Abstract
Background
Mobile stroke units (MSUs) reduce time to intravenous thrombolysis in acute ischemic stroke. Whether this advantage exists in densely populated urban areas with many proximate hospitals is unclear.
Methods and Results
We evaluated patients from the METRONOME (Metropolitan New York Mobile Stroke) registry with suspected acute ischemic stroke who were transported by a bi‐institutional MSU operating in Manhattan, New York, from October 2016 to September 2017. The comparison group included patients transported to our hospitals via conventional ambulance for acute ischemic stroke during the same hours of MSU operation (Monday to Friday, 9 am to 5 pm). Our exposure was MSU care, and our primary outcome was dispatch‐to‐thrombolysis time. We estimated mean differences in the primary outcome between both groups, adjusting for clinical, demographic, and geographic factors, including numbers of nearby designated stroke centers and population density. We identified 66 patients treated or transported by MSU and 19 patients transported by conventional ambulance. Patients receiving MSU care had significantly shorter dispatch‐to‐thrombolysis time than patients receiving conventional care (mean: 61.2 versus 91.6 minutes; P=0.001). Compared with patients receiving conventional care, patients receiving MSU care were significantly more likely to be picked up closer to a higher mean number of designated stroke centers in a 2.0‐mile radius (4.8 versus 2.7, P=0.002). In multivariable analysis, MSU care was associated with a mean decrease in dispatch‐to‐thrombolysis time of 29.7 minutes (95% CI, 6.9–52.5) compared with conventional care.
Conclusions
In a densely populated urban area with a high number of intermediary stroke centers, MSU care was associated with substantially quicker time to thrombolysis compared with conventional ambulance care.
Keywords: acute ischemic stroke, geocoding, mobile stroke unit, prehospital stroke care, tissue plasminogen activator
Subject Categories: Ischemic Stroke, Cerebrovascular Disease/Stroke, Health Services
Clinical Perspective
What Is New?
In the most densely populated US city, where many stroke centers exist, patients with suspected acute ischemic stroke treated in a mobile stroke unit received intravenous thrombolysis ≈30 minutes faster than patients with similar symptoms treated in an emergency department after transport by conventional ambulance.
This treatment advantage persisted despite adjustment for population density and number of intermediary stroke centers.
What Are the Clinical Implications?
Given the public health implications of faster stroke treatment, these results suggest that mobile stroke units may represent a potentially beneficial addition to stroke systems of care in dense cities.
Introduction
Prior studies have demonstrated that mobile stroke units (MSUs) are associated with faster tissue plasminogen activator (tPA) treatment times in acute ischemic stroke than conventional ambulance transport followed by treatment in emergency departments.1, 2, 3, 4, 5 Along with the time advantages associated with MSU care, authors have also noted the potential for high utilization of MSU programs in very densely populated metropolitan areas.6 In such areas, however, a given emergency scene location may be close to a high number of stroke‐capable hospitals, resulting in shorter travel times for emergency transport vehicles. This could potentially reduce or nullify the beneficial effect of MSU care on thrombolysis time that has been demonstrated in less densely populated regions.
New York is the most densely populated city in the United States.7 Within New York City, Manhattan is the most densely populated borough and contains the highest number of hospitals per unit area,8 making it an ideal setting in which to examine these questions. In a single MSU operating within Manhattan, we sought to analyze the difference in time to thrombolytic therapy between MSU and conventional ambulance care among patients with suspected acute ischemic stroke. We hypothesized that despite high population densities and high numbers of proximate designated stroke centers (DSCs), time from ambulance dispatch to treatment with thrombolytic therapy would remain faster among patients with suspected acute ischemic stroke treated with MSU care than in comparable patients receiving care in a conventional ambulance.
Methods
Design
We conducted a prospective cohort study of a New York City–based MSU program that was launched in October 2016 by New York Presbyterian (NYP) Hospital and the Fire Department of New York (FDNY). The program consisted of a single MSU operating exclusively in Manhattan on weekdays, 9 am to 5 pm, from 2 separate and nonintersecting medical center catchment areas (Weill Cornell Medical Center [WCMC] and Columbia University Irving Medical Center [CUIMC]; Figure 1) in alternating, 2‐week‐long periods referred to as “on‐weeks.”9 The MSU was on‐service within the Cornell catchment area for 2 weeks while it was simultaneously off‐service for the same period within the Columbia catchment area. The configuration of on‐ and off‐service centers was then inverted over the following 2 weeks, thereby constituting an alternating biweekly schedule. Further details regarding the design, workflow, and implementation of the NYP MSU program have been described elsewhere.10
Figure 1.
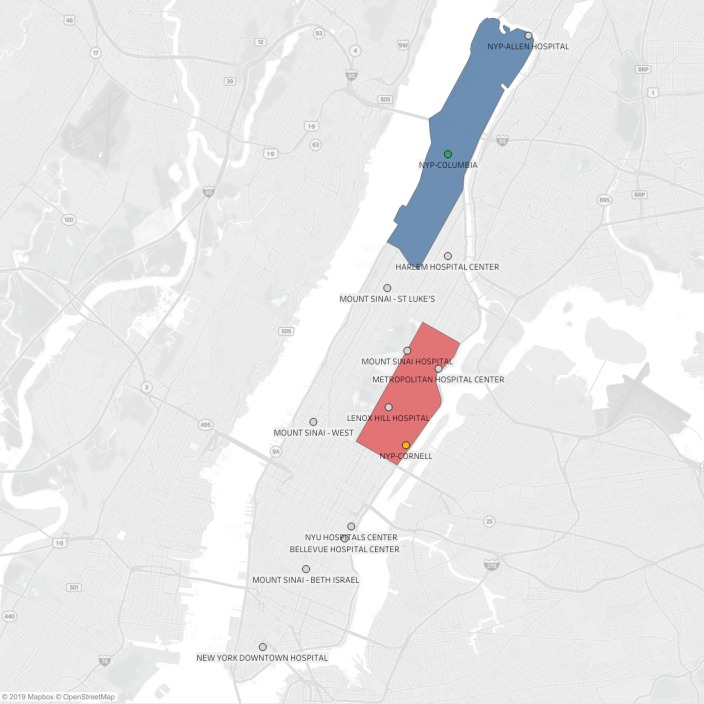
Map of Manhattan showing all Manhattan‐based designated stroke centers (DSCs) and mobile stroke unit catchment areas. Red and blue polygons designate Weill Cornell Medical Center and Columbia University Irving Medical Center catchment areas, respectively; orange and green circles designate Weill Cornell and Columbia DSC locations, respectively; gray circles designate DSCs; DSC names appear to the right of each circle. NYP indicates New York Presbyterian; NYU, New York University.
Patient Population
For this study, we used a prospectively recorded, bi‐institutional MSU registry, METRONOME (Metropolitan New York Mobile Stroke), which contains all patients who are treated or transported by the NYP MSU during on‐weeks and a comparison group of patients with suspected acute stroke who are transported by conventional ambulance to the “off‐week” medical center. The comparison group contained all patients who were transported by conventional ambulance for suspected acute ischemic stroke to the off‐service medical center campus while the MSU was operating within the on‐service medical center catchment area.
The comparison group was identified through 2 principal steps using predefined criteria. First, a vascular neurologist at each NYP medical campus prospectively reviewed an FDNY database of all emergency medical services (EMS) ambulance call reports of ambulance trips to each respective medical center's emergency department. All reports of ambulance transports that occurred during each campus’ 2 off‐weeks between 9 am and 5 pm, Monday through Friday, and were associated with an FDNY EMS call type of “CVA” (cerebrovascular accident) or “CVA‐C” (cerebrovascular accident–critical) were flagged for review. EMS call types are routinely recorded elements of a coding system used by EMS dispatch operators to classify the medical problem associated with each ambulance‐transported patient in New York City. After receiving an emergency telephone call, dispatch operators assign a call type of CVA to patients with stroke symptoms that are evaluated >5 hours from symptom onset and CVA‐C to patients with stroke symptoms that are evaluated within 5 hours of symptom onset or in whom the last known “well time” is unknown.11
Next, using our institution's electronic medical record and ambulance call reports, 3 vascular neurologists reviewed all available history and physical examination findings for each of the flagged patients and determined by consensus whether the patient would have been eligible for MSU transport had the MSU been available at the time of evaluation. If eligible for MSU care, the patient was added to the METRONOME registry as part of the conventional care group. The neurologists performing the determination were blinded to prehospital care‐related time metrics, including dispatch‐to‐thrombolysis time.
We included all MSU‐treated or ‐transported patients in the METRONOME registry and all patients in the comparison group between October 2016 and September 2017. We excluded patients who were missing zip codes or ≥1 time metric. The institutional review boards of Weill Cornell Medicine and CUIMC approved this analysis of institutional registry data with a waiver of informed consent. The data that support the findings of this study are available from the corresponding author on reasonable request.
Measurements
One vascular neurologist (B.R.K.) abstracted information on age, race, sex, accepting hospital, medical comorbidities, National Institutes of Health Stroke Scale (NIHSS), baseline functional status, medication use, receipt of tPA, final diagnosis, complications, and treatment‐related time metrics from the METRONOME registry. Final diagnosis was adjudicated by a group of 3 vascular neurologists. Two investigators (M.D.H. and E.R.K.) determined key geographic variables for both groups in several steps. First, pick‐up addresses were converted to geocoordinates using ArcGIS software (ESRI Inc). Using population data from the 2010 US Census and zip code shape files obtained from the US Census Bureau,12 population density was determined for each pick‐up zip code by dividing its population by the zip code area determined by ArcGIS. Next, using the US Department of Health and Human Services Data Warehouse13 geolocator file and DSC classifications published by the New York State Department of Health,14 the density of New York State Department of Health–accredited DSCs around each pick‐up was determined by counting the number of DSCs in a Cartesian‐coordinate 0.5‐, 1.0‐, and 2.0‐mile radius from each pick‐up location. Using ArcGIS, ambulance travel distances were then computed from the patient's pick‐up geocoordinates to the closest DSC and the hospital to which the patient was transported.
Statistical Analysis
We summarized all collected information for both groups using descriptive statistics, including mean, median, and standard deviation. We evaluated the differences in baseline characteristics and treatment time metrics between groups using the Student t test or the Wilcoxon rank sum test for continuous variables and the Fisher exact test or χ2 test for categorical variables. To compute the adjusted mean differences in dispatch‐to‐thrombolysis time, we first used targeted minimum loss‐based estimation15 to compute the adjusted mean differences in dispatch‐to‐ambulance arrival and ambulance arrival‐to‐thrombolysis times. We then added these 2 estimated mean differences to obtain the estimate of the mean difference in the primary outcome. Targeted minimum loss‐based estimation uses a preliminary estimator for the outcome regression and the propensity score to construct a doubly robust estimator that remains consistent under misspecification of either model. The estimator of the conditional distribution for the outcome measures and the model for predicting group membership as a function of covariates (ie, the propensity score) were estimated using a cross‐validation selector that chooses the best‐fitting model among several candidate models.16 This approach reflects the difference in outcome between both treatment groups after all possible measured confounding was removed.
Potential confounding factors included demographic characteristics (age, sex, race, ethnicity), clinical characteristics (use of telephone translator, baseline NIHSS, baseline modified Rankin scale, finger‐stick glucose, initial blood pressure, receipt of thrombolysis, presence of stroke risk factors, final diagnosis), and geographic characteristics (population density, number of DSCs in a 0.5‐, 1.0‐, and 2.0‐mile radius from pick‐up). The candidate models used for estimation of the propensity score and outcome regression were stepwise regression, stepwise regression with interaction terms, generalized linear models, and elastic‐net generalized linear models. All statistical analyses were performed by X.W. and I.L.D. in R v3.4.3 (R Foundation for Statistical Computing). Adjusted mean differences with 95% CIs were reported. All P values were from 2‐tailed tests, and results were deemed statistically significant at P<0.05.
Sensitivity Analysis
Because seasonal weather occurrences in the New York City area could differentially affect traffic patterns as well as the speed with which ambulances could reach patients and times in either treatment group, we conducted a post hoc sensitivity analysis adjusting the primary analysis for the season of patients’ ambulance transport date as an additional variable. Four seasons were defined by transport date ranges according to the astronomical calendar as follows: winter (December 21 to March 20), spring (March 21 to June 20), summer (June 21 to September 20), and autumn (September 21 to December 20).
Results
From October 2016 through September 2017, there were 85 patients who met study eligibility criteria, including 66 transported by the MSU and 19 transported by conventional ambulance. To identify the conventional care group, we reviewed 26 919 patient ambulance call reports at our institution. Most patients were excluded for not having a CVA or CVA‐C call type or not presenting within the MSU operating hours; a small minority was excluded after adjudication (Figure 2). Of the final study populations, 29 (43.9%) in the MSU group and 9 (47.4%) in the conventional care group were treated with intravenous tPA (P=0.48 for intergroup difference; Table 1). Although demographic and clinical characteristics, including rates of symptomatic intracranial hemorrhage, were not significantly different between groups (Tables 1 and 2), within the thrombolysis‐treated population, patients who underwent thrombolysis on the MSU were more likely to have a final diagnosis of stroke mimic than patients in the conventional care group. Compared with patients treated with conventional care, patients treated with MSU care had significantly shorter mean dispatch‐to‐thrombolysis, onset‐to‐thrombolysis, and ambulance arrival‐to‐thrombolysis times. Patients in the MSU group were significantly more likely to be picked up closer to a higher mean number of DSCs in a 2.0‐mile radius than patients in the conventional care group. However, there were no differences between groups in the number of DSCs within a 1.0‐ and 0.5‐mile radius of pick‐up, mean population density at pick‐up, or distances from pick‐up to the closest DSC. Finally, compared with patients in the conventional care group, patients in the MSU group had significantly longer ambulance arrival‐to‐hospital arrival times and were transported farther from pick‐up to the accepting hospital (Table 3).
Figure 2.
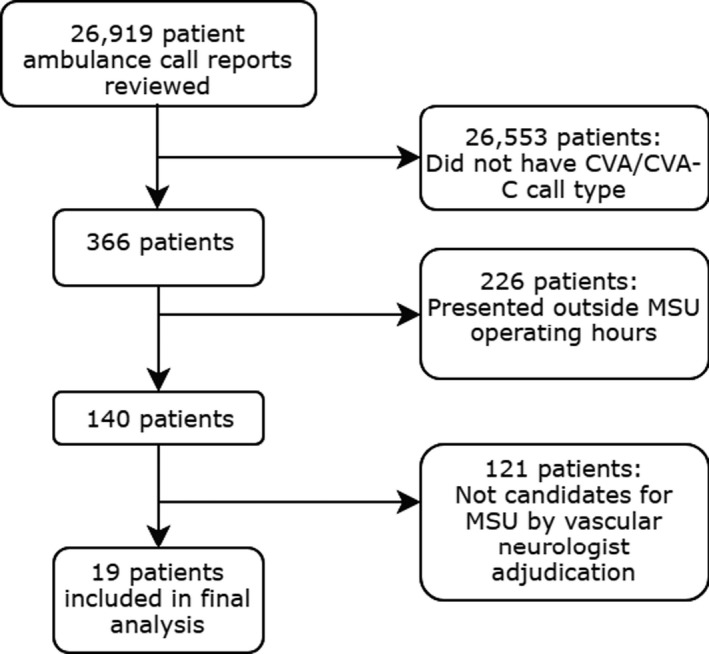
Comparison group selection process. CVA indicates cerebrovascular accident; CVA‐C, cerebrovascular accident–critical; MSU, mobile stroke unit.
Table 1.
Patient Characteristics Stratified by Treatment Group
| Characteristic | MSU Care (n=66) | Conventional Care (n=19) | P Value |
|---|---|---|---|
| Age, mean (SD) | 77.2 (16.2) | 71.6 (11.3) | 0.16 |
| Male sex | 28 (42.4) | 10 (52.6) | 0.60 |
| Racea | 0.47 | ||
| Black | 9 (13.6) | 3 (15.8) | |
| Asian | 4 (6.1) | 1 (5.3) | |
| Latino/Hispanic | 16 (24.2) | 8 (42.1) | |
| White | 31 (47.0) | 7 (36.8) | |
| Initial NIHSS, mean (SD) | 9.7 (8.3) | 10.4 (7.8) | 0.75 |
| Treatment with tPA | 29 (43.9) | 9 (47.4) | >0.99 |
| Translator used | 7 (10.6) | 5 (26.3) | 0.17 |
| Finger stick, mg/dL, mean (SD) | 141.0 (56.1) | 147.6 (67.0) | 0.69 |
| Initial systolic BP, mm Hg, mean (SD) | 166.0 (32.1) | 165.0 (31.3) | 0.88 |
| Initial diastolic BP, mm Hg, mean (SD) | 91.0 (20.2) | 86.0 (16.8) | 0.30 |
| Baseline mRSb | 0.28 | ||
| 0 | 31 (47.0) | 11 (57.9) | |
| 1 | 2 (3.0) | 1 (5.3) | |
| 2 | 3 (4.5) | 0 (0.0) | |
| 3 | 5 (7.6) | 3 (15.8) | |
| 4 | 12 (18.2) | 0 (0.0) | |
| 5 | 2 (3.0) | 0 (0.0) | |
| Post‐tPA symptomatic intracranial hemorrhage | 1 (1.5) | 0 (0.0) | 0.48 |
| Presence of stroke risk factors | |||
| History of stroke | 14 (21.2) | 8 (42.1) | 0.12 |
| Diabetes mellitus | 15 (22.7) | 8 (42.1) | 0.28 |
| Hypertension | 43 (65.2) | 14 (73.7) | 0.87 |
| Atrial fibrillation | 14 (21.2) | 3 (15.8) | 0.85 |
| Coronary artery disease | 10 (15.2) | 7 (36.8) | 0.08 |
| Active smoking | 2 (3.0) | 3 (15.8) | 0.11 |
| Peripheral vascular disease | 3 (4.5) | 0 (0.0) | >0.99 |
| Final diagnosis | 0.34 | ||
| Acute ischemic stroke | 31 (47.0) | 9 (47.4) | |
| Transient ischemic attack | 3 (4.5) | 2 (10.5) | |
| Intracerebral hemorrhage | 5 (7.6) | 1 (5.3) | |
| Stroke mimic | 27 (40.9) | 6 (31.6) | |
Data are reported as n (%) unless otherwise noted. BP indicates blood pressure; mRS, modified Rankin scale; MSU, mobile stroke unit; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
Race data missing for 6 MSU patients.
mRS data missing for 11 MSU and 4 conventional care patients at baseline evaluation.
Table 2.
Characteristics of Patients Treated With Intravenous Thrombolysis, Stratified by Treatment Group
| Characteristic | MSU Care (n=29) | Conventional Care (n=9) | P Value |
|---|---|---|---|
| Age, mean (SD) | 76.1 (15.6) | 73.1 (11.1) | 0.68 |
| Male sex | 15 (51.7) | 6 (66.7) | 0.48 |
| Racea | 0.65 | ||
| Black | 3 (10.3) | 1 (11.1) | |
| Asian | 2 (6.9) | 0 (0.0) | |
| Latino/Hispanic | 8 (27.6) | 5 (55.6) | |
| White | 13 (44.8) | 3 (33.3) | |
| Initial NIHSS, mean (SD) | 9.2 (6.9) | 12.9 (7.9) | 0.19 |
| Translator used | 5 (17.2) | 2 (22.2) | >0.99 |
| Finger stick, mg/dL, mean (SD) | 153.0 (65.0) | 154.0 (71.0) | 0.97 |
| Initial systolic BP, mm Hg, mean (SD) | 162.0 (28.0) | 161.0 (31.0) | 0.89 |
| Initial diastolic BP, mm Hg, mean (SD) | 88.0 (20.0) | 81.0 (18.0) | 0.34 |
| Baseline mRSb | 0.66 | ||
| 0 | 16 (55.2) | 5 (55.6) | |
| 1 | 2 (6.9) | 1 (11.1) | |
| 2 | 1 (3.4) | 0 (0.0) | |
| 3 | 1 (3.4) | 2 (22.2) | |
| 4 | 2 (6.9) | 0 (0.0) | |
| 5 | 2 (6.9) | 1 (11.1) | |
| Post‐tPA symptomatic intracranial hemorrhage | 1 (3.4) | 0 (0.0) | >0.99 |
| Presence of stroke risk factors | |||
| History of stroke | 8 (27.6) | 4 (44.4) | 0.44 |
| Diabetes mellitus | 10 (34.5) | 3 (33.3) | >0.99 |
| Hypertension | 19 (65.5) | 6 (66.7) | >0.99 |
| Atrial fibrillation | 6 (20.7) | 1 (11.1) | >0.99 |
| Coronary artery disease | 6 (20.7) | 5 (55.6) | 0.09 |
| Active smoking | 1 (3.4) | 1 (11.1) | 0.96 |
| Peripheral vascular disease | 2 (6.9) | 0 (0.0) | >0.99 |
| Final diagnosis | 0.57 | ||
| Acute ischemic stroke | 21 (72.4) | 8 (88.9) | |
| Stroke mimic | 8 (27.6) | 1 (11.1) | |
Data are reported as n (%) unless otherwise noted. BP indicates blood pressure; mRS, modified Rankin scale; MSU, mobile stroke unit; NIHSS, National Institutes of Health Stroke Scale; tPA, tissue plasminogen activator.
Race data missing for 6 MSU patients.
mRS data missing for 5 MSU patients and 1 conventional care patient who were lost to follow‐up.
Table 3.
Geographic Characteristics, Treatment Time Metrics, and Functional Outcomes of Thrombolysis‐Treated Patients, Stratified by Treatment Group
| Characteristic | MSU Care (n=29) | Conventional Care (n=9) | P Value |
|---|---|---|---|
| Population density of pick‐up zip code, 1000 people per square mile | 99.9 (31.1) | 90.2 (28.7) | 0.41 |
| Distance to accepting hospital, miles | 2.0 (1.0) | 1.15 (0.6) | 0.03 |
| Distance to closest DSC, miles | 0.8 (0.4) | 1.0 (0.3) | 0.22 |
| No. of DSCs in 0.5‐mile radius at pick‐up | 0.6 (0.5) | 0.4 (0.7) | 0.34 |
| No. of DSCs in 1.0‐mile radius at pick‐up | 1.5 (1.0) | 0.9 (0.6) | 0.10 |
| No. of DSCs in 2.0‐mile radius at pick‐up | 4.8 (1.3) | 2.7 (2.0) | 0.002 |
| Onset to tPA treatment time | 101.0 (46.5) | 143.9 (49.9) | 0.04 |
| Ambulance arrival to tPA treatment time | 48.3 (13.7) | 84.1 (36.6) | <0.001 |
| Dispatch to tPA treatment time | 61.2 (15.27) | 91.6 (39.2) | 0.001 |
| Dispatch to ambulance arrival time | 12.8 (10.8) | 7.4 (4.2) | 0.16 |
| Ambulance arrival to hospital arrival time | 70.4 (15.3) | 31.3 (19.3) | <0.001 |
| Scene departure to hospital arrival time | 12.4 (7.5) | 10.8 (15.6) | 0.68 |
Numbers are reported as mean (SD) and time metrics are reported in minutes, unless otherwise specified. DSC indicates designated stroke center; MSU, mobile stroke unit; tPA, tissue plasminogen activator.
In both unadjusted and adjusted analyses, none of the chosen covariates were predictive of dispatch‐to‐thrombolysis time. There was minimal difference in the point estimate of the primary outcome between the unadjusted and adjusted models (data not shown). Compared with patients in the conventional care group, patients in the MSU group had a mean decrease in dispatch‐to‐thrombolysis time of ≈29.7 minutes (95% CI, 6.9–52.5; Table 4). Within this estimated difference in dispatch‐to‐thrombolysis time, patients in the MSU group had a mean increase in dispatch‐to‐ambulance arrival time of 6.5 minutes (95% CI, 2.4–10.6; P=0.002), which was offset by a mean decrease in ambulance arrival‐to‐thrombolysis time of 36.2 minutes (95% CI, −58.5 to −13.9; P=0.001). The season of ambulance transport was evenly distributed between groups and patients who were treated by intravenous thrombolysis in both groups (χ2 test, P=0.236 and P=0.848, respectively). The results of the post hoc sensitivity analysis were similar to the results of the primary analysis (Table 5). Differences between groups with respect to dispatch‐to‐ambulance arrival time and ambulance arrival‐to‐thrombolysis times were similar to those found in the primary analysis.
Table 4.
Adjusted Intergroup Mean Difference in Primary Outcome and Related Time Intervals
| Outcome | Mean Difference Estimate | 95% CI | P Value |
|---|---|---|---|
| Dispatch to treatment with tPA | −29.7 | −52.5 to −6.9 | 0.01 |
| Dispatch to ambulance arrival | 6.5 | 2.4–10.5 | 0.002 |
| Ambulance arrival to treatment with tPA | −36.2 | −58.5 to −13.9 | 0.001 |
All times in minutes. Conventional care group estimate is subtracted from mobile stroke unit group estimate; model is adjusted for number of designated stroke centers per unit area, population density, and clinical and demographic characteristics. tPA indicates tissue plasminogen activator.
Table 5.
Sensitivity Analysis Adjusting for Season of Ambulance Transport Date
| Outcome | Mean Difference Estimate | 95% CI | P Value |
|---|---|---|---|
| Dispatch to treatment with tPA | −26.1 | −46.2 to −6.0 | 0.01 |
| Dispatch to ambulance arrival | 7.8 | 3.9–11.7 | <0.001 |
| Ambulance arrival to treatment with tPA | −33.9 | −53.4 to −14.5 | <0.001 |
All times in minutes. Conventional care group estimate is subtracted from mobile stroke unit group estimate; model is identical to that in Table 4 with the additional adjustment for season of transport. tPA indicates tissue plasminogen activator.
Discussion
In examining the role of MSU treatment in a densely populated urban area, we found that times from ambulance dispatch to thrombolytic therapy were significantly shorter for patients treated in the MSU compared with patients treated with conventional care at the same institution. These differences were found despite the result that patients in the non‐MSU group traveled shorter distances to accepting facilities and had shorter times from dispatch to scene arrival. Furthermore, in a regression model adjusting for potential confounders, the time‐saving benefit of MSU care persisted despite similarities in clinical, demographic, and geographic characteristics between treatment groups.
Our results are consistent with previously reported reductions in thrombolysis time achieved by MSU care programs from Europe (Berlin and Homburg, Germany) and the United States (Cleveland, OH, and Houston, TX).1, 2, 4, 5 However, the unique geographic characteristics of the New York City area are important when contextualizing our results and may reinforce the potential impact of MSU care in major metropolitan areas. The borough of Manhattan alone has a density of 69 000 people per square mile,17, 18 which represents nearly 5, 10, 20, and 50 times the population densities of Berlin,19, 20 Cleveland, Houston,18 and Homburg,21, 22 respectively. Our findings, taken together with the positive effect of population density on cost‐effectiveness of MSU programs23, 24 and the well‐established beneficial effect of shorter onset to thrombolysis time on postthrombolysis outcomes,25 may support the need for MSU systems of care that can cost‐effectively reduce treatment times in densely populated cities.
Prehospital stroke care systems in New York City are complex and dynamic, involving a large network of non‐MSU ambulances operated by the FDNY and multiple institutional and private agencies. Despite finding treatment time reductions that were consistent with those from other MSU programs, we found that fewer patients were transported by the NYP MSU over a 12‐month period than were reported in the published experiences of other MSU programs operating in less densely populated areas. This may be explained in part by our finding that 87 patients with CVA or CVA‐C designations were transported by conventional ambulances to both WCMC and CUIMC during MSU operating hours at either center. Because our MSU was the first of its kind in New York, it was also the only such unit operating in a given catchment area; considering that we found MSU transport and evaluation required ≈40 minutes more time than in a conventional ambulance, the finding that a number of patients with CVA and CVA‐C call types were transported to either medical center during MSU on‐hours is not entirely unexpected.
Nevertheless, it is unclear whether this population of patients constitutes true “misses” by the MSU. Despite the fact that CVA and CVA‐C call‐types were given to these patients, they did not receive a careful history and physical examination in the field by a neurologist; therefore, only a small subset of this group of patients may been deemed to have a diagnosis of acute stroke on arrival in the emergency department, of which even fewer may have qualified for thrombolytic treatment. In addition to the novelty of our unit in Manhattan, lower than expected activations could also have been caused by lack of FDNY dispatcher familiarity with our MSU. Our MSU was launched as an institutional unit authorized by the FDNY to operate on the EMS network in Manhattan rather than as an official municipal ambulance unit. Regardless, these numbers raise the possibility that recognition of acute stroke by emergency dispatchers may be insufficient.
Legislative efforts from many regions of the United States now require EMS routing of early stroke patients to DSCs,26 and DSC status has been shown to positively affect thrombolysis times.27 The important role played by DSCs in ensuring high‐quality care for patients with acute ischemic stroke, the high density of DSCs in New York City, and the resulting short travel times to such centers are also important factors in the interpretation of our results. Manhattan alone contains ≈0.6 DSC per square mile, which represents 10‐fold that availability in Cleveland28 and 14‐fold that of Berlin.19, 29 The high relative availability of DSCs from most emergency pick‐up locations in Manhattan and the resulting short travel distances to accepting DSCs could potentially reduce the relative time advantage of early computed tomography scanning and thrombolytic administration in MSU care in such urban areas and possibly surpass this advantage through door‐to‐tPA delay reduction programs that have been implemented at many urban hospital centers.30, 31 Despite the potentially adverse effects of many proximate DSCs and longer travel times from dispatch to emergency locations, our findings demonstrate faster dispatch‐to‐thrombolysis time among patients with acute ischemic stroke treated by MSU than in patients treated by conventional ambulance. Moreover, the longer distances traveled by MSU to accepting hospitals may have reflected the treatment advantages derived by the integration of the MSU clinical information system into the NYP electronic health record.10
The finding that patients in the MSU group were more likely to be picked up with a greater number of DSCs in a 2.0‐mile radius than patients in the conventional care group is likely due to a confluence of geographic factors. First, the WCMC catchment area contains twice (n=4) the number of DSCs as the CUIMC catchment area. Second, 47 (70.0%) MSU transports occurred in the WCMC catchment area and 14 (73.7%) of conventional care transports were to CUIMC. Finally, a 2.0‐mile radius (≈12 square miles) represents approximately half the land area of Manhattan and thus is likely to have included many of the island's DSCs. We were not able to determine the exact “catchment area” traveled by conventional ambulances that transported patients to either medical center campus, but taken together, the difference in the number of DSCs in a 2‐mile radius around MSU pick‐up addresses may simply have reflected the relatively higher number of DSCs in the WCMC catchment area combined with multiple additional sections of the island that also contain DSCs.
Finally, we found that more patients with a final diagnosis of a stroke mimic received thrombolysis on the MSU than did patients who were treated with thrombolysis in the conventional care group. Although the exact reason for this finding is uncertain, it is possible that this occurred because of the faster treatment times in the MSU group, whereby MSU‐treated patients had less time to spontaneously improve or more clearly manifest as a mimic. Alternatively, it is possible that the clinicians treating patients aboard the MSU were more aggressive in their administration of intravenous tPA, although we think this is unlikely because the same WCMC and CUIMC faculty rotated on the MSU and conventional hospital‐based stroke services during the study time period.
To our knowledge, this study is the first analysis of the effects of MSU treatment on time to thrombolytic therapy in an urban area as densely populated as New York City. In addition, this analysis appears to be the first that incorporates population density and geocoordinate calculations using availability of stroke center resources at a given ambulance pick‐up location. Moreover, this study included a diverse population taken from 2 academic medical institutions within an urban hospital system.
Despite these advantages, our study was limited by several factors. First, the overall study population size was small, and our comparison group was considerably smaller than the MSU group. However, although smaller, the comparison group was identified in a prospective manner using strict, predefined criteria, including EMS call types and stroke prenotification systems that were as similar as possible to those used to activate the MSU, ensuring as close a comparison as possible. Furthermore, at the time of inclusion in the registry, investigators were blinded to the primary outcome of dispatch‐to‐thrombolysis time among patients in the comparison group, eliminating some selection bias. Second, the use of CVA and CVA‐C call types to constitute the comparison group may have excluded patients who may have been candidates for the MSU had it been available at the time of evaluation. Third, we cannot completely exclude the possibility that the effect of MSU on treatment times may be systematically explained by external independent factors, and we were not able to adjust for potential confounding factors independent of MSU or conventional ambulance transport that could have affected the time to thrombolysis in either group. These include traffic, the day of the week and the time at which the patient was transported, and the distance traveled between the ambulance dispatch and patient pick‐up locations. Last, we lacked access to ambulance radio communications and ambulance call records of conventional ambulance transports to non‐NYP hospitals. This limited our ability to determine the exact reasons for transport of patients by conventional care ambulance to specific destinations, including either medical center campus during MSU operating hours.
In a densely populated urban center with high relative numbers of proximate stroke centers, MSU care was associated with a significant reduction in dispatch‐to‐thrombolysis time for suspected acute ischemic stroke compared with similar patients treated with standard ambulance care. Although the study population was small, these results suggest that an MSU care model may provide a time‐efficient approach to acute stroke care in a densely populated area such as New York City. However, further studies are necessary to investigate the impact of community education on onset‐to‐treatment times, and randomized studies investigating functional and economic outcomes are ongoing in diverse urban areas.32 Results of this work may shed light on the benefits of MSU care in similar metropolitan centers.
Sources of Funding
This work was supported by a grant from the William P. Carey Foundation.
Disclosures
Dr Fink serves as an editor for Relias Learning. The remaining authors have no disclosures to report.
(J Am Heart Assoc. 2019;8:e013529 DOI: 10.1161/JAHA.119.013529.)
References
- 1. Walter S, Kostopoulos P, Haass A, Keller I, Lesmeister M, Schlechtriemen T, Roth C, Papanagiotou P, Grunwald I, Schumacher H, Helwig S, Viera J, Körner H, Alexandrou M, Yilmaz U, Ziegler K, Schmidt K, Dabew R, Kubulus D, Liu Y, Volk T, Kronfeld K, Ruckes C, Bertsch T, Reith W, Fassbender K. Diagnosis and treatment of patients with stroke in a mobile stroke unit versus in hospital: a randomised controlled trial. Lancet Neurol. 2012;11:397–404. [DOI] [PubMed] [Google Scholar]
- 2. Ebinger M, Winter B, Wendt M, Weber JE, Waldschmidt C, Rozanski M, Kunz A, Koch P, Kellner PA, Gierhake D, Villringer K, Fiebach JB, Grittner U, Hartmann A, Mackert BM, Endres M, Audebert HJ; STEMO Consortium . Effect of the use of ambulance‐based thrombolysis on time to thrombolysis in acute ischemic stroke: a randomized clinical trial. JAMA. 2014;311:1622–1631. [DOI] [PubMed] [Google Scholar]
- 3. Itrat A, Taqui A, Cerejo R, Briggs F, Cho SM, Organek N, Reimer AP, Winners S, Rasmussen P, Hussain MS, Uchino K; Cleveland Pre‐Hospital Acute Stroke Treatment Group . Telemedicine in prehospital stroke evaluation and thrombolysis: taking stroke treatment to the doorstep. JAMA Neurol. 2016;73:162–168. [DOI] [PubMed] [Google Scholar]
- 4. Taqui A, Cerejo R, Itrat A, Briggs FB, Reimer AP, Winners S, Organek N, Buletko AB, Sheikhi L, Cho SM, Buttrick M, Donohue MM, Khawaja Z, Wisco D, Frontera JA, Russman AN, Hustey FM, Kralovic DM, Rasmussen P, Uchino K, Hussain MS. Reduction in time to treatment in prehospital telemedicine evaluation and thrombolysis. Neurology. 2017;88:1305–1312. [DOI] [PubMed] [Google Scholar]
- 5. Bowry R, Parker S, Rajan SS, Yamal JM, Wu TC, Richardson L, Noser E, Persse D, Jackson K, Grotta JC. Benefits of stroke treatment using a mobile stroke unit compared with standard management: the BEST‐MSU study run‐in phase. Stroke. 2015;46:3370–3374. [DOI] [PubMed] [Google Scholar]
- 6. Fassbender K, Grotta JC, Walter S, Grunwald IQ, Ragoschke‐Schumm A, Saver JL. Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. Lancet Neurol. 2017;16:227–237. [DOI] [PubMed] [Google Scholar]
- 7. New York City Department of City Planning Population Division . NYC 2010: results from the 2010 Census—population growth and race/Hispanic composition. New York City Department of City Planning, Population Division; April 27, 2018.
- 8. The American Hospital Directory. Individual Hospital Statistics for New York . [Web page]. Summary statistics for non‐federal, short‐term, acute‐care hospitals in the State of New York. Available at: https://www.ahd.com/states/hospital_NY.html. Accessed March 12, 2018.
- 9. Kummer BR, Lerario MP, Ganzman AC, Navi BB, Ribaudo D, Mir S, Pishanidar S, Mehendale R, Williams O, Kamel H, Marshall RS, Elkind MS, Fink ME. Establishing the first mobile stroke unit in New York City. Abstract presented at American Heart Association International Stroke Conference; February 22, 2017; Houston, TX, USA.
- 10. Kummer BR, Lerario MP, Navi BB, Ganzman AC, Ribaudo D, Mir SA, Pishanidar S, Lekic T, Williams O, Kamel H, Marshall RS, Hripcsak G, Elkind MSV, Fink ME. Clinical information systems integration in New York City's first mobile stroke unit. Appl Clin Inform. 2018;9:89–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Brandler ES, Sharma M, McCullough F, Ben‐Eli D, Kaufman B, Khandelwal P, Helzner E, Sinert RH, Levine SR. Prehospital stroke identification: factors associated with diagnostic accuracy. J Stroke Cerebrovasc Dis. 2015;24:2161–2166. [DOI] [PubMed] [Google Scholar]
- 12. U.S. Census Bureau . Maps & data: cartographic boundary shapefiles. Available at: https://www.census.gov/geo/maps-data/data/tiger-cart-boundary.html. Accessed November 5, 2018.
- 13. Health Resources & Services Administration . Health Resources & Services Administration Data Warehouse. Available at: https://datawarehouse.hrsa.gov/data/dataservices/arccatalogconnection.aspx. Accessed March 16, 2018.
- 14. New York State Department of Health . New York State health profiles—hospitals—designated centers. Available at: https://profiles.health.ny.gov/hospital/designated_centers/Stroke+Center. Accessed March 16, 2018.
- 15. van der Laan MJ, Gruber S. Targeted minimum loss based estimation of causal effects of multiple time point interventions. Int J Biostat. 2012;8:1–39. [DOI] [PubMed] [Google Scholar]
- 16. Pearl J. Understanding Propensity Scores. Causality: Models, Reasoning, and Inference. New York, NY: Cambridge University Press; 2009. [Google Scholar]
- 17. United States Census Bureau . United States Census QuickFacts on New York City. 2016. Available at: https://www.census.gov/quickfacts/fact/table/newyorkcitynewyork/PST045217. Accessed April 27, 2018.
- 18. United States Census Bureau . United States Census Bureau QuickFacts: Cleveland City, Ohio; Houston City, TX; New York City (Manhattan Borough), New York; United States. 2010. Available at: https://www.census.gov/quickfacts/fact/table/houstoncitytexas,clevelandcityohio,newyorkcitynewyork,US/PST045218. Accessed April 27, 2018.
- 19. The Berlin Brandenburg Office of Statistics . Population Census—basic data. 2016; Summary population statistics of the Berlin and Brandenburg areas in 2016. Available at: https://www.statistik-berlin-brandenburg.de/BasisZeitreiheGrafik/Bas-Bevoelkerungsstand.asp?Ptyp=300&Sageb=12015&creg=BBB&anzwer=6. Accessed March 16, 2018.
- 20. Federal Statistical Office and Statistical Office of the Lander . Census database—Berlin, population depending on age (five classes of years); Census from the adjusted stock of registers. 2011. Available at: https://ergebnisse.zensus2011.de. Accessed April 27, 2018.
- 21. Homburg District and University City . City profile—Homburg dates and facts. 2015. Available at: https://www.homburg.de/index.php/stadtprofil/stadtinfo/daten-und-fakten. Accessed April 27, 2018.
- 22. Federal Statistical Office and Statistical Office of the Lander . Census database—Homburg, Kreisstadt—population depending on age (five classes of years); Census from the adjusted stock of registers. 2011. Available at: https://ergebnisse.zensus2011.de. Accessed April 27, 2018.
- 23. Gyrd‐Hansen D, Olsen KR, Bollweg K, Kronborg C, Ebinger M, Audebert HJ. Cost‐effectiveness estimate of prehospital thrombolysis: results of the PHANTOM‐S study. Neurology. 2015;84:1090–1097. [DOI] [PubMed] [Google Scholar]
- 24. Dietrich M, Walter S, Ragoschke‐Schumm A, Helwig S, Levine S, Balucani C, Lesmeister M, Haass A, Liu Y, Lossius HM, Fassbender K. Is prehospital treatment of acute stroke too expensive? An economic evaluation based on the first trial. Cerebrovasc Dis. 2014;38:457–463. [DOI] [PubMed] [Google Scholar]
- 25. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H; on behalf of the American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease, Council on Clinical Cardiology . Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 26. Sanossian N, Liebeskind DS, Eckstein M, Starkman S, Stratton S, Pratt FD, Koenig W, Hamilton S, Kim‐Tenser M, Conwit R, Saver JL; Investigators F‐M, Coordinators . Routing ambulances to designated centers increases access to stroke center care and enrollment in prehospital research. Stroke. 2015;46:2886–2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Panezai S, Gezmu T, Kirmani J, Chukwuneke F, Bitra R, Mammo A, Gizzi M. Compliance with joint commission measures in state‐designated stroke centers. J Hosp Med. 2014;9:88–93. [DOI] [PubMed] [Google Scholar]
- 28. The Joint Commission . Quality Check Certification Data Download—Stroke Certification. Available at: https://www.qualitycheck.org/data-download/certification-data-download/. The Joint Commission, Accessed January 5, 2018.
- 29. Koch PM, Kunz A, Ebinger M, Geisler F, Rozanski M, Waldschmidt C, Weber JE, Wendt M, Winter B, Zieschang K, Bollweg K, Kaczmarek S, Endres M, Audebert HJ. Influence of distance to scene on time to thrombolysis in a specialized stroke ambulance. Stroke. 2016;47:2136–2140. [DOI] [PubMed] [Google Scholar]
- 30. Meretoja A, Strbian D, Mustanoja S, Tatlisumak T, Lindsberg PJ, Kaste M. Reducing in‐hospital delay to 20 minutes in stroke thrombolysis. Neurology. 2012;79:306–313. [DOI] [PubMed] [Google Scholar]
- 31. Ruff IM, Ali SF, Goldstein JN, Lev M, Copen WA, McIntyre J, Rost NS, Schwamm LH. Improving door‐to‐needle times: a single center validation of the target stroke hypothesis. Stroke. 2014;45:504–508. [DOI] [PubMed] [Google Scholar]
- 32. Yamal JM, Rajan SS, Parker SA, Jacob AP, Gonzalez MO, Gonzales NR, Bowry R, Barreto AD, Wu TC, Lairson DR, Persse D, Tilley BC, Chiu D, Suarez JI, Jones WJ, Alexandrov A, Grotta JC. Benefits of stroke treatment delivered using a mobile stroke unit trial. Int J Stroke. 2018;13:321–327. [DOI] [PubMed] [Google Scholar]


