Abstract
Background
Limited data exist to guide treatment for patients with heart failure with preserved ejection fraction and atrial fibrillation, including the important decision regarding rate versus rhythm control.
Methods and Results
We analyzed the Get With The Guidelines—Heart Failure (GWTG‐HF) registry linked to Medicare claims data from 2008 to 2014 to describe current treatments for rate versus rhythm control and subsequent outcomes in patients with heart failure with preserved ejection fraction and atrial fibrillation using inverse probability weighted analysis. Rhythm control was defined as use of an antiarrhythmic medication, cardioversion, or AF ablation or surgery. Rate control was defined as use of any combination of β‐blocker, calcium channel blocker, and digoxin without evidence of rhythm control. Among 15 682 fee‐for‐service Medicare patients, at the time of discharge, 1857 were treated with rhythm control and 13 825 with rate control, with minimal differences in baseline characteristics between groups. There was higher all‐cause death at 1 year in the rate control compared with the rhythm control group (37.5% and 30.8%, respectively, P<0.01). The lower 1‐year all‐cause death in the rhythm control group remained after risk adjustment (adjusted hazard ratio, 0.86; 95% CI, 0.75–0.98; P=0.02).
Conclusions
Rhythm control in patients aged 65 and older with heart failure with preserved ejection fraction and AF was associated with a lower risk of 1 year all‐cause mortality. Future prospective randomized studies are needed to explore this potential benefit.
Keywords: atrial fibrillation, heart failure with preserved ejection fraction, rate control, rhythm control
Subject Categories: Heart Failure, Atrial Fibrillation, Quality and Outcomes
Clinical Perspective
What Is New?
Atrial fibrillation is common in patients with heart failure and is associated with higher mortality, readmissions, and heart failure readmissions.
Patients aged 65 and older with heart failure with preserved ejection fraction and atrial fibrillation treated with rhythm control were found to have an associated lower 1‐year mortality compared with treatment with rate control.
What Are the Clinical Implications?
Rhythm control is a potential treatment strategy for patients with heart failure with preserved ejection fraction and atrial fibrillation that may provide benefit.
Introduction
Heart failure with preserved ejection fraction (HFpEF) accounts for one half of all heart failure (HF) visits and hospital admissions in the United States. Patients with HFpEF have similar risks of morbidity and all‐cause mortality as patients with heart failure with reduced ejection fraction (HFrEF).1, 2 Atrial fibrillation (AF) is the most common sustained arrhythmia in patients with HF, occurring in approximately one third of patients.3, 4, 5, 6 In patients with HFpEF, the prevalence of AF ranges from 15% to 41% in registries and clinical trials.6
Patients with HF and AF have worse outcomes than patients with HF without AF.7 The HF guidelines focus their recommendations on prevention of thromboembolism and symptom control with the goal to correct underlying causes of AF and HF and optimize HF management. The guidelines then differentiate patients who develop HF as a result of AF and patients who have HF and go on to develop AF.8 Patients with newly diagnosed HF in the presence of AF with rapid ventricular response are recommended to undergo rhythm control, as a rate‐related cardiomyopathy is a reversible cause of HF. There is no other specific recommendation for rate versus rhythm control in the HF guidelines.8 The AF guidelines recommend rhythm control only in patients who remain symptomatic despite rate control treatment.9 A recent meta‐analysis revealed that all‐cause mortality is significantly higher in patients with AF and HFrEF compared with patients with AF and HFpEF, yet stroke risk and HF hospitalization rates were similar among both groups.10 Similar comparisons in the GWTG‐HF (Get With The Guidelines—Heart Failure) registry extends these prior findings, demonstrating that AF is common in patients with HF and associated with higher mortality, readmissions, and HF readmissions.11
HFpEF and AF have shared risk factors, confer increased risks for adverse cardiovascular outcomes, and frequently occur together.6, 12 Despite accumulating epidemiologic data detailing worse outcomes in patients with HFpEF complicated by AF, there are no medical therapies that have been definitively shown to improve outcomes for patients with HFpEF. As a result, the guideline writing committees limit their scope to treatment of associated comorbidities.8, 13, 14, 15, 16 To describe current treatment patterns and subsequent outcomes in patients with HFpEF complicated by AF, we analyzed the GWTG‐HF registry linked to Medicare claims data.
Methods
Data Sources
The primary data source for this study used the American Heart Association's GWTG‐HF registry, which is an ongoing hospital‐based voluntary national HF registry that was established in 2005. All participating centers obtain institutional review board approval for the registry protocol, and given that the primary purpose of the registry is for quality improvement, a waiver for informed consent is granted under the Common Rule. The design and methodology of the GWTG‐HF registry have been described previously.17, 18 All of the clinical, demographic, and medication data were obtained from the GWTG‐HF registry and subsequently linked with Medicare claims data for additional medical history information and outcome data.19 We obtained the 2008–2014 Medicare data for this study from the Centers for Medicare and Medicaid Research. These data included the denominator data, which contains information about enrollment, demographics, and mortality, and the inpatient claims data, which include information about hospitalizations.
We analyzed fee‐for‐service Centers for Medicare and Medicaid Services claims data for Medicare‐enrolled patients to ascertain long‐term patient outcomes following discharge. The following research identifiable files were used: (1) Denominator: The denominator file contains Medicare enrollment information as well as demographic data for each beneficiary, including date of birth, sex, and race/ethnicity. A unique beneficiary identifier is present in all research identifiable files and was used to link multiple inpatient claims on the beneficiary level. (2) Inpatient: Medicare Part A inpatient visit data were obtained. The inpatient research identifiable files contain information on dates of hospital admission and discharge, International Classification of Diseases, Ninth Revision (ICD‐9) diagnosis and procedure codes, hospital identifiers, and beneficiary demographic information.
Study Population
We included patients aged 65 years and older who were discharged alive from a GWTG‐HF hospitalization based on the treating physician's clinical diagnosis of heart failure successfully linked to Medicare claims data with a discharge date between January 1, 2008, and December 31, 2013. If multiple linked hospitalizations existed for a patient, the earliest was selected for analysis. Patients were required to be enrolled in fee‐for‐service Medicare at the index hospitalization and for the year prior, in order to ascertain data required for assigning patients into the appropriate study group.
Study Groups
Patients were required to have a clinical diagnosis of HFpEF and AF in the GWTG‐HF registry data set. Two groups of patients were identified on the basis of (1) rate control strategy or (2) rhythm control strategy to treat AF. Patients who did not meet either of these criteria were not included in this analysis (ie, patients who may have had HFpEF and AF but were not receiving rate or rhythm control treatments). Patients with HFpEF were required to have a documented ejection fraction ≥50%, or in the small number of patients where ejection fraction was qualified but not quantified, patients with normal or mildly impaired systolic function were classified as HFpEF as characterized in previous GWTG‐HF analyses.20 AF diagnosis required documentation of medical history of AF within the registry or diagnosis of AF at presentation or during the present hospitalization. Patients with transient episodes of AF that were entirely reversible and terminated within 8 weeks following coronary angiography or transient and entirely reversible because of thyrotoxicosis were not included. The rate control group was defined by the exclusive use of β‐blockers, calcium channel blockers, and/or digoxin in patients with known AF and HFpEF. The rhythm control group was defined by patients who had a history of or current hospitalization for AF ablation/pulmonary vein isolation treatment (in hospital); ongoing treatment with amiodarone, sotalol, tikosyn, or other antiarrhythmic therapy; or elective cardioversion (in hospital). Patients in the rhythm control group were also allowed to be treated with rate control agents including calcium channel blockers, β‐blockers, and/or digoxin.
Outcomes of Interest
The primary outcome of interest was all‐cause mortality. Secondary outcomes of interest included all‐cause mortality or readmission, all‐cause readmission, ischemic stroke readmissions, HF readmissions, other cardiovascular readmissions, and bleeding readmissions. Any hospitalization following discharge from the index hospitalization was included except for transfers to another hospital. This analysis used Medicare inpatient claims for ascertainment of readmission and used the denominator file for the ascertainment of mortality. Mortality was identified using the date of death in the Medicare denominator files, if applicable. Readmission was identified using the Medicare inpatient claims files. All‐cause readmission included any hospitalization following discharge from the index hospitalization, except transfers to another hospital and admissions for rehabilitation (diagnosis‐related group 945 or ICD‐9, Clinical Modification diagnosis code of V57.xx). All outcomes were assessed at 30 days and 1 year after discharge index date.
Patient Characteristics
The data, analytical methods, and study materials will not be made available to other researchers for purposes of reproducing the results or replicating the procedure. GWTG‐HF registry data were used to describe the baseline characteristics of study patients as of the discharge date from the index hospitalization. These characteristics were also used for risk adjustment. Patient characteristics of interest include demographics (age, sex, race), medical history (anemia, ischemic history, coronary artery disease, chronic renal insufficiency, chronic obstructive pulmonary disease/asthma, diabetes mellitus, hyperlipidemia, hypertension, stroke/transient ischemic attack, peripheral vascular disease, prior heart failure), laboratory data (serum creatinine), vital signs (discharge heart rate, systolic blood pressure), ejection fraction, hospital characteristics (region, hospital type, number of beds) and discharge medications (angiotensin‐converting enzyme, angiotensin receptor blocker, aspirin, and anticoagulation therapy).
Statistical Analysis
For each study group, we summarized patient demographics, physical and laboratory findings, medical history, and therapies using frequencies and percentages for categorical variables and medians and 25th and 75th percentiles for continuous variables with differences between groups tested using a chi‐square or Fisher's exact test. Baseline patient characteristics were compared using the Wilcoxon rank‐sum test for continuous variables and chi‐square tests for categorical variables.
Next, we described the observed outcomes according to group. For mortality, we calculated incidence based on Kaplan–Meier estimates and tested for differences between groups using log‐rank tests. For readmission, we calculated incidence based on estimates from cumulative incidence function, which accounts for the competing risk of mortality, and we tested for differences between groups using Gray tests. To account for potential confounding by characteristics influencing treatment decisions and outcomes, we used inverse probability of treatment weighting. The probability of rhythm control treatment use was estimated using age, sex, race, prior ischemic history, hypertension, hyperlipidemia, smoking history, prior cerebrovascular accident/transient ischemic attack, diabetes mellitus, renal insufficiency, anemia, peripheral vascular disease, prior heart failure, chronic obstructive pulmonary disease, other evidence‐based discharge therapy, and hospital characteristics. Digoxin use status was not included in any model. Each subject was then weighted by the inverse of this treatment propensity in survival models. The distribution of baseline patient characteristics for each treatment group was assessed after weighting to determine adequacy of the propensity score. Specifically, short‐term outcomes (within 30 days) were analyzed using the GWTG‐HF registry data only, which included patients’ baseline characteristics measured at or shortly after admission, including laboratory results and hospital characteristics. Long‐term outcomes were analyzed using GWTG‐HF data linked to Centers for Medicare and Medicaid Services data, which allowed us to identify and ascertain death; readmissions and the associated regression models included all important patient hospital results, including laboratory results and medication characteristics. To account for clustering of patients within hospitals, all regression models included the generalized estimating equation variance estimation procedure.
The binary treatment was rhythm control (yes) versus rate control (no). Then, we estimated the hazard ratios between study groups and each outcome using a Cox proportional hazard regression, weighted by the inverse probability of treatment. Multiple imputation by fully conditional specification was performed for missing values. For missing medical history, it was assumed that the medical condition did not occur. Variables with missing rate >40% were not included in the model. We also performed Cox proportional hazards models to assess the association of rhythm control versus rate control with other outcomes of interest, including all‐cause readmission, stroke readmission, bleeding readmission, HF readmission, and other cardiovascular readmission. For all models, significance tests and CIs were based on robust standard errors to account for the clustering of patients by hospital. We reported estimates and 95% CIs and used 2‐tailed tests with α=0.05 to establish statistical significance.
Results
Baseline Demographics
There were 15 682 patients in the GWTG‐HF registry who were enrolled in fee‐for‐service Medicare discharged between January 1, 2008, and December 31, 2013, with HFpEF and AF and received rate or rhythm control treatment. Of 15 682 patients with HFpEF, 1857 received rhythm control and 13 825 received rate control. There were differences in age (median age 81 for rhythm control and 83 for rate control) but other variables were similar, including sex (65.8% female in both groups), comorbidities, vital signs, and laboratory values.
Rate Versus Rhythm Control
There were no differences in treatment with aldosterone antagonist and anticoagulation use between groups at discharge. The rate control group had higher rates of treatment with calcium channel blockers (27.5% versus 25.5%), β‐blockers (89.4% versus 69.5%), and digoxin (17.1% versus 12.2%). The rhythm control group was prescribed higher rates of loop diuretics at discharge (76.4% versus 55.4%). The specific treatments of rate versus rhythm control in patients with HFpEF and AF are reported in Table 1. Patients could receive >1 rate control treatment or >1 rhythm control treatment. Of 1857 patients on rhythm control, 67.2% were treated with amiodarone (62.5% with amiodarone only), 11.7% with sotalol (10.6% with sotalol only), 13.6% with in‐hospital cardioversion (7.1% with cardioversion only), 11.4% with other antiarrhythmic (10.5% other antiarrhythmic only), 2.0% with dofetilide, and 1% with AF ablation or surgery (in hospital). Of 13 825 patients with HFpEF and AF receiving rate control, 89.4% were treated with β‐blockers (63.3% β‐blockers only), 25.3% with calcium channel blockers (6.3% calcium channel blockers only) and 17.1% with digoxin (2.2% digoxin only). The rate control group included 9.2% of patients treated with β‐blockers and digoxin, 13.3% of patients who were treated with β‐blockers and calcium channel blockers, and 3.6% of patients who were treated with β‐blockers, calcium channel blockers, and digoxin.
Table 1.
Patient Characteristics and Treatments in Patients With HFpEF and AF Receiving Rhythm Versus Rate Control
| Variable | Overall | Rhythma | Ratea |
|---|---|---|---|
| 15 682 | 1857 | 13 825 | |
| Age, median y | 83.0 | 81.0 | 83.0 |
| Sex, female | 65.8 | 65.8 | 65.8 |
| Anemia | 22.9 | 23.3 | 22.8 |
| Smoking | 5.3 | 6.4 | 5.2 |
| Atrial flutter | 3.8 | 5.9 | 3.5 |
| Coronary artery disease | 46.1 | 48.7 | 45.8 |
| Chronic obstructive pulmonary disease or asthma | 32 | 32.3 | 32.0 |
| Cerebrovascular accident/transient ischemic attack | 19.1 | 17.5 | 19.3 |
| Diabetes mellitus | 36.1 | 36.0 | 36.2 |
| Peripheral vascular disease | 13.2 | 13.4 | 13.1 |
| Prior heart failure | 66.8 | 65.8 | 67.0 |
| Dyslipidemia | 50.4 | 57.9 | 49.4 |
| Hypertension | 82.1 | 83.8 | 81.9 |
| Renal insufficiencyb | 19.4 | 18.5 | 19.5 |
| Dialysis | 2.2 | 2.8 | 2.2 |
| Systolic blood pressure, median | 141 | 141 | 141 |
| Weight, kg | 76.2 | 78.0 | 76.0 |
| Heart rate, bpm | 80.0 | 78.0 | 80.0 |
| Ejection fraction, % | 58.0 | 58.0 | 58.0 |
| QRS duration | 98.0 | 100.0 | 98.0 |
| Sodium, mEq/L | 138.0 | 138.0 | 138.0 |
| Blood urea nitrogen, mg/dL | 24.0 | 24.0 | 25.0 |
| Serum creatinine, mg/dL | 1.2 | 1.2 | 1.2 |
| Brain natriuretic peptide, pg/mL | 559.0 | 594.0 | 553.0 |
| Hemoglobin, g/dL | 11.5 | 11.4 | 11.5 |
| Medications at discharge | |||
| Aldosterone antagonist | 10.5 | 11.6 | 10.3 |
| Loop diuretic | 57.9 | 76.4 | 55.4 |
| β‐Blockers | 87.1 | 69.5 | 89.4 |
| Calcium channel blocker | 25.5 | 27.5 | 25.3 |
| Digoxin | 16.6 | 12.2 | 17.1 |
| Anticoagulation | 56.4 | 56 | 56.5 |
| Length of stay, d | 4 | 5.0 | 4.0 |
| In hospital procedures | |||
| Cardioversion | 1.6 | 13.6 | 0 |
| AF ablation or surgery | 0.12 | 1.0 | 0 |
| Treatment strategyc | |||
| Rhythm control | 11.8 | 100 | ··· |
| Amiodarone | 8.0 | 67.2 | ··· |
| Dofetilide | 0.24 | 2.0 | ··· |
| Sotalol | 1.4 | 11.7 | ··· |
| Other antiarrhythmic | 1.3 | 11.4 | ··· |
| AF ablation or surgeryd | 0.12 | 1.0 | ··· |
| Cardioversion procedured | 1.6 | 13.6 | ··· |
| Rate control | 88.2 | ··· | 100 |
| β‐Blocker | 78.8 | ··· | 89.4 |
| Calcium channel blocker | 22.2 | ··· | 25.3 |
| Digoxin | 15.1 | ··· | 17.1 |
AF indicates atrial fibrillation; HFpEF, heart failure with preserved ejection fraction.
Reported as % unless stated otherwise.
Serum creatinine >2.
Patients in each strategy group can fall into multiple subcategories.
In hospital procedure.
Outcomes
Outcomes for patients at 30 days and 1 year according to rate versus rhythm control are shown in Table 2. The 30‐day outcomes for all‐cause death were similar for rate versus rhythm control (6.5% versus 5.4%, respectively; P=0.07). There was a significantly lower risk of 1‐year mortality in patients receiving rhythm control versus rate control (37.5% versus 30.8%; P<0.01). There was no difference in other cardiovascular readmissions at 30 days and 1 year in rate control (7.6% and 28.1%, respectively) versus rhythm control (8.7% and 30.8%, respectively) group (P=0.09 for both). There was also no significant difference in 30‐day death/readmission, all‐cause readmission, ischemic stroke readmission, HF readmission, or bleeding readmission (all P≥0.2). Specifically, there was no difference in 30‐day death/readmission rates (25.6% and 25.5% for rate and rhythm control, respectively, P=0.9); however, there were lower rates of death/readmission at 1 year in the rhythm control group (70.1%) versus rate control group (74.1%) (P<0.01). There were lower rates of 1‐year all‐cause readmissions, ischemic stroke readmissions, and HF readmissions (all P<0.05) in the rhythm control group. Despite the lower all‐cause death at 1 year in the rhythm control group, there were numerically higher (but not statistically significant differences) other cardiovascular readmissions in the rhythm control group at 30 days (8.7%) and 1 year (30.8%) compared with the rate control group at 30 days (7.6%) and 1 year (28.1%) (P=0.09 for both).
Table 2.
Outcomes for Patients at 30 Days and 1 Year for Patients With HFpEF and AF Treated With Rhythm Control (Ref=Rate Control)
| Outcome | 30‐Day | 1‐Year | ||||
|---|---|---|---|---|---|---|
| Rate Control | Rhythm Control | P Value | Rate Control | Rhythm Control | P Value | |
| All‐cause death | 6.5 | 5.4 | 0.07 | 37.5 | 30.8 | <0.01 |
| Death/readmission | 25.6 | 25.5 | 0.90 | 74.1 | 70.1 | <0.01 |
| All‐cause readmissions | 22.2 | 22.6 | 0.68 | 64.6 | 62.0 | 0.02 |
| Ischemic stroke readmissions | 0.3 | 0.2 | 0.20 | 2.3 | 1.56 | 0.02 |
| HF readmissions | 7.4 | 7.2 | 0.69 | 27.7 | 26.3 | 0.05 |
| Other cardiovascular readmissions | 7.6 | 8.7 | 0.09 | 28.1 | 30.8 | 0.09 |
| Bleeding readmissions | 0.9 | 0.8 | 0.74 | 4.74 | 4.25 | 0.19 |
AF indicates atrial fibrillation; HF, heart failure; HFpEF, heart failure with preserved ejection fraction.
All data presented in %.
The unadjusted and adjusted 1‐year hazard ratios for patients with HFpEF and AF treated with rhythm control compared with rate control are presented in Figure 1. Unadjusted analysis revealed that there was no difference in all‐cause death at 30 days in the rhythm control group (hazard ratio, 0.83; 95% CI, 0.67–1.02; P=0.07); however, there was lower all‐cause death at 1 year in the rhythm control group (hazard ratio, 0.78; 95% CI, 0.72–0.85; P<0.0001).
Figure 1.
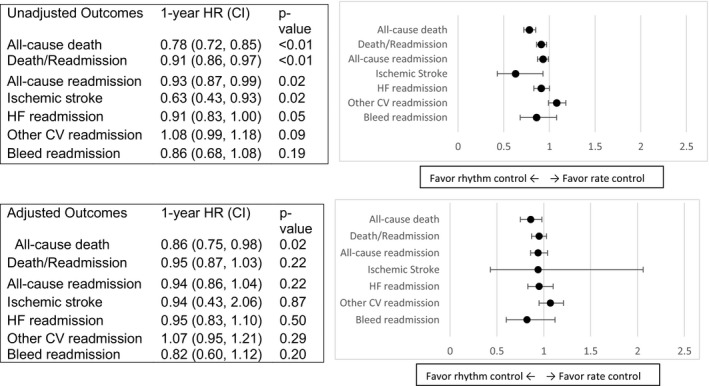
Unadjusted and adjusted HRs for patients with HFpEF and AF treated with rhythm control (ref=rate control). AF indicates atrial fibrillation; CV, cardiovascular; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio.
The lower 1‐year all‐cause death in the rhythm control group remained after multivariable adjustment (hazard ratio, 0.86; 95% CI, 0.75–0.98; P=0.02). A sensitivity analysis was also performed that revealed there were no significant differences found in the unadjusted and adjusted analyses when the 11.4% of the patients in the rhythm control group on other antiarrhythmic agents were excluded from the original analysis. However, while there were numerically increased cardiovascular readmissions in the rhythm control group (hazard ratio, 1.15; 95% CI 0.98–1.36, P‐value 0.09), there were no lower rates of 1‐year death/readmission, all‐cause readmission, ischemic stroke readmission, HF readmission, other cardiovascular readmission, or bleeding readmission after multivariable adjustment (all P≥0.2).
The Kaplan–Meier survival curves are presented for all‐cause death and all‐cause death/readmission in Figures 2 and 3, respectively.
Figure 2.
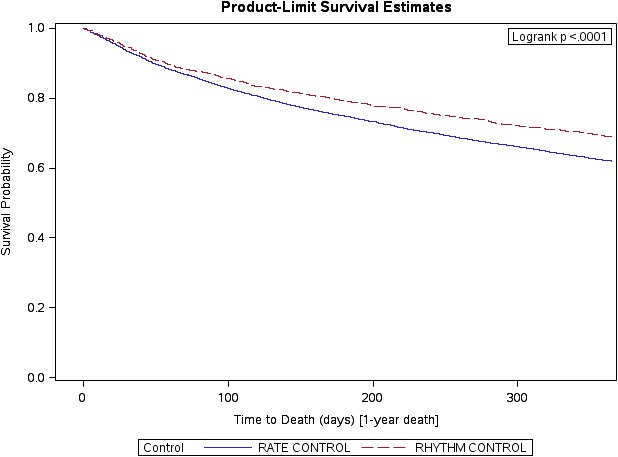
All‐cause death with rhythm control in patients with HFpEF and AF (ref=rate control). AF indicates atrial fibrillation; HFpEF, heart failure with preserved ejection fraction.
Figure 3.

All‐cause death or all‐cause hospitalization with rhythm control in patients with HFpEF and AF (ref=rate control). AF indicates atrial fibrillation; HFpEF, heart failure with preserved ejection fraction.
Discussion
In a population of inpatient HF patients aged 65 years and older, we found that patients with HFpEF and AF treated with rhythm control had lower 1‐year mortality compared with treatment with rate control. Our results suggest that there may be a potential opportunity to improve outcomes in patients with HFpEF and AF; however, future randomized studies are needed to explore this potential benefit.
The most recent full and focused updated HF guidelines do not specifically guide clinicians how to approach rhythm control in patients with HFpEF.8, 14 Landmark studies have confirmed that rate control is noninferior to rhythm control, and there is no difference in lenient versus strict rate control in patients with AF. However, the generalizability of these results to patients with HFpEF is unclear.21, 22, 23 Lam and colleagues recently demonstrated the strong association of reduced higher exercise intolerance, natriuretic peptide elevation, and left atrial remodeling in patients with HFpEF and AF compared with patients without AF, suggesting that treatments to reduce the burden of AF may be beneficial in patients with HFpEF.12 The differential response that patients with HFpEF demonstrate in response to successful HF therapies targeting patients with HFrEF and the dearth of current treatments in this population suggest that future research should evaluate rhythm control treatment as a potential therapy to improve outcomes in patients with AF and HFpEF.24
There are only 2 antiarrhythmic medications not specifically contraindicated in patients with HF: amiodarone and dofetilide, which were utilized in 67.2% and 2.0% of our studied patients, respectively. These medications have challenging safety profiles and narrow therapeutic indices, limiting their use. Other rhythm control medications, including the class Ic agents propafenone and flecainide as well as dronedarone, are considered harmful in patients with HFrEF, and these warnings are extended to include patients with HFpEF.25, 26 Yet there is limited evidence that these rhythm control agents, which are available to patients without HF, are deleterious to patients with HFpEF. Interestingly, we found that dronedarone and class Ic agents were used in 11.4% of our rhythm control group.
Catheter ablation for AF has been shown to be safe but has not been studied specifically in a randomized population of patients with AF and HFpEF. Catheter ablation of AF has been shown to be superior to amiodarone in achieving freedom from AF in long‐term follow‐up as well as reduced unplanned hospitalization and mortality in patients with HFrEF.27 However, national trends among Medicare fee‐for‐service beneficiaries have demonstrated more frequent use of AF ablation for AF, with associated improved outcomes of lower rates of in‐hospital mortality, 30‐day readmission, 30‐day mortality, and 1‐year mortality.28, 29
Although only 1% of patients in our study were treated with AF ablation, the increasing use of catheter ablation certainly includes AF with coexisting HFpEF and portends a potential treatment option that may portend benefit.
A recent study that examined rate control versus rhythm control in postoperative cardiovascular surgery revealed no differences in outcomes; rhythm control was safe and equally effective through 2 months of follow‐up.30 This is a strategy still frequently used in clinical practice as the rhythm control group in our analysis included 13.6% of patients who underwent cardioversion. Our analysis findings mirror this study and multiple other studies examining cardioversion and rhythm control demonstrating no difference in short‐term outcomes of 30‐day death/readmission, ischemic stroke readmissions, HF readmissions, and bleeding readmissions. However, the observed safety of rhythm control in the short term combined with the associated 6.7% lower all‐cause mortality suggests that a potential benefit from rhythm control may exist and should be further studied.
There are important limitations to this study. This was a retrospective observational analysis of an inpatient registry, with data collected at multiple volunteer participating sites. The findings may not be generalizable to younger patients (age <65), those not enrolled in Medicare, or those cared for in centers that differ from those participating in GWTG‐HF. There is potential for bias related to unknown and unmeasured underlying health status in each group of our patients. Although we adjusted for clinically relevant covariates in patients with HFpEF and AF, other measured and unmeasured variables may have influenced these results. The specific subtype of cardiomyopathy is not available and may contribute to differential outcomes among groups. AF treatment is not static, and the associated patient status in our analysis as rate or rhythm control reflects their status at time of data capture and may not appropriately reflect their previous failure in the other category (eg, current treatment with rate control because previous rhythm control strategy failed). Similarly, the strictness of rate control was not available for adjustment, which may have biased the outcomes (strict rate control has been shown to increase mortality). Another important limitation of this analysis is the inability to analyze patients with AF with regard to duration of AF and/or permanent versus paroxysmal AF, which may be associated with worse outcomes. Patients in the rate control group may be on β‐blockers or calcium channel blockers primarily for use in hypertension or angina. Similarly, patients assigned to the rhythm control group may have antiarrhythmic treatment for ventricular arrhythmias or amiodarone use for rate control in refractory AF tachycardia. Furthermore, the time of initiation and duration of therapy of amiodarone was unavailable and thus difficult to ascertain if amiodarone may have been used as a rate control agent independent of use as a rhythm control agent.
Conclusions
Our findings highlight that patients aged 65 and older with HFpEF and AF treated with rhythm control have an associated lower 1‐year mortality compared with treatment with rate control, even after risk adjustment. Rhythm control is a potential treatment strategy for this population that may provide benefit. Future randomized studies are needed to explore the potential benefits of different rhythm control treatments in patients with HFpEF and AF.
Sources of Funding
This project was supported by cooperative agreement number U19HS021092 from the Agency for Healthcare Research and Quality. The GWTG‐HF program is provided by the American Heart Association. GWTG‐HF is sponsored, in part, by Amgen Cardiovascular and has been funded in the past through support from Medtronic, GlaxoSmithKline, Ortho‐McNeil, and the American Heart Association Pharmaceutical Roundtable.
Disclosures
Dr Devore has received research support from the AHA, Amgen, and Novartis and has served as a consultant for Novartis. Dr Sharma is supported by the Alberta Innovates Health Solution Clinician Scientist Fellowship; has received research support from Roche, Takeda, Canadian Cardiovascular Society Bayer Vascular Research grant, and the European Society of Cardiology young investigator grant. Dr Piccini has received research grants from Johnson & Johnson/Janssen Pharmaceuticals and Boston Scientific Corp; has received grants to his institution from Janssen, Boston Scientific, ARCA biopharma, St. Jude Medical, ResMed, and Gilead; has received other research support from Johnson & Johnson/Janssen Pharmaceuticals; and has received consultant/advisory board fees from Forest Laboratories, Medtronic, GlaxoSmithKline, Amgen, and Johnson & Johnson/Janssen Pharmaceuticals. Dr Allen has received grants from the American Heart Association, National Institutes of Health, and the Patient‐Centered Outcomes Research Institute; and has received consultancy fees from Janssen and Novartis. Dr Peterson reported receiving research grants from Eli Lilly and Janssen and serving as a consultant for Astra Zeneca, Boehringer Ingelheim, Janssen, and Sanofi. Dr Peterson has received research grants from the American Heart Association, the American College of Cardiology, Janssen Pharmaceuticals, Eli Lilly, and the Society of Thoracic Surgeons; has served as a consultant to or on the advisory board of Merck & Co., Boehringer Ingelheim, Genentech, Sanofi‐Aventis, and Janssen Pharmaceuticals; and has received personal fees from AstraZeneca, Bayer, Regeneron, and Valeant. Dr Fonarow has received research funding from the National Institutes of Health and has served as a consultant for Amgen, Janssen, Novartis, Medtronic, and St. Jude Medical. Dr Hernandez has received research funding from Janssen, Novartis, Portola, and Bristol‐Myers Squibb; and has served as a consultant for Amgen, AstraZeneca, Bayer, Boston Scientific, Bristol‐Myers Squibb, Gilead, Janssen, Merck, and Novartis. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e011560 DOI: 10.1161/JAHA.118.011560.)
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER III, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Executive summary: heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:399–410. [DOI] [PubMed] [Google Scholar]
- 2. Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–259. [DOI] [PubMed] [Google Scholar]
- 3. Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, Das SR, de Ferranti S, Després JP, Fullerton HJ, Howard VJ, Huffman MD, Isasi CR, Jiménez MC, Judd SE, Kissela BM, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Magid DJ, McGuire DK, Mohler ER III, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Rosamond W, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Woo D, Yeh RW, Turner MB. Executive summary: heart disease and stroke statistics—2016 update: a report from the American Heart Association. Circulation. 2016;133:447–454. [DOI] [PubMed] [Google Scholar]
- 4. Fonarow GC, Stough WG, Abraham WT, Albert NM, Gheorghiade M, Greenberg BH, O'Connor CM, Sun JL, Yancy CW, Young JB. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE‐HF Registry. J Am Coll Cardiol. 2007;50:768–777. [DOI] [PubMed] [Google Scholar]
- 5. Maggioni AP1, Dahlström U, Filippatos G, Chioncel O, Leiro MC, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Metra M, Parissis J, Persson H, Ponikowski P, Rauchhaus M, Voors A, Nielsen OW, Zannad F, Tavazzi L; Heart Failure Association of ESC (HFA) . EURObservational Research Programme: the Heart Failure Pilot Survey (ESC‐HF Pilot). Eur J Heart Fail. 2010;12:1076–1084. [DOI] [PubMed] [Google Scholar]
- 6. Kotecha D, Lam CS, Van Veldhuisen DJ, Van Gelder IC, Voors AA, Rienstra M. Heart failure with preserved ejection fraction and atrial fibrillation: vicious twins. J Am Coll Cardiol. 2016;68:2217–2228. [DOI] [PubMed] [Google Scholar]
- 7. Mountantonakis SE, Grau‐Sepulveda MV, Bhatt DL, Hernandez AF, Peterson ED, Fonarow GC. Presence of atrial fibrillation is independently associated with adverse outcomes in patients hospitalized with heart failure: an analysis of Get with the Guidelines—Heart Failure. Circ Heart Fail. 2012;5:191–201. [DOI] [PubMed] [Google Scholar]
- 8. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation. 2013;128:e240–e327. [DOI] [PubMed] [Google Scholar]
- 9. January CT, Wann LS, Alpert JS, Calkins H, Cigarroa JE, Cleveland JC Jr, Conti JB, Ellinor PT, Ezekowitz MD, Field ME, Murray KT, Sacco RL, Stevenson WG, Tchou PJ, Tracy CM, Yancy CW. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014;130:2071–2104. [DOI] [PubMed] [Google Scholar]
- 10. Kotecha D, Chudasama R, Lane DA, Kirchhof P, Lip GY. Atrial fibrillation and heart failure due to reduced versus preserved ejection fraction: a systematic review and meta‐analysis of death and adverse outcomes. Int J Cardiol. 2016;203:660–666. [DOI] [PubMed] [Google Scholar]
- 11. Khazanie P, Liang L, Qualls LG, Curtis LH, Fonarow GC, Hammill BG, Hammill SC, Heidenreich PA, Masoudi FA, Hernandez AF, Piccini JP. Outcomes of Medicare beneficiaries with heart failure and atrial fibrillation. JACC Heart Fail. 2014;2:41–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lam CS, Rienstra M, Tay WT, Liu LC, Hummel YM, van der Meer P, de Boer RA, Van Gelder IC, van Veldhuisen DJ, Voors AA, Hoendermis ES. Atrial fibrillation in heart failure with preserved ejection fraction: association with exercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. JACC Heart Fail. 2017;5:92–98. [DOI] [PubMed] [Google Scholar]
- 13. Lindenfeld J, Albert NM, Boehmer JP, Collins SP, Ezekowitz JA, Givertz MM, Katz SD, Klapholz M, Moser DK, Rogers JG, Starling RC, Stevenson WG, Tang WH, Teerlink JR, Walsh MN. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail. 2010;16:e1–e194. [DOI] [PubMed] [Google Scholar]
- 14. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2016 ACC/AHA/HFSA focused update on new pharmacological therapy for heart failure: an update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Circulation. 2016;134:e282–e293. [DOI] [PubMed] [Google Scholar]
- 15. Mentz RJ, Kelly JP, von Lueder TG, Voors AA, Lam CS, Cowie MR, Kjeldsen K, Jankowska EA, Atar D, Butler J, Fiuzat M, Zannad F, Pitt B, O'Connor CM. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J Am Coll Cardiol. 2014;64:2281–2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly JP, Mentz RJ, Mebazaa A, Voors AA, Butler J, Roessig L, Fiuzat M, Zannad F, Pitt B, O'Connor CM, Lam CSP. Patient selection in heart failure with preserved ejection fraction clinical trials. J Am Coll Cardiol. 2015;65:1668–1682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. LaBresh KA, Gliklich R, Liljestrand J, Peto R, Ellrodt AG. Using “Get With The Guidelines” to improve cardiovascular secondary prevention. Jt Comm J Qual Saf. 2003;29:539–550. [DOI] [PubMed] [Google Scholar]
- 18. LaBresh KA, Ellrodt AG, Gliklich R, Liljestrand J, Peto R. Get with the guidelines for cardiovascular secondary prevention: pilot results. Arch Intern Med. 2004;164:203–209. [DOI] [PubMed] [Google Scholar]
- 19. Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, Devore AD, Yancy CW, Fonarow GC. Heart failure with preserved, borderline, and reduced ejection fraction: 5‐year outcomes. J Am Coll Cardiol. 2017;70:2476–2486. [DOI] [PubMed] [Google Scholar]
- 21. Van Gelder IC, Hagens VE, Bosker HA, Kingma JH, Kamp O, Kingma T, Said SA, Darmanata JI, Timmermans AJ, Tijssen JG, Crijns HJ. A comparison of rate control and rhythm control in patients with recurrent persistent atrial fibrillation. N Engl J Med. 2002;347:1834–1840. [DOI] [PubMed] [Google Scholar]
- 22. Wyse DG, Waldo AL, DiMarco JP, Domanski MJ, Rosenberg Y, Schron EB, Kellen JC, Greene HL, Mickel MC, Dalquist JE, Corley SD. A comparison of rate control and rhythm control in patients with atrial fibrillation. N Engl J Med. 2002;347:1825–1833. [DOI] [PubMed] [Google Scholar]
- 23. Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma‐Kadijk JA, Cornel JH, Kamp O, Tukkie R, Bosker HA, Van Veldhuisen DJ, Van den Berg MP. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. [DOI] [PubMed] [Google Scholar]
- 24. Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, Connolly SJ, Dubuc M, Ducharme A, Guerra PG, Hohnloser SH, Lambert J, Le Heuzey JY, O'Hara G, Pedersen OD, Rouleau JL, Singh BN, Stevenson LW, Stevenson WG, Thibault B, Waldo AL. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. [DOI] [PubMed] [Google Scholar]
- 25. Echt DS, Liebson PR, Mitchell LB, Peters RW, Obias‐Manno D, Barker AH, Arensberg D, Baker A, Friedman L, Greene HL. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324:781–788. [DOI] [PubMed] [Google Scholar]
- 26. Kober L, Torp‐Pedersen C, McMurray JJ, Gøtzsche O, Lévy S, Crijns H, Amlie J, Carlsen J; Dronedarone Study Group . Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med. 2008;358:2678–2687. [DOI] [PubMed] [Google Scholar]
- 27. Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, Casella M, Pelargonio G, Narducci ML, Schweikert R, Neuzil P, Sanchez J, Horton R, Beheiry S, Hongo R, Hao S, Rossillo A, Forleo G, Tondo C, Burkhardt JD, Haissaguerre M, Natale A. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC Multicenter Randomized Trial. Circulation. 2016;133:1637–1644. [DOI] [PubMed] [Google Scholar]
- 28. Machino‐Ohtsuka T, Seo Y, Ishizu T, Sugano A, Atsumi A, Yamamoto M, Kawamura R, Machino T, Kuroki K, Yamasaki H, Igarashi M, Sekiguchi Y, Aonuma K. Efficacy, safety, and outcomes of catheter ablation of atrial fibrillation in patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. 2013;62:1857–1865. [DOI] [PubMed] [Google Scholar]
- 29. Freeman JV, Wang Y, Akar J, Desai N, Krumholz H. National trends in atrial fibrillation hospitalization, readmission, and mortality for Medicare beneficiaries, 1999–2013. Circulation. 2017;135:1227–1239. [DOI] [PubMed] [Google Scholar]
- 30. Gillinov AM, Bagiella E, Moskowitz AJ, Raiten JM, Groh MA, Bowdish ME, Ailawadi G, Kirkwood KA, Perrault LP, Parides MK, Smith RL II, Kern JA, Dussault G, Hackmann AE, Jeffries NO, Miller MA, Taddei‐Peters WC, Rose EA, Weisel RD, Williams DL, Mangusan RF, Argenziano M, Moquete EG, O'Sullivan KL, Pellerin M, Shah KJ, Gammie JS, Mayer ML, Voisine P, Gelijns AC, O'Gara PT, Mack MJ; CTSN . Rate control versus rhythm control for atrial fibrillation after cardiac surgery. N Engl J Med. 2016;374:1911–1921. [DOI] [PMC free article] [PubMed] [Google Scholar]


