure after adrenalectomy44 but this reduction of blood pressure could last for a long time.45, 46 In contrast, the aldosterone level remains high in patients with APAs using an MRA, even though the mineralocorticoid has been blocked. The doses and kinds of antihypertensive agents used have also been shown to increase with time.40, 47
Several studies have shown that the structural cardiac changes seen in patients with APAs without adrenalectomy, mostly LV hypertrophy/hyperplasia, are resolved or greatly improved after adrenalectomy.48, 49, 50, 51, 52 LV mass and LV mass index are also shown to significantly decrease after adrenalectomy, as seen in a prospective study of 6.4 years.48, 52
With medical and MRA treatment for APAs, the patient's drug compliance is vital, and poor medication compliance was associated with higher levels of aldosterone, which could lead to further deterioration of PA and associated complications.
Adrenalectomy in patients with APAs could lower the prevalence of new‐onset atrial fibrillation, which is deeply related to CHF and mortality, and consequently was associated with a lower risk of new‐onset CHF and mortality.
Our study showed the evidence that adrenalectomy is the treatment of choice for patients with APAs suitable for operation; the treatment goal is to prevent the morbidity and mortality associated with hypertension, hypokalemia, and cardiovascular damage, especially CHF.
Strengths and Limitations
This study has several strengths. First, we provided a large cohort of patients with APAs and a long‐term follow‐up by using a population‐based database, which carries a low risk of selection bias. Second, our investigation analysis targeted patients in real‐world practice and provided clinical support to previous echocardiography findings and smaller‐scale clinical observational and in vitro studies.22 Third, patients with APAs were compared with their respective EH control patients, via proper propensity score matching, and both groups had comparable baseline cardiovascular risk profiles.
However, there are also some weaknesses in our retrospective cohort analysis that should be acknowledged. First, the data retrieved and examined in this study were from population‐based health insurance registration data, where the diagnostic results were not “controlled” as ideally as some single‐ or multicenter clinical studies could have achieved. These findings may shed light on some heterogeneity of findings in previous studies, as targeted treatment has not always been accounted for in the studies. It is unlikely that prospective randomized trials of adrenalectomy for prevention of CHF among patients with APAs will ever be undertaken because of the sheer number of patients that would require randomization to have adequate numbers of the patients with APAs. Second, as a claims‐based analysis, we could not eliminate the possibility of selection bias and residual confounding. The propensity score matching prompted a rigorous adjustment, but the confounding inherent to unmeasured factors affecting treatment assignment still could not be fully accounted for. Third, it is possible that the diagnosis of PA on the basis of only the ICD code may not be correct. However, we located patients with PA using both diagnosis with 255.1x and existence of an MRA prescription, and this model has been examined in previous studies with meticulous sensitivity and specificity. Fourth, actual blood pressure control cannot be acquired in this registry. Fifth, because of patients’ and treating physicians’ choice, a few cases choose second‐class medical care, and this choice is associated with poor clinical outcomes. Therefore, a prospective study is needed to investigate the risk of new‐onset CHF in patients with APAs and explore the effect of adrenalectomy on new‐onset CHF.
Conclusions
For patients with APAs, adrenalectomy can be associated with a lower risk of incidental CHF and all‐cause mortality in a long‐term follow‐up.
Appendix
Membership of the Taiwan Primary Aldosteronism Investigation (TAIPAI) Study Group
Tai‐Shuan Lai, Vin‐Cent Wu, Shao‐Yu Yang, Kao‐Lang Liu, Chin‐Chen Chang, Bo‐Chiag Lee, Shuo‐Meng Wang, Kuo‐How Huang, Po‐Chih Lin, Yen‐Hung Lin, Lian‐Yu Lin, Shih‐Cheng Liao, Ruoh‐Fang Yen, and Ching‐Chu Lu (National Taiwan University Hospital, Taipei, Taiwan); Chieh‐Kai Chan (NTUH Hsin‐Chu branch); Leay‐Kiaw Er, Ya‐Hui Hu, Chia‐Hui Chang, Che‐Hsiung Wu, and Yao‐Chou Tsai (Taipei Tzu Chi Hospital, Buddhist Tzu Chi Medical Foundation, Taipei, Taiwan); Shih‐Chieh Jeff Chueh (Cleveland Clinic Institute of Urology and Kidneys); Chen‐Hsun Ho (Taipei Medical University‐Shuang Ho Hospital, Ministry of Health and Welfare); Wei‐Chieh Huang (New Taipei City Hospital); Ying‐Ying Chen (MacKay Memorial Hospital); and Kwan‐Dun Wu (National Taiwan University Hospital, Taipei, Taiwan NTUH, Director of Coordinating Center).
Sources of Funding
This work was supported by a grant from Taipei Medical University (grant number, 105‐TMU‐NTUST‐105‐03) and Ministry of Science and Technology (MOST) of the Republic of China (Taiwan) (grant number, MOST 106‐2321‐B‐182‐002).
Disclosures
None.
Supporting information
Data S1. Identification of primary aldosteronism from the National Health Insurance Research Databases.
Table S1. Details of Diagnostic Procedures From National Health Insurance Data12
Table S2. Details of Diagnostic Procedures From TAIPAI Database12
Table S3. Baseline Characteristics of All Study Population
Table S4. Incidence Rate of CHF, Mortality, and HRs (PA and EH)
Acknowledgments
Role of the sponsors: Some of the authors are employed by 2 organizations financially supporting the study: National Taiwan University Hospital and National Health Research Institutes. The other funding organization, National Science Council, neither played a role in the design and conduct of the study; in the collection, management, analysis, and interpretation of the data; nor in the preparation, review, and approval of the manuscript. We thank Mr Eric B. Chueh for English editing of the manuscript.
Wei Chieh Huang drafted the manuscript and collected data. Chun‐Yao Huang and Vin‐Cent Wu provided the original conception and design of the study, modified the statistical models critically, and provided technical and statistical support during the analyses. All the authors interpreted and had full access to the data, revised the manuscript critically, and approved the final article.
(J Am Heart Assoc. 2019;8:e012410 DOI: 10.1161/JAHA.119.012410.)
Contributor Information
Chun‐Yao Huang, Email: cyhuang@h.tmu.edu.tw.
Vin‐Cent Wu, Email: q91421028@ntu.edu.tw.
TAIPAI Study Group:
Tai‐Shuan Lai, Shao‐Yu Yang, Kao‐Lang Liu, Chin‐Chen Chang, Bo‐Chiag Lee, Shuo‐Meng Wang, Kuo‐How Huang, Lian‐Yu Lin, Shih‐Cheng Liao, Ruoh‐Fang Yen, Ching‐Chu Lu, Chieh‐Kai Chan, Leay‐Kiaw Er, Ya‐Hui Hu, Chia‐Hui Chang, Yao‐Chou Tsai, and Chen‐Hsun Ho
References
- 1. Monticone S, Burrello J, Tizzani D, Bertello C, Viola A, Buffolo F, Gabetti L, Mengozzi G, Williams TA, Rabbia F, Veglio F, Mulatero P. Prevalence and clinical manifestations of primary aldosteronism encountered in primary care practice. J Am Coll Cardiol. 2017;69:1811–1820. [DOI] [PubMed] [Google Scholar]
- 2. Horton R. Aldosterone: review of its physiology and diagnostic aspects of primary aldosteronism. Metabolism. 1973;22:1525–1545. [DOI] [PubMed] [Google Scholar]
- 3. Nicholls MG, Ramsay LE, Boddy K, Fraser R, Morton JJ, Robertson JI. Mineralocorticoid‐induced blood pressure, electrolyte, and hormone changes, and reversal with spironolactone, in healthy men. Metabolism. 1979;28:584–593. [DOI] [PubMed] [Google Scholar]
- 4. Young WF Jr. Minireview: primary aldosteronism–changing concepts in diagnosis and treatment. Endocrinology. 2003;144:2208–2213. [DOI] [PubMed] [Google Scholar]
- 5. Milliez P, Girerd X, Plouin PF, Blacher J, Safar ME, Mourad JJ. Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism. J Am Coll Cardiol. 2005;45:1243–1248. [DOI] [PubMed] [Google Scholar]
- 6. Tomaschitz A, Ritz E, Pieske B, Rus‐Machan J, Kienreich K, Verheyen N, Gaksch M, Grubler M, Fahrleitner‐Pammer A, Mrak P, Toplak H, Kraigher‐Krainer E, Marz W, Pilz S. Aldosterone and parathyroid hormone interactions as mediators of metabolic and cardiovascular disease. Metabolism. 2014;63:20–31. [DOI] [PubMed] [Google Scholar]
- 7. Conn JW, Knopf RF, Nesbit RM. Clinical characteristics of primary aldosteronism from an analysis of 145 cases. Am J Surg. 1964;107:159–172. [DOI] [PubMed] [Google Scholar]
- 8. Suzuki T, Abe H, Nagata S, Saitoh F, Iwata S, Ashizawa A, Kuramochi M, Omae T. Left ventricular structural characteristics in unilateral renovascular hypertension and primary aldosteronism. Am J Cardiol. 1988;62:1224–1227. [DOI] [PubMed] [Google Scholar]
- 9. Rossi G, Boscaro M, Ronconi V, Funder JW. Aldosterone as a cardiovascular risk factor. Trends Endocrinol Metab. 2005;16:104–107. [DOI] [PubMed] [Google Scholar]
- 10. Wu VC, Yang SY, Lin JW, Cheng BW, Kuo CC, Tsai CT, Chu TS, Huang KH, Wang SM, Lin YH, Chiang CK, Chang HW, Lin CY, Lin LY, Chiu JS, Hu FC, Chueh SC, Ho YL, Liu KL, Lin SL, Yen RF, Wu KD; TAIPAI Study Group . Kidney impairment in primary aldosteronism. Clin Chim Acta. 2011;412:1319–1325. [DOI] [PubMed] [Google Scholar]
- 11. Kuo CC, Wu VC, Tsai CW, Wu KD; Taiwan Primary Aldosteronism Investigation Study Group . Relative kidney hyperfiltration in primary aldosteronism: a meta‐analysis. J Renin Angiotensin Aldosterone Syst. 2011;12:113–122. [DOI] [PubMed] [Google Scholar]
- 12. Rossi GP, Sechi LA, Giacchetti G, Ronconi V, Strazzullo P, Funder JW. Primary aldosteronism: cardiovascular, renal and metabolic implications. Trends Endocrinol Metab. 2008;19:88–90. [DOI] [PubMed] [Google Scholar]
- 13. Plante GE. Vascular response to stress in health and disease. Metabolism. 2002;51:25–30. [DOI] [PubMed] [Google Scholar]
- 14. Funder JW. Aldosterone, mineralocorticoid receptors and vascular inflammation. Mol Cell Endocrinol. 2004;217:263–269. [DOI] [PubMed] [Google Scholar]
- 15. Nicoletti A, Michel JB. Cardiac fibrosis and inflammation: interaction with hemodynamic and hormonal factors. Cardiovasc Res. 1999;41:532–543. [DOI] [PubMed] [Google Scholar]
- 16. Sowers JR, Whaley‐Connell A, Epstein M. Narrative review: the emerging clinical implications of the role of aldosterone in the metabolic syndrome and resistant hypertension. Ann Intern Med. 2009;150:776–783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hannemann A, Meisinger C, Bidlingmaier M, Doring A, Thorand B, Heier M, Belcredi P, Ladwig KH, Wallaschofski H, Friedrich N, Schipf S, Ludemann J, Rettig R, Peters J, Volzke H, Seissler J, Beuschlein F, Nauck M, Reincke M. Association of plasma aldosterone with the metabolic syndrome in two German populations. Eur J Endocrinol. 2011;164:751–758. [DOI] [PubMed] [Google Scholar]
- 18. Fallo F, Veglio F, Bertello C, Sonino N, Della Mea P, Ermani M, Rabbia F, Federspil G, Mulatero P. Prevalence and characteristics of the metabolic syndrome in primary aldosteronism. J Clin Endocrinol Metab. 2006;91:454–459. [DOI] [PubMed] [Google Scholar]
- 19. Matrozova J, Steichen O, Amar L, Zacharieva S, Jeunemaitre X, Plouin PF. Fasting plasma glucose and serum lipids in patients with primary aldosteronism: a controlled cross‐sectional study. Hypertension. 2009;53:605–610. [DOI] [PubMed] [Google Scholar]
- 20. Rossi GP, Sacchetto A, Visentin P, Canali C, Graniero GR, Palatini P, Pessina AC. Changes in left ventricular anatomy and function in hypertension and primary aldosteronism. Hypertension. 1996;27:1039–1045. [DOI] [PubMed] [Google Scholar]
- 21. Goff DC Jr, Pandey DK, Chan FA, Ortiz C, Nichaman MZ. Congestive heart failure in the United States: is there more than meets the I(CD code)? The Corpus Christi Heart Project. Arch Intern Med. 2000;160:197–202. [DOI] [PubMed] [Google Scholar]
- 22. Wu VC, Hu YH, Wu CH, Kao CC, Wang CY, Yang WS, Lee HH, Chang YS, Lin YH, Wang SM, Chen L, Wu KD; TAIPAI Study Group . Administrative data on diagnosis and mineralocorticoid receptor antagonist prescription identified patients with primary aldosteronism in Taiwan. J Clin Epidemiol. 2014;67:1139–1149. [DOI] [PubMed] [Google Scholar]
- 23. Wu VC, Wu CH, Huang TM, Wang CY, Lai CF, Shiao CC, Chang CH, Lin SL, Chen YY, Chen YM, Chu TS, Chiang WC, Wu KD, Tsai PR, Chen L, Ko WJ; NSARF Group . Long‐term risk of coronary events after AKI. J Am Soc Nephrol. 2014;25:595–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Wu VC, Chueh SJ, Chen L, Chang CH, Hu YH, Lin YH, Wu KD, Yang WS; TAIPAI Study Group . Risk of new‐onset diabetes mellitus in primary aldosteronism: a population study over 5 years. J Hypertens. 2017;35:1698–1708. [DOI] [PubMed] [Google Scholar]
- 25. Wang WJ, Chao CT, Huang YC, Wang CY, Chang CH, Huang TM, Lai CF, Huang HY, Shiao CC, Chu TS, Chen YM, Wu VC, Ko WJ, Wu KD; National Taiwan University Study Group on Acute Renal Failure . The impact of acute kidney injury with temporary dialysis on the risk of fracture. J Bone Miner Res. 2014;29:676–684. [DOI] [PubMed] [Google Scholar]
- 26. Lai TS, Wang CY, Pan SC, Huang TM, Lin MC, Lai CF, Wu CH, Wu VC, Chien KL; National Taiwan University Hospital Study Group on Acute Renal Failure . Risk of developing severe sepsis after acute kidney injury: a population‐based cohort study. Crit Care. 2013;17:R231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lin CC, Lai MS, Syu CY, Chang SC, Tseng FY. Accuracy of diabetes diagnosis in health insurance claims data in Taiwan. J Formos Med Assoc. 2005;104:157–163. [PubMed] [Google Scholar]
- 28. Cheng CL, Kao YH, Lin SJ, Lee CH, Lai ML. Validation of the National Health Insurance Research Database with ischemic stroke cases in Taiwan. Pharmacoepidemiol Drug Saf. 2011;20:236–242. [DOI] [PubMed] [Google Scholar]
- 29. Wu VC, Hu YH, Er LK, Yen RF, Chang CH, Chang YL, Lu CC, Chang CC, Lin JH, Lin YH, Wang TD, Wang CY, Tu ST, Jeff Chueh SC, Chang CC, Tseng FY, Wu KD; TAIPAI Group . Case detection and diagnosis of primary aldosteronism—the consensus of Taiwan Society of Aldosteronism. J Formos Med Assoc. 2017;116:993–1005. [DOI] [PubMed] [Google Scholar]
- 30. Wu VC, Wang SM, Chang CH, Hu YH, Lin LY, Lin YH, Chueh SC, Chen L, Wu KD. Long term outcome of aldosteronism after target treatments. Sci Rep. 2016;6:32103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lee BC, Chang CC, Liu KL, Chang YC, Wu VC, Huang KH. Evaluation of right adrenal vein anatomy by Dyna computed tomography in patients with primary aldosteronism. Sci Rep. 2016;6:28305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wu VC, Chang HW, Liu KL, Lin YH, Chueh SC, Lin WC, Ho YL, Huang JW, Chiang CK, Yang SY, Chen YM, Wang SM, Huang KH, Hsieh BS, Wu KD; TAIPAI Study Group . Primary aldosteronism: diagnostic accuracy of the losartan and captopril tests. Am J Hypertens. 2009;22:821–827. [DOI] [PubMed] [Google Scholar]
- 33. Yen RF, Wu VC, Liu KL, Cheng MF, Wu YW, Chueh SC, Lin WC, Wu KD, Tzen KY, Lu CC; TAIPAI Study Group . 131I‐6beta‐iodomethyl‐19‐norcholesterol SPECT/CT for primary aldosteronism patients with inconclusive adrenal venous sampling and CT results. J Nucl Med. 2009;50:1631–1637. [DOI] [PubMed] [Google Scholar]
- 34. Chuang SC, Wu GJ, Lu YS, Lin CH, Hsiung CA. Associations between medical conditions and breast cancer risk in Asians: a nationwide population‐based study in Taiwan. PLoS One. 2015;10:e0143410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Dai YX, Chen MH, Chen TJ, Lin MH. Patterns of psychiatric outpatient practice in Taiwan: a nationwide survey. Int J Environ Res Public Health. 2016;13:E955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Wu CS, Lai MS, Gau SS, Wang SC, Tsai HJ. Concordance between patient self‐reports and claims data on clinical diagnoses, medication use, and health system utilization in Taiwan. PLoS One. 2014;9:e112257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Funder JW, Carey RM, Mantero F, Murad MH, Reincke M, Shibata H, Stowasser M, Young WF Jr. The management of primary aldosteronism: case detection, diagnosis, and treatment: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2016;101:1889–1916. [DOI] [PubMed] [Google Scholar]
- 38. Vaidya A, Malchoff CD, Auchus RJ; AACE Adrenal Scientific Committee . An individualized approach to the evaluation and management of primary aldosteronism. Endocr Pract. 2017;23:680–689. [DOI] [PubMed] [Google Scholar]
- 39. Catena C, Colussi G, Nadalini E, Chiuch A, Baroselli S, Lapenna R, Sechi LA. Cardiovascular outcomes in patients with primary aldosteronism after treatment. Arch Intern Med. 2008;168:80–85. [DOI] [PubMed] [Google Scholar]
- 40. Rossi GP, Cesari M, Cuspidi C, Maiolino G, Cicala MV, Bisogni V, Mantero F, Pessina AC. Long‐term control of arterial hypertension and regression of left ventricular hypertrophy with treatment of primary aldosteronism. Hypertension. 2013;62:62–69. [DOI] [PubMed] [Google Scholar]
- 41. Wu CH, Yang YW, Hung SC, Tsai YC, Hu YH, Lin YH, Chu TS, Wu KD, Wu VC. Effect of treatment on body fluid in patients with unilateral aldosterone producing adenoma: adrenalectomy versus spironolactone. Sci Rep. 2015;5:15297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Mulatero P, Monticone S, Bertello C, Viola A, Tizzani D, Iannaccone A, Crudo V, Burrello J, Milan A, Rabbia F, Veglio F. Long‐term cardio‐ and cerebrovascular events in patients with primary aldosteronism. J Clin Endocrinol Metab. 2013;98:4826–4833. [DOI] [PubMed] [Google Scholar]
- 43. Strauch B, Petrak O, Zelinka T, Wichterle D, Holaj R, Kasalicky M, Safarik L, Rosa J, Widimsky J Jr. Adrenalectomy improves arterial stiffness in primary aldosteronism. Am J Hypertens. 2008;21:1086–1092. [DOI] [PubMed] [Google Scholar]
- 44. Group TS , Wu VC, Chueh SC, Chang HW, Lin LY, Liu KL, Lin YH, Ho YL, Lin WC, Wang SM, Huang KH, Hung KY, Kao TW, Lin SL, Yen RF, Chen YM, Hsieh BS, Wu KD. Association of kidney function with residual hypertension after treatment of aldosterone‐producing adenoma. Am J Kidney Dis. 2009;54:665–673. [DOI] [PubMed] [Google Scholar]
- 45. Sywak M, Pasieka JL. Long‐term follow‐up and cost benefit of adrenalectomy in patients with primary hyperaldosteronism. Br J Surg. 2002;89:1587–1593. [DOI] [PubMed] [Google Scholar]
- 46. Letavernier E, Peyrard S, Amar L, Zinzindohoue F, Fiquet B, Plouin PF. Blood pressure outcome of adrenalectomy in patients with primary hyperaldosteronism with or without unilateral adenoma. J Hypertens. 2008;26:1816–1823. [DOI] [PubMed] [Google Scholar]
- 47. Ori Y, Chagnac A, Korzets A, Zingerman B, Herman‐Edelstein M, Bergman M, Gafter U, Salman H. Regression of left ventricular hypertrophy in patients with primary aldosteronism/low‐renin hypertension on low‐dose spironolactone. Nephrol Dial Transplant. 2013;28:1787–1793. [DOI] [PubMed] [Google Scholar]
- 48. Catena C, Colussi G, Lapenna R, Nadalini E, Chiuch A, Gianfagna P, Sechi LA. Long‐term cardiac effects of adrenalectomy or mineralocorticoid antagonists in patients with primary aldosteronism. Hypertension. 2007;50:911–918. [DOI] [PubMed] [Google Scholar]
- 49. Yoshitomi Y, Nishikimi T, Abe H, Yoshiwara F, Suzuki T, Ashizawa A, Nagata S, Kuramochi M, Matsuoka H, Omae T. Comparison of changes in cardiac structure after treatment in secondary hypertension. Hypertension. 1996;27:319–323. [DOI] [PubMed] [Google Scholar]
- 50. Giacchetti G, Ronconi V, Turchi F, Agostinelli L, Mantero F, Rilli S, Boscaro M. Aldosterone as a key mediator of the cardiometabolic syndrome in primary aldosteronism: an observational study. J Hypertens. 2007;25:177–186. [DOI] [PubMed] [Google Scholar]
- 51. Lin YH, Huang KH, Lee JK, Wang SM, Yen RF, Wu VC, Chung SD, Liu KL, Chueh SC, Lin LY, Ho YL, Chen MF, Wu KD; Group Ts . Factors influencing left ventricular mass regression in patients with primary aldosteronism post adrenalectomy. J Renin Angiotensin Aldosterone Syst. 2011;12:48–53. [DOI] [PubMed] [Google Scholar]
- 52. Matsumura K, Fujii K, Oniki H, Oka M, Iida M. Role of aldosterone in left ventricular hypertrophy in hypertension. Am J Hypertens. 2006;19:13–18. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Identification of primary aldosteronism from the National Health Insurance Research Databases.
Table S1. Details of Diagnostic Procedures From National Health Insurance Data12
Table S2. Details of Diagnostic Procedures From TAIPAI Database12
Table S3. Baseline Characteristics of All Study Population
Table S4. Incidence Rate of CHF, Mortality, and HRs (PA and EH)
Data Availability Statement
We are unable to make our data, analytic methods, and study materials available to other researchers because of the following limitation: The Taiwanese Government allows researchers to analyze the National Health Insurance (NHI) data only from locations within Taiwan. Currently, the government owns 2 centers that store NHI data for academic use: the data science center of health and welfare (https://dep.mohw.gov.tw/DOS/np-2497-113.html) and the information integration and application service center of the NHI Administration (https://www.nhi.gov.tw/Content_List.aspx?n=468220093AFEB6DF&topn=CA428784F9ED78C9). Researchers in Taiwan are required to submit their research plans and institutional review board approvals for the proposed studies and to pay for using such data. The amount of payment depends on the volume of data retrieved.


